The potassium channel subunit KV1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells
eLife assessment
This study provides direct evidence showing that KV1.8 channels provide the basis for several potassium currents in the two types of sensory hair cells found in the mouse vestibular system. This is an important finding because the nature of the channels underpinning the unusual potassium conductance gK,L in type I hair cells has been under scrutiny for many years. The experimental evidence is compelling and the analysis is rigorous. The study will be of interest to cell and molecular biologists as well as vestibular and auditory neuroscientists.
https://doi.org/10.7554/eLife.94342.4.sa0Important: Findings that have theoretical or practical implications beyond a single subfield
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Compelling: Evidence that features methods, data and analyses more rigorous than the current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
In amniotes, head motions and tilt are detected by two types of vestibular hair cells (HCs) with strikingly different morphology and physiology. Mature type I HCs express a large and very unusual potassium conductance, gK,L, which activates negative to resting potential, confers very negative resting potentials and low input resistances, and enhances an unusual non-quantal transmission from type I cells onto their calyceal afferent terminals. Following clues pointing to KV1.8 (Kcna10) in the Shaker K channel family as a candidate gK,L subunit, we compared whole-cell voltage-dependent currents from utricular HCs of KV1.8-null mice and littermate controls. We found that KV1.8 is necessary not just for gK,L but also for fast-inactivating and delayed rectifier currents in type II HCs, which activate positive to resting potential. The distinct properties of the three KV1.8-dependent conductances may reflect different mixing with other KV subunits that are reported to be differentially expressed in type I and II HCs. In KV1.8-null HCs of both types, residual outwardly rectifying conductances include KV7 (Knq) channels. Current clamp records show that in both HC types, KV1.8-dependent conductances increase the speed and damping of voltage responses. Features that speed up vestibular receptor potentials and non-quantal afferent transmission may have helped stabilize locomotion as tetrapods moved from water to land.
Introduction
The receptor potentials of hair cells (HCs) are strongly shaped by large outwardly rectifying K+ conductances that are differentially expressed according to HC type. Here, we report that a specific voltage-gated K+ (KV) channel subunit participates in very different KV channels dominating the membrane conductances of type I and II HCs in amniote vestibular organs.
Type I HCs occur only in amniote vestibular organs. Their most distinctive features are that they are enveloped by a calyceal afferent terminal (Wersall, 1956; Lysakowski and Goldberg, 2004) and that they express gK,L (Correia and Lang, 1990; Rennie and Correia, 1994; Rüsch and Eatock, 1996a): a large non-inactivating conductance with an activation range from –100 to –60 mV, far more negative than other ‘low-voltage-activated’ KV channels. HCs are known for their large outwardly rectifying K+ conductances, which repolarize membrane voltage following a mechanically evoked perturbation and in some cases contribute to sharp electrical tuning of the HC membrane. gK,L is unusually large and unusually negatively activated, and therefore strongly attenuates and speeds up the receptor potentials of type I HCs (Correia et al., 1996; Rüsch and Eatock, 1996b). In addition, gK,L augments non-quantal transmission from type I HC to afferent calyx by providing open channels for K+ flow into the synaptic cleft (Contini et al., 2012; Contini et al., 2017; Contini et al., 2020; Govindaraju et al., 2023), increasing the speed and linearity of the transmitted signal (Songer and Eatock, 2013).
Type II HCs have compact afferent synaptic contacts (boutons) where the receptor potential drives quantal release of glutamate. They have fast-inactivating (A-type, gA) and delayed rectifier (gDR) conductances that are opened by depolarization above resting potential (Vrest).
The unusual properties of gK,L have long attracted curiosity about its molecular nature. gK,L has been proposed to include ‘M-like’ KV channels in the KV7 and/or erg channel families (Kharkovets et al., 2000; Hurley et al., 2006; Holt et al., 2007). The KV7.4 subunit was of particular interest because it contributes to the low-voltage-activated conductance, gK,n, in cochlear outer HCs, but was eventually eliminated as a gK,L subunit by experiments on KV7.4-null mice (Spitzmaul et al., 2013).
Several observations suggested the KV1.8 (KCNA10) subunit as an alternative candidate for gK,L. KV1.8 is highly expressed in vestibular sensory epithelia (Carlisle et al., 2012), particularly HCs (Lee et al., 2013; Scheffer et al., 2015; McInturff et al., 2018), with slight expression elsewhere (skeletal muscle, Lee et al., 2013; kidney, Yao et al., 2002). Kcna10–/– mice show absent or delayed vestibular-evoked potentials, the synchronized activity of afferent nerve fibers sensitive to fast linear head motions (Lee et al., 2013). Unique among KV1 channels, KV1.8 has a cyclic nucleotide-binding domain (Lang et al., 2000) with the potential to explain gK,L’s known cGMP dependence (Behrend et al., 1997; Chen and Eatock, 2000).
Our comparison of whole-cell currents and immunohistochemistry in type I HCs from Kcna10–/– and Kcna10+/+,+/– mouse utricles showed that KV1.8 expression is necessary for gK,L. More surprisingly, KV1.8 expression is also required for A-type and delayed rectifier conductances of type II HCs. In both HC types, eliminating the KV1.8-dependent major conductances revealed a smaller delayed rectifier conductance involving KV7 channels. Thus, the distinctive outward rectifiers that produce such different receptor potentials in type I and II HCs both include KV1.8 and KV7 channels.
Results
We compared whole-cell voltage-activated K+ currents in type I and II HCs from homozygous knockout (Kcna10–/–) animals and their wildtype (Kcna10+/+) or heterozygote (Kcna10+/–) littermates. We immunolocalized KV1.8 subunits in the utricular epithelium and pharmacologically characterized the residual K+ currents of Kcna10–/– animals. Current clamp experiments demonstrated the impact of KV1.8-dependent currents on passive membrane properties.
We recorded from three utricular zones: lateral extrastriola (LES), striola, and medial extrastriola (MES); striolar and extrastriolar zones have many structural and functional differences and give rise to afferents with different physiology (reviewed in Goldberg, 2000; Eatock and Songer, 2011). Recordings are from 412 type I and II HCs (53% LES, 30% MES, 17% striola) from mice between postnatal day (P) 5 and P370. We recorded from such a wide age range to test for developmental or senescent changes in the impact of the null mutation. Above P18, we did not see substantial changes in KV channel properties, as reported (González-Garrido et al., 2021).
Kcna10–/– animals appeared to be healthy and to develop and age normally, as reported (Lee et al., 2013), and HCs were healthy (see Methods for criteria).
KV1.8 is necessary for gK,L in type I HCs
The large low-voltage-activated conductance, gK,L, in Kcna10+/+,+/– type I HCs produces distinctive whole-cell current responses to voltage steps, as highlighted by our standard type I voltage protocol (Figure 1A). From a holding potential within the gK,L activation range (here –74 mV), the cell is hyperpolarized to –124 mV, negative to EK and the activation range, producing a large inward current through open gK,L channels that rapidly decays as the channels deactivate. We use the large transient inward current as a hallmark of gK,L. The hyperpolarization closes all gK,L channels, and then the activation function is probed with a series of depolarizing steps, obtaining the maximum conductance from the peak tail current at –44 mV (Figure 1A). We detected no difference between the Boltzmann parameters of gK,L G–V curves from Kcna10+/– and Kcna10+/+ type I HCs.
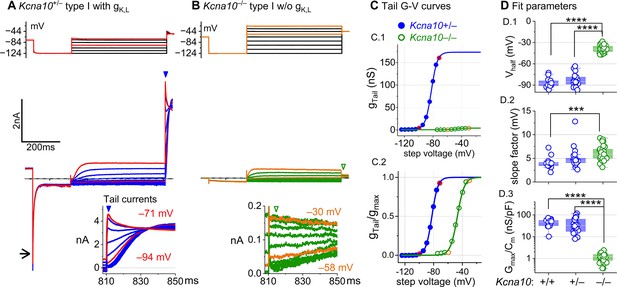
Kcna10–/– type I hair cells (HCs) lacked gK,L, the dominant conductance in mature Kcna10+/+,++/–– type I HCs.
