Flagellar Growth: Boarder control on the IFT train
Cilia and flagella are cylindrical organelles that are present at the surface of many eukaryotic cells, where they detect changes in the local environment and—when they beat—help the cells to move. An individual cilium or flagellum grows by adding new protein subunits to its tip, using a special mechanism to move proteins from the body of the cell to the tip of the organelle.
Intraflagellar transport, or IFT, was discovered by Joel Rosenbaum and co-workers at Yale University twenty years ago while they were studying the green alga Chlamydomonas, which is a classic model for flagellum studies (Kozminski et al., 1993). In this form of transport, complexes containing about 20 IFT proteins are moved from the base to the tip of the flagella, and are then recycled back towards the base. This movement can be compared to trains travelling on microtubule tracks. At the time, it was proposed that the cargoes, or passengers, on the IFT ‘train’ are the precursors of the axoneme that forms the core of the flagellum (Figure 1A). This very reasonable hypothesis is supported by the observation that construction of the flagellum is inhibited if a single IFT protein is missing (Pazour et al., 2000).
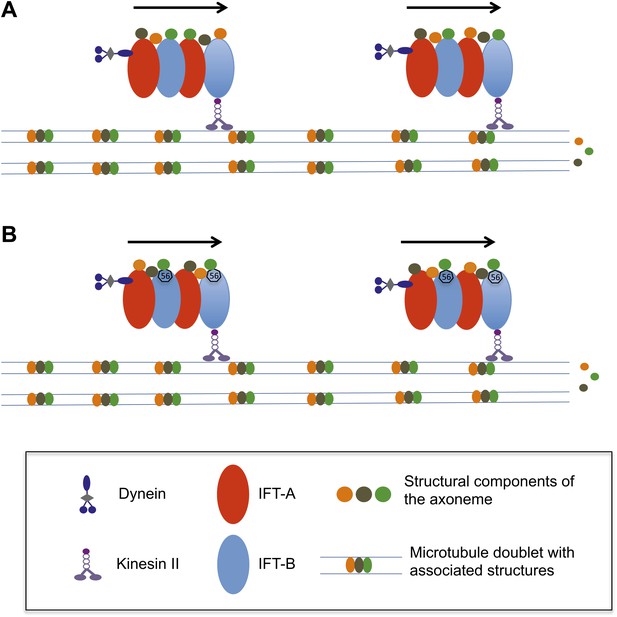
How does the axoneme at the core of a flagellum grow?
(A) The precursor proteins that will become part of the axoneme are loaded onto the IFT trains. These trains are formed of tightly bound IFT proteins (red and blue ovals) and are powered towards the end of the flagellum by kinesin II molecular motors. The precursor proteins (orange, brown and green circles) are randomly distributed within the IFT trains. (B) According to the new findings by Ishikawa et al., a protein called IFT56 (shown here as a black heptagon) could function as a specific adaptor ensuring transport of a limited but specific subset of axoneme components (in this case, the green one).
However, demonstrating the presence of passengers on IFT trains turned out to be very tricky. IFT trains were purified from different organisms, confirming that IFT proteins were tightly bound together, but the presence of cargoes could not be shown convincingly. This suggests that association is transitory and cannot survive biochemical purification. So are the passengers hiding?
Now, in eLife, Hiroaki Ishikawa and Wallace Marshall, both from the University of California, San Francisco, and co-workers in the US, Japan and Germany report on a new IFT protein called IFT56 (also known as DYF13, TTC26B or PIFTC3) that could deliver a specific set of proteins that power the movement of flagella (Ishikawa et al., 2014). IFT56 would therefore function as a train conductor selecting particular proteins to board the train (Figure 1B). This exciting proposal is based on exhaustive analysis of cilia and flagella in zebrafish and Chlamydomonas when IFT56 expression was prevented. A mutation leading to the production of a severely truncated IFT56 protein did not interfere with train speed or frequency, but resulted in the formation of slightly shorter flagella with reduced motility. Proteomic analyses revealed that these flagella contained reduced amounts of several proteins associated with the generation or control of flagellum beating. Although the model is based on indirect evidence, the recent report that cargo proteins can finally be visualised (Wren et al., 2013) means that it is now possible to test this hypothesis: in other words, we will be able to unmask the passengers.
If IFT56 really acts as a conductor, how does it function? Recent data indicate that only a minority of the trains transport cargoes (Wren et al., 2013), despite the presence of IFT56 on all of them. So what controls loading? Is it simply the availability of cargoes or are some proteins marked in some way to indicate that they should be sent to the flagellum? In other words, do passengers need tickets to gain access to the train? This ticket could be a single post-translational modification such as phosphorylation.
The absence of IFT56 affects different organisms in different ways. In protozoa called trypanosomes, an absence of IFT56 causes flagella to go missing (Absalon et al., 2008; Franklin and Ullu, 2010), but in zebrafish (Zhang et al., 2012) and the green alga it only results in shorter flagella. This absence could impact the stability or movement of the IFT train. Perhaps in some species, the conductor is also an engineer, assisting train formation and function.