Representative voltage-evoked currents in (A) a P22 Kcna10+/– type I HC and (B) a P29 Kcna10–/– type I HC. (A) Arrow, transient inward current that is a hallmark of gK,L. Arrowheads, tail currents, magnified in insets. For steps positive to the midpoint voltage, tail currents are very large. As a result, K+ accumulation in the calyceal cleft reduces driving force on K+, causing currents to decay rapidly, as seen in A (Lim et al., 2011). Note that the voltage protocol (top) in B extends to more positive voltages. (C) Activation (G–V) curves from tail currents in A and B; symbols, data; curves, Boltzmann fits (Equation 1). (D) Fit parameters from mice >P12 show big effect of Kcna10–/– and no difference between Kcna10+/– and Kcna10+/+. (D.1), Tukey’s test: +/+ vs –/–, p<1E-9; +/– vs –/–, p<1E-9. (D.2), Tukey’s test: +/+ vs –/–, p=9.4E-4. (D.3), Tukey’s test: +/+ vs –/–, p<1E-9; +/– vs –/–, p<1E-9. Asterisks: ***p < 0.001; and ****p < 0.0001. Line, median; Box, interquartile range; Whiskers, outliers. See Table 1 for statistics.
Type I hair cell KV activation voltage dependence.
Mean ± SEM (number of cells). g is effect size, Hedge’s g. KWA is Kruskal–Wallis ANOVA.
| Zone | Kcna10 | Tail V1/2, mV* | Tail S, mV† | Tail gmax, nS‡ | Tail gmax/Cm, nS/pF§ | Age (median, range) |
|---|---|---|---|---|---|---|
| Extrastriola | +/+ | –85 ± 2 (12) | 4.3 ± 0.4 (12) | 270 ± 40 (11) | 47 ± 8 (11) | 22, 14–287 |
| +/– | –83 ± 1 (40) | 5.2 ± 0.3 (40) | 210 ± 20 (40) | 37 ± 4 (40) | 19, 13–259 | |
| –/– | –40.2 ± 0.9 (26) | 5.7 ± 0.3 (26) | 5.4 ± 0.3 (26) | 1.11 ± 0.08 (26) | 45, 14–277 | |
| Striola | +/+ | –87 ± 3 (6) | 4.3 ± 0.3 (6) | 310 ± 70 (6) | 41 ± 7 (6) | 40, 15–59 |
| +/– | –88 ± 2 (3) | 4.7 ± 0.9 (3) | 270 ± 60 (3) | 44 ± 6 (3) | 19, 14–20 | |
| –/– | –38 ± 1 (13) | 6.2 ± 0.4 (13) | 6.5 ± 0.6 (13) | 1.5 ± 0.1 (13) | 202, 14–370 |
-
*
–/– vs +/+: two-way ANOVA, p < 1E−9, g 7.7; –/– vs +/–: two-way ANOVA, p < 1E−9, g 6.8.
-
†
–/– vs +/+: two-way ANOVA, p = 8.4E−4, g 1.2.
-
‡
–/– vs +/+: two-way ANOVA, p < 1E−9, g 3.7; –/– vs +/–: two-way ANOVA, p < 1E−9, g 2.1.
-
§
–/– vs +/+: two-way ANOVA, p < 1E−9, g 3.6; –/– vs +/–: two-way ANOVA, p < 1E−9, g 2.0.
For a similar voltage protocol, Kcna10–/– type I HCs (Figure 1B) produced no inward transient current at the step from holding potential to –124 mV and much smaller depolarization-activated currents during the iterated steps, even at much more positive potentials. Figure 1C compares the conductance–voltage (G–V, activation) curves fit to tail currents (Equation 1; see insets in Figure 1A, B): the maximal conductance (gmax) of the Kcna10–/– HC was over 10-fold smaller (Figure 1C.1), and the curve was positively shifted by >40 mV (Figure 1C.2). Figure 1D shows the G–V Boltzmann fit parameters for type I HCs from mice >P12, an age at which type I HCs normally express gK,L (Rüsch et al., 1998).
In type I HCs from Kcna10+/+,+,– mice, the G–V parameters of outwardly rectifying currents transitioned over the first 15–20 postnatal days from values for a conventional delayed rectifier, activating positive to resting potential, to gK,L values (Figure 1—figure supplement 1A), as previously described (Rüsch et al., 1998; Géléoc et al., 2004; Hurley et al., 2006). Between P5 and P10, some type I HCs have not yet acquired the physiologically defined conductance, gK,L. No effects of KV1.8 deletion were seen in the delayed rectifier currents of immature type I HCs (Figure 1—figure supplement 1B), showing that they were not immature forms of the KV1.8-dependent gK,L channels.
Kcna10–/– type I HCs had a much smaller residual delayed rectifier that activated positive to resting potential, with Vhalf ~–40 mV and gmax density of 1.3 nS/pF. No additional K+ conductance activated up to +40 mV, and G–V parameters did not change much with age from P5 to P370. We characterize this KV1.8-independent delayed rectifier later. A much larger non-gK,L delayed rectifier conductance (gDR,I) was reported in our earlier publication on mouse utricular type I HCs (Rüsch et al., 1998). This current was identified as that remaining after ‘blocking’ gK,L with 20 mM external Ba2+. Our new data suggest that there is no large non-gK,L conductance, and that instead high Ba2+ positively shifted the apparent voltage dependence of gK,L.
KV1.8 strongly affects type I passive properties and responses to current steps
While the cells of Kcna10–/– and Kcna10+/– epithelia appeared healthy, type I HCs had smaller membrane capacitances (Cm): 4–5 pF in Kcna10–/– type I HCs, ~20% smaller than Kcna10+/– type I HCs (~6 pF) and ~30% smaller than Kcna10+/+ type I HCs (6–7 pF; Table 2). While Cm scales with surface area, the lack of change in soma sizes by deletion of KV1.8 (Supplementary file 1b) suggests that surface area was not different. Instead, C may be higher in Kcna10+/+ cells because of gK,L for two reasons. First, highly expressed trans-membrane proteins (see discussion of gK,L channel density in Chen and Eatock, 2000) can affect membrane thickness (Mitra et al., 2004), which is inversely proportional to specific Cm. Second, resistive current through gK,L could contaminate estimations of capacitive current, which is calculated from the decay time constant of transient current evoked by a small voltage step negative to –90 mV, where we measured Cm (see Methods).
Type I hair cell passive membrane properties in the extrastriola (ES) and striola (S).
Mean ± SEM (number of cells). g is effect size, Hedge’s g. KWA is Kruskal–Wallis ANOVA.
| Zone | Kcna10 | Vrest, mV*, † | Rinput, MΩ‡ | , ms§ | Cm, pF¶ | Age (median, range) |
|---|---|---|---|---|---|---|
| ES | +/+ | –84 ± 3 (6) | 44 ± 6 (6) | 0.24 ± 0.03 (6) | 6.1 ± 0.4 (13) | 20, 14–287 |
| +/– | –88.0 ± 0.7 (28) | 55 ± 5 (24) | 0.32 ± 0.03 (23) | 5.8 ± 0.2 (44) | 21, 16–29 | |
| –/– | –63 ± 2 (15) | 1400 ± 100 (15) | 6.4 ± 0.6 (15) | 5.0 ± 0.2 (27) | 45, 14–202 | |
| S | +/+ | –87 ± 2 (4) | 50 ± 8 (4) | 0.30 ± 0.04 (4) | 7.4 ± 0.7 (7) | 43, 40–59 |
| +/– | –87 ± 3 (3) | 38 ± 8 (2) | 0.21 ± 0.01 (2) | 5.9 ± 0.6 (3) | 19, 19–20 | |
| –/– | –74 ± 5 (5) | 1000 ± 300 (4) | 4.2 ± 1.0 (4) | 4.4 ± 0.2 (14) | 202, 24–370 |
-
*
Striolar –/– vs ES –/–: two-way ANOVA, p = 0.006, g 1.2; striolar –/– vs striolar +/+,+/–: two-way ANOVA, p = 0.005, g 1.7.
-
†
–/– vs +/+: two-way ANOVA, p < 1E−9, g 2.3; –/– vs +/–: two-way ANOVA, p < 1E−9, g 3.4.
-
‡
–/– vs +/+: two-way ANOVA, p < 1E−9, g 3.1; –/– vs +/–: two-way ANOVA, p < 1E−9, g 3.9.
-
§†
–/– vs +/+: two-way ANOVA, p < 1E−9, g 2.7; –/– vs +/–: two-way ANOVA, p < 1E−9, g 3.4.
-
¶‡
–/– vs +/+: two-way ANOVA, p = 3E−7, g 1.5; –/– vs +/–: two-way ANOVA, p = 1.3E−4, g 1.0; +/–vs +/+: two-way ANOVA, p = 0.048, g 0.6.
Basolateral conductances help set the resting potential and passive membrane properties that regulate the time course and gain of voltage responses to small currents. To examine the effect of KV1.8 on these properties, we switched to current clamp mode and measured resting potential (Vrest), input resistance (Rin, equivalent to voltage gain for small current steps, ΔV/ΔI), and membrane time constant (). In Kcna10–/– type I HCs, Vrest was much less negative (Figure 2A.1), Rin was greater by ~20-fold (Figure 2A.2), and membrane charging times were commensurately longer (Figure 2A.3).
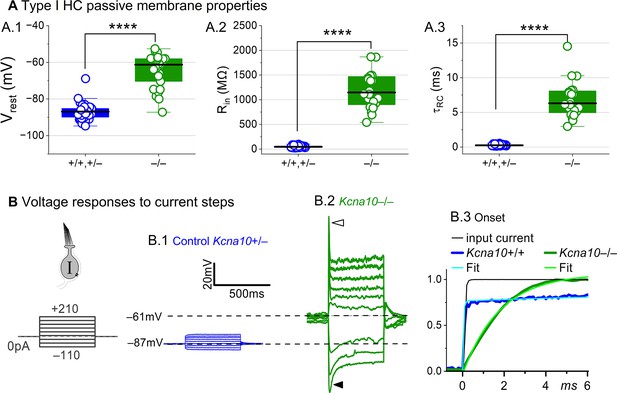
Kcna10–/– type I hair cells (HCs) had much longer membrane charging times and higher input resistances (voltage gains) than Kcna10+/+,+/– type I HCs.
(A) gK,L strongly affects passive membrane properties: (A.1) Vrest, Tukey’s test p<1E-9, (A.2) Rin, input resistance, Tukey’s test p<1E-9, and (A.3) membrane time constant, , Tukey’s test p<1E-9. See Table 2 for all statistics. (B) Current clamp responses to the same scale from (B.1) Kcna10+/– and (B.2) Kcna10–/– type I cells, both P29. Filled arrowhead (B.2), sag indicating IH activation. Open arrowhead, Depolarization rapidly decays as IDR activates. (B.3) First 6 ms of voltage responses to 170 pA injection, normalized to steady-state value; curves, double-exponential fits (Kcna10+/+, 40 μs and 2.4 ms) and single-exponential fits (Kcna10–/–, 1.1 ms). Asterisks, ****p < 0.0001. Line, median; Box, interquartile range; Whiskers, outliers.
The differences between the voltage responses of Kcna10+/+,+/– and Kcna10–/– type I HCs are expected from the known impact of gK,L on Vrest and Rin (Correia and Lang, 1990; Ricci et al., 1996; Rüsch and Eatock, 1996b; Songer and Eatock, 2013). The large K+-selective conductance at Vrest holds Vrest close to EK (K+ equilibrium potential) and minimizes gain (ΔV/ΔI), such that voltage-gated conductances are negligibly affected by the input current and the cell produces approximately linear, static responses to iterated current steps. For Kcna10–/– type I HCs, with their less negative Vrest and larger Rin, positive current steps evoked a fast initial depolarization (Figure 2B.2), activating residual delayed rectifiers and repolarizing the membrane toward EK. Negative current steps evoked an initial hyperpolarization followed by a slowly repolarizing ‘sag’ (Figure 2B.2) as the HCN1 channels open (Rüsch and Eatock, 1996b; Horwitz et al., 2011).
Overall, comparison of the Kcna10+/+,+/– and Kcna10–/– responses shows that with KV1.8 (gK,L), the voltage response of the type I HC is smaller but better reproduces the time course of the input current.
KV1.8 is necessary for both inactivating and non-inactivating KV currents in type II HCs
Type II HCs also express KV1.8 mRNA (McInturff et al., 2018; Orvis et al., 2021). Although their outwardly rectifying conductances (gA and gDR) differ substantially in voltage dependence and size from gK,L, both conductances were strongly affected by the null mutation: gA was eliminated and the delayed rectifier was substantially smaller. Below we describe gA and gDR in Kcna10+/+,+/– type II HCs and the residual outward-rectifying current in Kcna10–/– type II HCs.
Kcna10+/+,+/– type II HCs. Most (81/84) Kcna10+/+,+/– type II HCs expressed a rapidly activating, rapidly inactivating A-type conductance (gA). We define A current as the outwardly rectifying current that inactivates by over 30% within 200 ms. gA was more prominent in extrastriolar zones, as reported (Holt et al., 1999; Weng and Correia, 1999).
We compared the activation and inactivation time course and inactivation prominence for 200 ms steps from –124 to ~30 mV. Outward currents fit with Equation 3 yielded fast inactivation time constants () of ~30 ms in LES (Figure 3A.2). was faster in LES than in MES or striola (Figure 3A.3) and fast inactivation was a larger fraction of the total inactivation in LES than striola (~0.5 vs 0.3, Figure 3A.4).
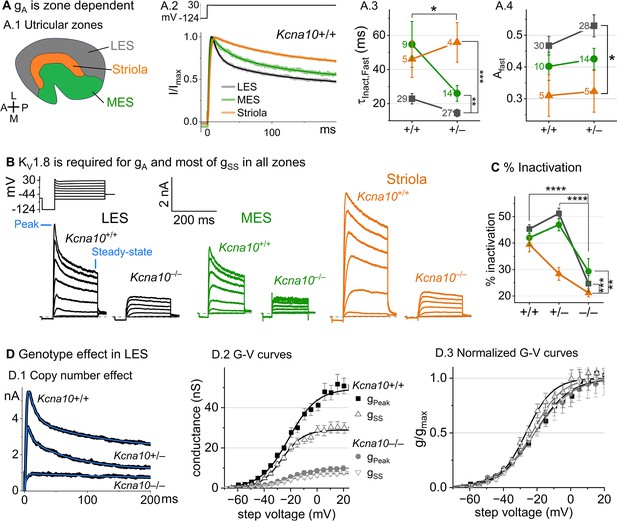
Kcna10–/– type II hair cells (HCs) in all zones of the sensory epithelium lacked the major rapidly inactivating conductance, gA, and had less delayed rectifier conductance.
Activation and inactivation varied with epithelial zone and genotype. (A) gA inactivation time course varied across zones. (A.1) Zones of the utricular epithelium: lateral extrastriola (LES), medial extrastriola (MES), and striola (S). (A.2) Normalized currents evoked by steps from –124 to +30 mV with overlaid fits of Equation 3. (A.3) was faster in Kcna10+/– (n=45) than Kcna10+/+ (n=43) HCs (KWA, p=0.027), and faster in LES (n=56) than MES (n=23, KWA, p=0.002) or S (n=9, KWA, p=2E-4). Point label is number of cells. Brackets show post hoc pairwise comparisons between two zones (vertical brackets) and horizontal brackets compare two genotypes; see Table 3 for statistics on kinetics. (A.4) Fast inactivation was a greater fraction of total inactivation in LES (n=58) than striola (n=10, Tukey’s test p=0.0041). (B) Exemplars; ages, left to right, P312, P53, P287, P49, P40, P154. (C) % inactivation at 30 mV was much lower in Kcna10–/– (n=37) than Kcna10+/– (n=47, Tukey’s HSD, p<1E-9) and Kcna10+/+ (n=44, Tukey’s HSD, p<1E-9). % inactivation was lower in striola (n=16) than LES (n=77, Tukey’s HSD, p=3E-5) and MES (n=36, Tukey’s HSD, p=0.0011). 2-way ANOVA detected interaction between zone and genotype, p=0.026 (Table 3). (D) Exemplar currents and G–V curves from LES type II HCs show a copy number effect. (D.1) Exemplar currents evoked by steps from –124 to +30 mV fit with Equation 3. (D.2) Averaged peak and steady-state conductance–voltage data points from LES cells (+/+, n=37; –/–, n=20) were fit with Boltzmann equations (Equation 1) and normalized by gmax in (D.3). Asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. Error bars, SEM. See Table 4 for statistics on voltage dependence.
Type II hair cell KV currents: activation and inactivation time course at +30 mV.