Alternatively, such a difference could reflect how the stability of the flagellum depends on the elements that power flagellar beating. For example, in Leishmania, the cousins of trypanosomes, a modification to the molecular motor results in the construction of much shorter flagella (Harder et al., 2010). This same modification in Chlamydomonas does not have this effect (Kamiya, 1988). In this case, the different phenotypes would be due to the nature of the flagellum itself–IFT56 and the IFT train would not play a direct role in determining them.
Intriguingly, IFT56 is also associated with IFT trains in immotile cilia that do not possess the motility elements discussed above (Blacque et al., 2005). This may seem to contradict the model proposed by Ishikawa et al. but could be explained by the conductor specialising to detect and transport any cargoes that possess the same ticket. In these conditions, IFT56 could ship very different protein complexes providing a single recognition element is shared between them.
Twenty years after the discovery of intraflagellar transport, we are now getting the first insights about putative cargoes. In the future, progress in live imaging, functional genomics and better understanding of the structure of IFT trains should illuminate the mechanisms by which cargoes are recognised, loaded and delivered to their destination.
References
-
Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomesMolecular Biology of the Cell 19:929–944.https://doi.org/10.1091/mbc.E07-08-0749
-
Functional genomics of the cilium, a sensory organelleCurrent Biology 15:935–941.https://doi.org/10.1016/j.cub.2005.04.059
-
Mutations at twelve independent loci result in absence of outer dynein arms in Chylamydomonas reinhardtiiThe Journal of Cell Biology 107:2253–2258.https://doi.org/10.1083/jcb.107.6.2253
-
A motility in the eukaryotic flagellum unrelated to flagellar beatingProceedings of the National Academy of Sciences of the United States of America 90:5519–5523.https://doi.org/10.1073/pnas.90.12.5519
-
A differential cargo-loading model of ciliary length regulation by IFTCurrent Biology 23:2463–2471.https://doi.org/10.1016/j.cub.2013.10.044
-
Knockdown of ttc26 disrupts ciliogenesis of the photoreceptor cells and the pronephros in zebrafishMolecular Biology of the Cell 23:3069–3078.https://doi.org/10.1091/mbc.E12-01-0019
Article and author information
Author details
Publication history
Copyright
© 2014, Fort and Bastin
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 403
- views
-
- 60
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
Expansion microscopy (ExM) enables nanoscale imaging using a standard confocal microscope through the physical, isotropic expansion of fixed immunolabeled specimens. ExM is widely employed to image proteins, nucleic acids, and lipid membranes in single cells; however, current methods limit the number of samples that can be processed simultaneously. We developed High-throughput Expansion Microscopy (HiExM), a robust platform that enables expansion microscopy of cells cultured in a standard 96-well plate. Our method enables ~4.2 x expansion of cells within individual wells, across multiple wells, and between plates. We also demonstrate that HiExM can be combined with high-throughput confocal imaging platforms to greatly improve the ease and scalability of image acquisition. As an example, we analyzed the effects of doxorubicin, a known cardiotoxic agent, on human cardiomyocytes (CMs) as measured by the Hoechst signal across the nucleus. We show a dose-dependent effect on nuclear DNA that is not observed in unexpanded CMs, suggesting that HiExM improves the detection of cellular phenotypes in response to drug treatment. Our method broadens the application of ExM as a tool for scalable super-resolution imaging in biological research applications.
-
- Cell Biology
- Developmental Biology
Eukaryotic cells depend on exocytosis to direct intracellularly synthesized material toward the extracellular space or the plasma membrane, so exocytosis constitutes a basic function for cellular homeostasis and communication between cells. The secretory pathway includes biogenesis of secretory granules (SGs), their maturation and fusion with the plasma membrane (exocytosis), resulting in release of SG content to the extracellular space. The larval salivary gland of Drosophila melanogaster is an excellent model for studying exocytosis. This gland synthesizes mucins that are packaged in SGs that sprout from the trans-Golgi network and then undergo a maturation process that involves homotypic fusion, condensation, and acidification. Finally, mature SGs are directed to the apical domain of the plasma membrane with which they fuse, releasing their content into the gland lumen. The exocyst is a hetero-octameric complex that participates in tethering of vesicles to the plasma membrane during constitutive exocytosis. By precise temperature-dependent gradual activation of the Gal4-UAS expression system, we have induced different levels of silencing of exocyst complex subunits, and identified three temporarily distinctive steps of the regulated exocytic pathway where the exocyst is critically required: SG biogenesis, SG maturation, and SG exocytosis. Our results shed light on previously unidentified functions of the exocyst along the exocytic pathway. We propose that the exocyst acts as a general tethering factor in various steps of this cellular process.