Mean ± SEM. g is effect size, Hedge’s g. KWA is Kruskal–Wallis ANOVA.
| Zone | Kcna10 | at 30 mV, ms*, † | at 30 mV, ms ‡, § | Fast inactivation prominence¶ | Inactivation %**,†† | N cells | Age (median, range) |
|---|---|---|---|---|---|---|---|
| LES | +/+ | 2.11 ± 0.09 | 23 ± 3 | 0.46 ± 0.03 | 45 ± 2 | 30 | 46, 14–312 |
| +/– | 1.64 ± 0.09 | 15 ± 2 | 0.53 ± 0.03 | 51 ± 2 | 27 | 29, 13–280 | |
| –/– | 4.4 ± 0.5 | NA | NA | 25 ± 3 | 21 | 128, 15–355 | |
| MES | +/+ | 2.8 ± 0.5 | 50 ± 10 | 0.40 ± 0.04 | 42 ± 3 | 9 | 94, 22–296 |
| +/– | 2.2 ± 0.2 | 90 ± 60 | 0.42 ± 0.03 | 47 ± 2 | 15 | 24, 13–52 | |
| –/– | 10 ± 7 | NA | NA | 29 ± 5 | 10 | 84, 28–355 | |
| Striola | +/+ | 2.7 ± 0.3 | 50 ± 10 | 0.31 ± 0.07 | 39 ± 3 | 5 | 45, 40–287 |
| +/– | 2.9 ± 0.4 | 300 ± 200 | 0.3 ± 0.06 | 28 ± 2 | 5 | 19, 14–30 | |
| –/– | 7 ± 2 | NA | NA | 22 ± 2 | 6 | 202, 29–298 |
-
*
–/– vs +/+: KWA, p = 0.0048, g 0.6; –/– vs +/–: KWA, p = 2.3E−7, g 0.6.
-
†
Striola vs LES: KWA, p = 5.7E−4, g 1.0.
-
‡
+/– vs +/+: KWA, p = 0.027, g 0.2.
-
§
LES vs MES: KWA, p = 0.0018, g 0.3; LES vs Striola: KWA, p = 1.9E−4, g 0.8.
-
¶
LES vs Striola: two-way ANOVA, p = 0.0041, g 0.7.
-
**
–/– vs +/+: two-way ANOVA, p < 1E−9, g 1.7; –/– vs +/–: two-way ANOVA, p < 1E−9, g 1.8.
-
††
Striola vs LES: two-way ANOVA, p = 3.4E−5, g 0.9; Striola vs MES: two-way ANOVA, p = 0.0011, g 1.0; interaction between genotype and zone: two-way ANOVA, p = 0.026.
To show voltage dependence of activation, we generated G–V curves for peak currents (sum of A-current and delayed rectifier) and steady-state currents measured at 200 ms, after gA has mostly inactivated (Figure 3D.2). Kcna10+/– HCs had smaller currents than Kcna10+/+ HCs, reflecting a smaller gDR (Figure 3D) and faster fast inactivation (Figure 3A.3). As discussed later, these effects may relate to effects of the Kcna10 gene dosage on the relative numbers of different KV1.8 heteromers.
For Kcna10+/+ and Kcna10+/– HCs, the voltage dependence as summarized by Vhalf and slope factor (S) was similar. Relative to gSS, gPeak had a more positive Vhalf (~–21 vs ~–26) and greater S (~12 vs ~9, Figure 3D, Table 4). Because gPeak includes channels with and without fast inactivation, the shallower gPeak–V curve may reflect a more heterogeneous channel population. Only gPeak showed zonal variation, with more positive Vhalf in LES than striola (~–20 vs ~–24 mV, Figure 3D, Table 4). We later suggest that variable subunit composition may drive zonal variation in gPeak.
Type II hair cell KV currents: activation voltage dependence.
Mean ± SEM. g is effect size, Hedge’s g. KWA is Kruskal–Wallis ANOVA.
| Zone | Kcna10 | Peak V1/2, mV** | Peak S, mV††, ‡ | A-type gmax/Cm, nS/pF§ § | SS Vhalf, mV¶ ¶ | SS S, mV**** | SS gmax/Cm, nS/pF †† ‡ ‡ | N cells | Age (median, range) |
|---|---|---|---|---|---|---|---|---|---|
| LES | +/+ | –19.8 ± 0.6 | 11.8 ± 0.4 | 4.0 ± 0.3 | –25.0 ± 0.5 | 8.7 ± 0.3 | 7.1 ± 0.8 | 37 | 46, 14–312 |
| +/– | –19.8 ± 0.8 | 12.8 ± 0.4 | 3.8 ± 0.3 | –26.8 ± 0.8 | 8.7 ± 0.3 | 4.9 ±0.4 | 35 | 29, 13–280 | |
| –/– | –18 ± 1 | 11.7 ± 0.4 | 0.37 ± 0.05 | –19 ± 1 | 12.1 ± 0.5 | 1.8 ±0.2 | 20 | 128, 15–355 | |
| MES | +/+ | –22 ± 1 | 11 ± 0.7 | 4.1 ± 0.7 | –26 ± 1 | 8.3 ± 0.5 | 9 ±1 | 11 | 94, 22–296 |
| +/– | –21 ± 1 | 11.8 ± 0.4 | 3.6 ± 0.5 | –27 ± 1 | 9.0 ± 0.3 | 5.9 ±0.7 | 16 | 24, 13–52 | |
| –/– | –19 ± 1 | 10.8 ± 0.6 | 0.6 ± 0.1 | –20 ± 1 | 10.7 ± 0.7 | 2.5 ±0.3 | 15 | 84, 28–355 | |
| Striola | +/+ | –24 ± 1 | 9.6 ± 0.5 | 5 ± 1 | –26.6 ± 0.9 | 8.2 ± 0.4 | 12 ±1 | 7 | 45, 40–287 |
| +/– | –25 ± 2 | 9.4 ± 0.4 | 2.6 ± 0.6 | –28 ± 2 | 8.2 ± 0.3 | 10±2 | 6 | 19, 14–30 | |
| –/– | –21.3 ± 0.9 | 10.3 ± 0.5 | 0.7 ± 0.1 | –21.7 ± 0.8 | 10.5 ± 0.6 | 3.9±0.5 | 8 | 202, 29–298 |
-
*
Striola vs LES: two-way ANOVA, p = 0.00116, g 0.9.
-
†
Striola vs MES: two-way ANOVA, p = 0.016, g 0.8; Striola vs LES: two-way ANOVA, p = 7.5E−6, g 1.2.
-
‡
–/– vs +/–: two-way ANOVA, p = 0.036, g 0.5.
-
§
–/– vs +/+: Welch ANOVA, p < 1E−9, g 2.3; –/– vs +/–: Welch ANOVA, p < 1E−9, g 2.3.
-
¶
–/– vs +/+: two-way ANOVA, p < 1E−9, g 1.4; –/– vs +/–: two-way ANOVA, p < 1E−9, g 1.6.
-
**
–/– vs +/+: two-way ANOVA, p < 1E−9, g 1.4; –/– vs +/–: two-way ANOVA, p = 4.5E−7, g 1.1.
-
††
–/– vs +/+: Welch ANOVA, p < 1E−9, g 1.6; –/– vs +/–: Welch ANOVA, p < 1E−9, g 1.3; +/+vs +/–: Welch ANOVA, p = 0.007, g 1.6.
-
‡ ‡
Striola vs LES: one-way ANOVA, p = 0.001, g (0.9); Striola vs MES: one-way ANOVA, p = 0.01, g 0.8.
Kcna10–/– type II HCs. Kcna10–/– type II HCs from all zones were missing gA and 30–50% of gDR (Figure 3B–D). The residual delayed rectifier (1.3 nS/pF) had a more positive Vhalf than gDR in Kcna10+/+,+/– HCs (~–20 vs ~–26 mV, Figure 3D.2). We refer to the KV1.8-dependent delayed rectifier component as gDR(KV1.8) and to the residual, KV1.8-independent delayed rectifier component as gDR(Kv7) because, as we show later, it includes KV7 channels.
Figure 3—figure supplement 2 shows the development of KV1.8-dependent and -independent KV currents in type II HCs with age from P5 to over P300. In Kcna10+/+,+/– type II HCs, gA was present at all ages with a higher % inactivation after P18 than at P5–P10 (Figure 3—figure supplement 2A.4). gPeak did not change much above P12 except for a compression of conductance density from P13 to P370 (partial correlation coefficient = –0.4, p = 2E−5, Figure 3—figure supplement 2A.3).
We saw small rapidly inactivating outward currents in a minority of Kcna10–/– type II HCs (23%, 7/30), all >P12 and extrastriolar (Figure 3—figure supplement 3). These currents overlapped with gA in percent inactivation, inactivation kinetics, and activation voltage dependence but were very small. As discussed later, we suspect that these currents flow through homomers of inactivating KV subunits that in control HCs join with KV1.8 subunits and confer inactivation on the heteromeric conductance.
KV1.8 affects type II passive properties and responses to current steps
In type II HCs, absence of KV1.8 did not change Vrest (Figure 4A.1) because gA and gDR both activate positive to rest, but significantly increased Rin and (Figure 4A.2 and A.3).
Kcna10–/– type II hair cells (HCs) had larger, slower voltage responses and more electrical resonance.
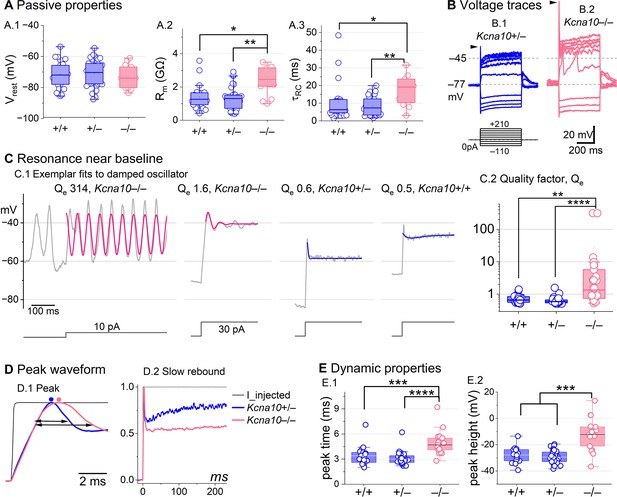
(A) Passive membrane properties near resting membrane potential: (A.1) Resting potential. Rinput (A.2) and (A.3) were obtained from single-exponential fits to voltage responses <15 mV. Rinput and were higher in Kcna10–/– (n=13) than Kcna10+/+ (n=22, KWA p=0.015; p=0.016) and Kcna10+/– (n=33, KWA p=0.002; p=0.008; see Table 5). (B) Exemplar voltage responses to iterated current steps (bottom) illustrate key changes in gain and resonance with KV1.8 knockout. (B.1) Kcna10+/– type II HC (P24, LES) and (B.2) Kcna10–/– type II HC (P53, LES). Arrowheads, depolarizing transients. (C) Range of resonance illustrated for Kcna10–/– type II HCs (left, pink curves fit to Equation 5) and controls (right, blue fits). (C.1) Resonant frequencies, left to right: 19.6, 18.4, 34.4, and 0.3 Hz. Leftmost cell resonated spontaneously (before step). (C.2) Tuning quality (Qe; Equation 6) was higher for Kcna10–/– (n=26) type II HCs (KWA: p = 0.0064 vs Kcna10+/+, n=23; p = 7E-8 vs Kcna10+/–, n=45). (D) Kcna10–/– type II HCs had higher, slower peaks and much slower rebound potentials in response to large (170 pA) current steps. (D.1) Normalized to show initial depolarizing transient (filled circles, times of peaks; horizontal arrows, peak width at half-maximum). (D.2) Longer time scale to highlight how null mutation reduced post-transient rebound. (E) In Kcna10–/– HCs (n=19), depolarizing transients evoked by a +90 pA step were slower to peak (E.1) than in Kcna10+/+ (n=19, 2-way ANOVA Tukey’s p<1E-9) and Kcna10+/– (n=34, 2-way ANOVA Tukey’s p<1E-9) and (E.2) larger than in Kcna10+/+ (n=19, KWA p=0.006) and Kcna10+/– (n=34, KWA p=2E-4). Asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. Line, median; Box, interquartile range; Whiskers, outliers.
Type II hair cell passive membrane properties in the extrastriola (ES) and striola (S).
Mean ± SEM (number of cells). g is effect size, Hedge’s g. KWA is Kruskal–Wallis ANOVA. Peak height and time were measured from responses to 170 pA input from rest.
| Zone | Kcna10 | Vrest, mV | Rinput, GΩ* | , ms† | Peak height, mV‡ | Peak time, ms§ | Cm, pF | Age (median, range) |
|---|---|---|---|---|---|---|---|---|
| ES | +/+ | –71 ± 2 (19) | 1.4 ± 0.2 (16) | 11 ± 3 (16) | –20 ± 2 (15) | 2.5 ± 0.2 (15) | 4.7 ± 0.2 (50) | 45, 16–312 |
| +/– | –71 ± 2 (34) | 1.2 ± 0.1 (27) | 9 ± 1 (27) | –20 ± 1 (30) | 2.44 ± 0.08 (30) | 4.6 ± 0.1 (52) | 27, 13–280 | |
| –/– | –76 ± 2 (9) | 2.3 ± 0.3 (7) | 16 ± 3 (7) | 2 ± 6 (7) | 3.6 ± 0.3 (7) | 4.6 ± 0.2 (35) | 53, 15–154 | |
| S | +/+ | –73.1 ± 1.0 (6) | 1.4 ± 0.1 (6) | 9 ± 1 (6) | –20 ± 2 (5) | 2.7 ± 0.1 (5) | 4.6 ± 0.2 (7) | 45, 40–224 |
| +/– | –71 ± 3 (5) | 1.4 ± 0.3 (6) | 7 ± 2 (6) | –20 ± 2 (6) | 2.3 ± 0.1 (6) | 4.8 ± 0.2 (6) | 19, 19–30 | |
| –/– | –68 ± 2 (6) | 3.0 ± 0.7 (6) | 26 ± 10 (6) | 2 ± 7 (4) | 4 ± 1 (4) | 4.4 ± 0.3 (7) | 178, 29–298 |
-
*
–/– vs +/+: KWA, p = 0.015, g 1.2; –/– vs +/–: KWA, p = 0.002, g 1.5.
-
†
–/– vs +/+: KWA, p = 0.016, g 0.7; –/– vs +/–: KWA, p = 0.008, g 1.2.
-
‡
–/– vs +/+: KWA, p = 0.006, g 2.1; –/– vs +/–: KWA, p = 2E−4, g 2.6.
-
§
–/– vs +/+: two-way ANOVA, p < 1E−9, g 1.3; –/– vs +/–: two-way ANOVA, p < 1E−9, g 1.9.
Positive current steps evoked an initial depolarizing transient in both Kcna10+/+ and Kcna10–/– type II HCs, but the detailed time course differed (Figure 4B). Both transient and steady-state responses were larger in Kcna10–/–, consistent with their larger Rin values.
Absence of KV1.8 increased the incidence of sharp electrical resonance in type II HCs. Electrical resonance, which manifests as ringing responses to current steps, can support receptor potential tuning (Ashmore, 1983; Fettiplace, 1987; Hudspeth and Lewis, 1988; Ramanathan and Fuchs, 2002). Larger Rin values made Kcna10–/– type II HCs more prone to electrical resonance; Figure 4C.1 shows a striking example. Median resonance quality (Qe, sharpness of tuning) was greater in Kcna10–/– (1.33, n=26) than Kcna10+/+ (0.66, n = 23) or Kcna10+/– (0.59, n = 44) type II HCs.
KV1.8 affected the time course of the initial peak in response to much larger current injections (Figure 4D, E). Fast activation of gA in control type II HCs rapidly repolarizes the membrane and then inactivates, allowing the constant input current to progressively depolarize the cell, producing a slow rebound (Figure 4D.2). This behavior has the potential to counter mechanotranduction adaptation (Vollrath and Eatock, 2003).
KV1.8 immunolocalized to basolateral membranes of both type I and II HCs
If KV1.8 is a pore-forming subunit in the KV1.8-dependent conductances gK,L, gA, and gDR, it should localize to HC membranes. Figure 5 compares KV1.8 immunoreactivity in Kcna10+/+ and Kcna10–/– utricles, showing specific immunoreactivity along the basolateral membranes of both HC types in Kcna10+/+ utricles. To identify HC type and localize the HC membrane, we used antibodies against KV7.4 (KCNQ4), an ion channel densely expressed in the calyceal ‘inner-face’ membrane next to the synaptic cleft (Hurley et al., 2006; Lysakowski et al., 2011), producing a cup-like stain around type I HCs (Figure 5A). KV1.8 immunoreactivity was present in HC membrane apposing KV7.4-stained calyx inner face in Kcna10+/+ utricles (Figure 5A.1 and A.2) and not in Kcna10–/– utricles (Figure 5A.3).
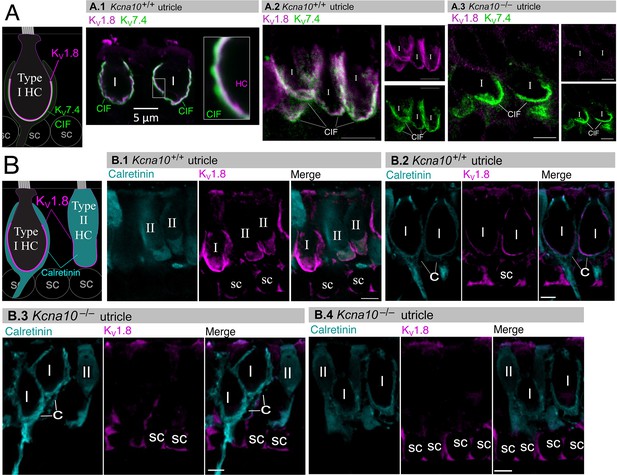
Type I and II hair cell (HC) basolateral membranes show specific immunoreactivity to Kv1.8 antibody (magenta).
Antibodies for KV7.4 (A, green) and calretinin (B, cyan) were used as counterstains for calyx membrane (Kv7.4), type II HC cytoplasm (calretinin) and cytoplasm of striolar calyx-only afferents (calretinin). (A) Left, Cartoon showing KV7.4 on the calyx inner face membrane (CIF) and KV1.8 on the type I HC membrane. SC, supporting cell nuclei. A.1–3, Adult mouse utricle sections. KV7.4 antibody labeled calyces on two KV1.8-positive type I HCs (A.1), four KV1.8-positive type I HCs (A.2), and two KV1.8-negative type I HCs from a Kcna10–/– mouse (A.3). (B) Left, Cartoon showing cytoplasmic calretinin stain in calyx-only striolar afferents and most type II HCs, and KV1.8 on membranes of both HC types. In wildtype utricles, KV1.8 immunolocalized to basolateral membranes of type I and II HCs (extrastriola, B.1). KV1.8 immunolocalized to type I HCs (striola, B.2). Staining of supporting cell (SC) membranes by Kv1.8 antibody was non-specific, as it was present in Kcna10–/– tissue (striola, B.3 and B.4). All scale bars 5 µm.
In other experiments, we used antibodies against calretinin (CALB2), a cytosolic calcium-binding protein expressed by many type II HCs and also by striolar calyx-only afferents (Desai et al., 2005; Lysakowski et al., 2011, Figure 5B). An HC is type II if it is calretinin-positive (Figure 5B.1) or if it lacks a KV7.4- or calretinin-positive calyceal cup (Figure 5A.2 and B.3, rightmost cells). HC identification was confirmed with established morphological indicators: for example, type II HCs tend to have basolateral processes (feet) (Pujol et al., 2014) and, in the extrastriola, more apical nuclei than type I HCs.
Previously, Carlisle et al., 2012 reported KV1.8-like immunoreactivity in many cell types in the inner ear. In contrast, Lee et al., 2013 found that gene expression reporters indicated expression only in HCs and some supporting cells. Here, comparison of control and null tissue showed selective expression of HC membranes, and that some supporting cell staining is non-specific.
KV1.4 may also contribute to gA
Results with the KV1.8 knockout suggest that type II HCs have an inactivating KV1 conductance that includes KV1.8 subunits. KV1.8, like most KV1 subunits, does not show fast inactivation as a heterologously expressed homomer (Lang et al., 2000; Ranjan et al., 2019; Dierich et al., 2020), nor do the KV1.8-dependent channels in type I HCs, as we show, and in cochlear inner HCs (Dierich et al., 2020). KV1 subunits without intrinsic inactivation can produce rapidly inactivating currents by associating with KVβ1 or KVβ3 subunits. KVβ1 (Kcnb1) is present in type II HCs alongside KVβ2 (Kcnb2) (McInturff et al., 2018; Jan et al., 2021; Orvis et al., 2021), which does not confer rapid inactivation (Dwenger et al., 2022).
Another possibility is that in type II HCs, KV1.8 subunits heteromultimerize with KV1.4 subunits—the only KV1 subunits which, when expressed as a homomer, have complete N-type (fast) inactivation (Stühmer et al., 1989). Multiple observations support this possibility. KV1.4 has been linked to gA in pigeon type II HCs (Correia et al., 2008) and is the second-most abundant KV1 transcript in mammalian vestibular HCs, after KV1.8 (Scheffer et al., 2015). KV1.4 is expressed in type II HCs but not type I HCs (McInturff et al., 2018; Orvis et al., 2021), and is not found in striolar HCs (Jan et al., 2021; Orvis et al., 2021), where even in type II HCs, inactivation is slower and less extensive (Figure 3A).
Functional heteromers form between KV1.4 and other KV1.x and/or KVβ1 (Imbrici et al., 2006; Correia et al., 2008; Al Sabi et al., 2011). Although KV1.4 and KV1.8 heteromers have not been studied directly, gA’s inactivation time course ( of ~30 ms +30 mV, Figure 3A) and voltage dependence (Vhalf –41 mV, Figure 6B) are consistent with these other KV1.4-containing heteromers.
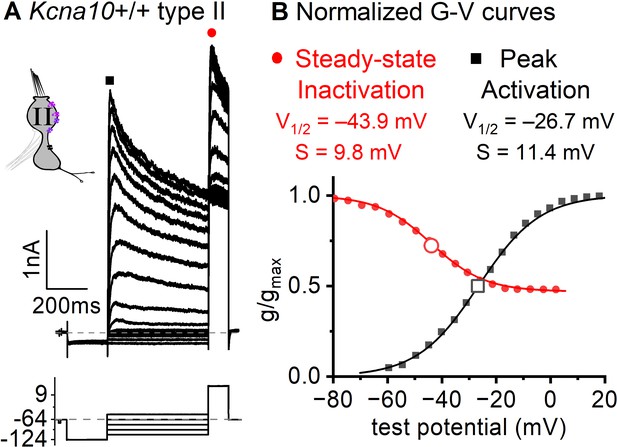
Inactivation curve of gA in extrastriolar type II hair cells (HCs).
(A) Modified voltage protocol measured accumulated steady-state inactivation at the tail potential. 100 μM ZD7288 in bath prevented contamination by HCN current. (B) Voltage dependence of gA’s steady-state inactivation (h∞ curve) and peak activation are consistent with KV1.4 heteromers. Curves, Boltzmann fits (Equation 1). Average fit parameters from Kcna10+/+,+/– type II HCs, P40–P210, median P94. Inactivation: Vhalf, –42 ± 2 mV (n = 11); S, 11 ± 1 mV. Activation: Vhalf, –23 ± 1 mV (n = 11); S, 11.2 ± 0.4 mV.
KV7 channels contribute a small delayed rectifier in type I and II HCs
In Kcna10–/– HCs, absence of IK,L and IA revealed smaller delayed rectifier K+ currents that, unlike IK,L, activated positive to resting potential and, unlike IA, lacked fast inactivation. Candidate channels include members of the KV7 (KCNQ, M-current) family, which have been identified previously in rodent vestibular HCs (Kharkovets et al., 2000; Rennie et al., 2001; Hurley et al., 2006; Scheffer et al., 2015).
We tested for KV7 contributions in Kcna10–/– type I HCs, Kcna10–/– type II HCs, and Kcna10+/+,+/– type II HCs of multiple ages by applying XE991 at 10 µM (Figure 7A), a dose selective for KV7 channels (Brown et al., 2002) and close to the IC50 (Alexander et al., 2019). In Kcna10–/– HCs of both types, 10 µM XE991 blocked about half of the residual KV conductance (Figure 7B.1), consistent with KV7 channels conducting most or all of the non-KV1.8 delayed rectifier current. In all tested HCs (P8–355, median P224), the XE991-sensitive conductance did not inactivate substantially within 200 ms at any voltage, consistent with KV7.2, 7.3, 7.4, and 7.5 currents (Wang, 1998; Kubisch et al., 1999; Schroeder et al., 2000; Jensen et al., 2007; Xu et al., 2007). We refer to this component as gDR(KV7). The voltage dependence and gmax density (gmax/Cm) of gDR(KV7) were comparable across HC types and genotypes (Figure 7B.2–4). Although KV7.4 was not detectable in HCs during immunostaining (Figure 5), KV7.4 has been shown in type I HCs with immunogold labeling (Kharkovets et al., 2000; Hurley et al., 2006).
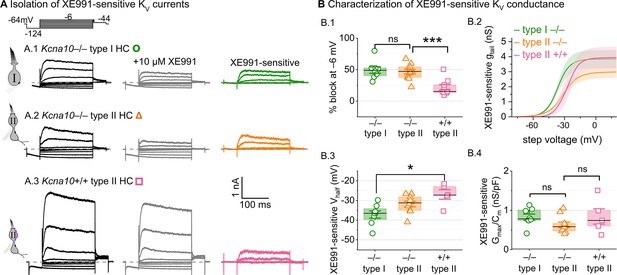
A KV7-selective blocker, XE991, reduced residual delayed rectifier currents in Kcna10–/– type I and II hair cells (HCs).
(A) XE991 (10 μM) partly blocked similar delayed rectifier currents in type I and II Kcna10–/– HCs and a type II Kcna10+/+ HC. (B) Properties of XE991-sensitive conductance, DR(KV7). (B.1) % Block of steady-state current. (B.2) Mean tail G–V curves for Kcna10–/– type I HCs (n = 8), Kcna10–/– type II HCs (9), and Kcna10+/+ type II HCs (5); shading is ± SEM. (B.3) Vhalf was less negative in Kcna10+/+ type II than Kcna10–/– type I HC (p = 0.01, KWA). (B.4) Conductance density was similar in all groups (ANOVA), non-significant at 0.4 power (left), 0.2 power (right). Asterisks: *p < 0.05 and ***p < 0.001. Line, median; Box, interquartile range; Whiskers, outliers.
These results are consistent with similar KV7 channels contributing a relatively small delayed rectifier in both HC types. In addition, the similarity of XE991-sensitive currents of Kcna10+/+ and Kcna10–/– type II HCs indicates that knocking out KV1.8 did not cause general effects on ion channel expression. We did not test XE991 on Kcna10+/+,+/– type I HCs because gK,L runs down in ruptured patch recordings (Rüsch and Eatock, 1996a; Chen and Eatock, 2000; Hurley et al., 2006), which could contaminate the XE991-sensitive conductance obtained by subtraction.
In one striolar Kcna10–/– type I HC, XE991 also blocked a small conductance that activated negative to rest (Figure 7—figure supplement 1A, B). This conductance (Vhalf ~ = –100 mV, Figure 7—figure supplement 1C) was detected only in Kcna10–/– type I HCs from the striola (5/23 vs 0/45 extrastriolar). The Vhalf and were similar to values reported for KV7.4 channels in cochlear HCs (Wong et al., 2004; Dierich et al., 2020). This very negatively activating KV7 conductance coexisted with the larger more positively activating KV7 conductance (Figure 7—figure supplement 1C) and was too small (<0.5 nS/pF) to contribute significantly to gK,L (~10–100 nS/pF, Figure 1D).
Other channels
While our data are consistent with KV1.8- and KV7-containing channels carrying most of the outward-rectifying current in mouse utricular HCs, there is evidence in other preparations for additional channels, including KV11 (KCNH, Erg) channels in rat utricular type I HCs (Hurley et al., 2006) and BK (KCNMA1) channels in rat utricle and rat and turtle semicircular canal HCs (Schweizer et al., 2009; Contini et al., 2020).
BK is expressed in mouse utricular HCs (McInturff et al., 2018; Jan et al., 2021; Orvis et al., 2021). However, Ca2+-dependent currents have not been observed in mouse utricular HCs, and we found little to no effect of the BK-channel blocker iberiotoxin at a dose (100 nM) well beyond the IC50: percent blocked at –30 mV was 2 ± 6% (3 Kcna10–/– type I HCs); 1 ± 5% (5 Kcna10+/+,+/– type II HCs); 7% and 14% (2 Kcna10–/– type II HCs). We also did not see N-shaped I–V curves typical of many Ca2+-dependent K+ currents. In our ruptured-patch recordings, Ca2+-dependent BK currents and erg channels may have been eliminated by wash-out of the HCs’ small CaV currents (Bao et al., 2003) or cytoplasmic second messengers (Hurley et al., 2006).
To check whether the constitutive KV1.8 knockout has strong non-specific effects on channel trafficking, we examined the summed HCN and fast inward rectifier currents (IH and IKir) at –124 mV, and found them similar across genotypes (Figure 7—figure supplement 2). The gK,L knockout allowed identification of zonal differences in IH and IKir in type I HCs, previously examined in type II HCs (Masetto and Correia, 1997; Levin and Holt, 2012). In type I HCs from both control and null utricles, IH and IKir were less prevalent in striola than extrastriola, and, when present, the combined inward current was smaller (Figure 7—figure supplement 2).
Discussion
We have shown that constitutive knockout of KV1.8 eliminated gK,L in type I HCs, and gA and much of gDR in type II HCs. KV1.8 immunolocalized specifically to the basolateral membranes of type I and II HCs. We conclude that KV1.8 is a pore-forming subunit of gK,L, gA, and part of gDR [gDR(KV1.8)]. We suggest that fast inactivation of gA may arise from heteromultimerization of non-inactivating KV1.8 subunits and inactivating KV1.4 subunits. Finally, we showed that a substantial component of the residual delayed rectifier current in both type I and II HCs comprises KV7 channels.
KV1.8 is expressed in HCs from mammalian cochlea (Dierich et al., 2020), avian utricle (Scheibinger et al., 2022), and zebrafish (Erickson and Nicolson, 2015). Our work suggests that in anamniotes, which lack type I cells and gK,L, KV1.8 contributes to gA and gDR, which are widespread in vertebrate HCs (reviewed in Meredith and Rennie, 2016). KV1.8 expression has not been detected in rodent brain but is reported in the pacemaker nucleus of weakly electric fish (Smith et al., 2018).
KV1.8 subunits may form homomultimers to produce gK,L in type I HCs
Recent single-cell expression studies on mouse utricles (McInturff et al., 2018; Jan et al., 2021; Orvis et al., 2021) have detected just one KV1 subunit, KV1.8, in mouse type I HCs. Given that KV1.8 can only form multimers with KV1 family members, and given that gK,L channels are present at very high density (~150 per μm2 in rat type I, Chen and Eatock, 2000), it stands to reason that most or all of the channels are KV1.8 homomers. Other evidence is consistent with this proposal. gK,L (Rüsch and Eatock, 1996a) and heterologously expressed KV1.8 homomers in oocytes (Lang et al., 2000) are non-inactivating and blocked by millimolar Ba2+ and 4-aminopyridine and >10 mM tetraethyl ammonium. Unlike channels with KV1.1, KV1.2, and KV1.6 subunits, gK,L is not sensitive to 10 nM α-dendrotoxin (Rüsch and Eatock, 1996a). gK,L and heterologously expressed KV1.8 channels have similar single-channel conductances (~20 pS for gK,L at positive potentials, Chen and Eatock, 2000; 11 pS in oocytes, Lang et al., 2000). gK,L is inhibited—or positively voltage-shifted—by cGMP (Behrend et al., 1997; Chen and Eatock, 2000), presumably via the C-terminal cyclic nucleotide-binding domain of KV1.8.
A major novel property of gK,L is that it activates 30–60 mV negative to type II KV1.8 conductances and most other low-voltage-activated KV channels (Ranjan et al., 2019). The very negative activation range is a striking difference between gK,L and known homomeric KV1.8 channels. Heterologously expressed homomeric KV1.8 channels have an activation Vhalf of –10 to 0 mV (X. laevis oocytes, Lang et al., 2000; Chinese hamster ovary cells, Dierich et al., 2020). In cochlear inner HCs, currents attributed to KV1.8 (by subtraction of other candidates) have a near-zero activation Vhalf (–4 mV, Dierich et al., 2020).
Possible factors in the unusually negative voltage dependence of gK,L include:
(1) Elevation of extracellular K+ by the enveloping calyceal terminal, unique to type I HCs (Lim et al., 2011; Contini et al., 2012; Spaiardi et al., 2020; Govindaraju et al., 2023). High K+ increases conductance though gK,L channels (Contini et al., 2020), perhaps through K+-mediated relief of C-type inactivation (López-Barneo et al., 1993; Baukrowitz and Yellen, 1995). We note, however, that gK,L is open at rest even in neonatal mouse utricles cultured without innervation (Rüsch et al., 1998) and persists in dissociated type I HCs (Chen and Eatock, 2000; Hurley et al., 2006).
(2) The high density of gK,L (~50 nS/pF in striolar Kcna10+/+ HCs) implies close packing of channels, possibly represented by particles (12–14 nm) seen in freeze-fracture electron microscopy of the type I HC membrane (Gulley and Bagger-Sjöbäck, 1979; Sousa et al., 2009). Such close channel packing might hyperpolarize in situ voltage dependence of gK,L, as proposed for KV7.4 channels in outer HCs (Perez-Flores et al., 2020). Type I HC-specific partners that may facilitate this close packing include ADAM11 (McInturff et al., 2018), which clusters presynaptic KV1.1 and KV1.2 to enable ephaptic coupling at a cerebellar synapse (Kole et al., 2015).
(3) Modulation by accessory subunits. Type I HCs express KVβ1 (McInturff et al., 2018; Orvis et al., 2021), an accessory subunit that can confer fast inactivation and hyperpolarize activation Vhalf by ~10 mV. KVβ1 might interact with KV1.8 to shift voltage dependence negatively. Arguments against this possibility include that gK,L lacks fast inactivation (Rüsch and Eatock, 1996a; Hurley et al., 2006; Spaiardi et al., 2017) and that cochlear inner HCs co-express KV1.8 and KVβ1 (Liu et al., 2018) but their KV1.8 conductance has a near-0 Vhalf (Dierich et al., 2020).
KV1.8 subunits may combine with different subunits to produce gA and KV1.8-dependent gDR in type II HCs
The KV1.8-dependent conductances of type II HCs vary in their fast and slow inactivation. In not showing fast inactivation (Lang et al., 2000; Ranjan et al., 2019; Dierich et al., 2020), heterologously expressed KV1.8 subunits resemble most other KV1 family subunits, with the exception of KV1.4 (for comprehensive review, see Ranjan et al., 2019). KV1.4 is a good candidate to provide fast inactivation based on immunolocalization and voltage dependence (Figures 4 and 6). We suggest that gA and gDR(KV1.8) are KV1.8-containing channels that may include a variable number of KV1.4 subunits and KVβ2 and KVβ1 accessory subunits.
KV1.4–KV1.8 heteromeric assembly could account for several related observations. The faster in Kcna10+/– relative to Kcna10+/+ type II HCs (Figure 3A.3, Figure 3—figure supplement 1A.1) could reflect an increased ratio of KV1.4–KV1.8 subunits and therefore more N-terminal inactivation domains per heteromeric channel. Zonal variation in the extent and speed of N-type inactivation (Figure 3A) might arise from differential expression of KV1.4. The small fast-inactivating conductance in ~20% of extrastriolar Kcna10–/– type II HCs (Figure 3—figure supplement 3) might flow through KV1.4 homomers.
Fast inactivation may also receive contribution from KVβ subunits. KVβ1 is expressed in type II HCs (McInturff et al., 2018; Jan et al., 2021; Orvis et al., 2021), and, together with KV1.4, has been linked to gA in pigeon vestibular HCs (Correia et al., 2008). KVβ2, also expressed in type II HCs (McInturff et al., 2018; Orvis et al., 2021), accelerates but does not confer fast inactivation.
We speculate that gA and gDR(KV1.8) have different subunit composition: gA may include heteromers of KV1.8 with other subunits that confer rapid inactivation, while gDR(KV1.8) may comprise homomeric KV1.8 channels, given that they do not have N-type inactivation.
KV1.8 relevance for vestibular function
In both type I and II utricular HCs, KV1.8-dependent channels strongly shape receptor potentials in ways that promote temporal fidelity rather than electrical tuning (Lewis, 1988), consistent with the utricle’s role in driving reflexes that compensate for head motions as they occur. This effect is especially pronounced for type I HCs, where the current-step evoked voltage response reproduces the input with great speed and linearity (Figure 2).
gK,Ldominates passive membrane properties in mature Kcna10+/+,+/– type I HCs such that Kcna10–/– type I HCs are expected to have receptor potentials with higher amplitudes but lower low-pass corner frequencies, closer to those of type II HCs and immature HCs of all types (Correia et al., 1996; Rüsch and Eatock, 1996a; Songer and Eatock, 2013). In Kcna10–/– epithelia, we expect the lack of a large basolateral conductance open at rest to reduce the speed and gain of non-quantal transmission, which depends on K+ ion efflux from the type I HC to change electrical and K+ potentials in the synaptic cleft (Govindaraju et al., 2023). In HCs, K+ enters the mechanosensitive channels of the hair bundle from the K+-rich apical endolymph and exits through basolateral potassium conductances into the more conventional low-K+ perilymph. For the type I-calyx synapse, having in the HC a large, non-inactivating K+ conductance open across the physiological range of potentials avoids channel gating time and allows for instantaneous changes in current into the cleft and fast afferent signaling (Pastras et al., 2023).
In contrast, mature type II HCs face smaller synaptic contacts and have KV1.8-dependent currents that are not substantially activated at resting potential. They do affect the time course and gain of type II HC responses to input currents, speeding up depolarizing transients, producing a repolarizing rebound during the step, and reducing resonance.
Type I and II vestibular HCs are closely related, such that adult type II HCs acquire type I-like properties upon deletion of the transcription factor Sox2 (Stone et al., 2021). In normal development of the two cell types, the Kcna10 gene generates biophysically distinct and functionally different ion channels, presenting a natural experiment in functional differentiation of sensory receptor cells.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-Kv1.8 (Rabbit polyclonal) | Alomone | Cat# APC-157, lot# 0102, RRID:AB_2341039 | 1:200 or 1:400 |
| Antibody | Anti-calretinin (goat polyclonal) | Millipore | Cat# AB1550, lot# 9669, RRID:AB_90764 | 1:600 |
| Antibody | Anti-Kv7.4 (mouse IgG1 monoclonal) | NeuroMab | Cat# 2HK-65, RRID:AB_2131828 | 1:200 |
| Peptide, recombinant protein | Iberiotoxin | Alomone | STI-400 | 100 nM (water) |
| Chemical compound, drug | XE991 | Sigma | X2254 | 100 µM (water) |
| Chemical compound, drug | ZD7288 | Tocris | APN18035-2 | 100 µM (water) |
| Peptide, recombinant protein | Bovine serum albumin | Fisher | BP671 | 1 mg/ml (water) |
Preparation
Request a detailed protocolAll procedures for handling animals followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of the University of Chicago (Animal Care and Use Procedure #72360) and the Office of Animal Care and Institutional Biosafety at the University of Illinois Chicago (Protocol for Animal Use #17106). Most mice belonged to a transgenic line with a knockout allele of Kcna10 (referred to here as Kcna10–/–). Our breeding colony was established with a generous gift of such animals from Sherry M. Jones and Thomas Friedman. These animals are described in their paper (Lee et al., 2013). Briefly, the Texas A&M Institute for Genomic Medicine generated the line on a C57BL/6;129SvEv mixed background by replacing Exon 3 of the Kcna10 gene with an IRES-bGeo/Purocassette. Mice in our colony were raised on a 12:12 hr light–dark cycle with access to food and water ad libitum.
Semi-intact utricles were prepared from ~150 male and ~120 female mice, postnatal days (P) 5–375, for same-day recording. HC KV channel data were pooled across sexes as most results did not appear to differ by sex; an exception was that gK,L had a more negative Vhalf in males (Supplementary file 1a), an effect not clearly related to age, copy number, or other properties of the activation curve.
Preparation, stimulation, and recording methods followed our previously described methods for the mouse utricle (Vollrath and Eatock, 2003). Mice were anesthetized through isoflurane inhalation. After decapitation, each hemisphere was bathed in ice-cold, oxygenated Liebowitz-15 (L15) media. The temporal bone was removed, the labyrinth was cut to isolate the utricle, and the nerve was cut close to the utricle. The utricle was treated with proteinase XXIV (100 μg/ml, ~10 min, 22°C) to facilitate removal of the otoconia and attached gel layer and mounted beneath two glass rods affixed at one end to a coverslip.
Electrophysiology
Request a detailed protocolWe used the HEKA Multiclamp EPC10 with Patchmaster acquisition software, filtered by the integrated HEKA filters: a 6-pole Bessel filter at 10 kHz and a second 4-pole Bessel filter at 5 kHz, and sampled at 10–100 kHz. Recording electrodes were pulled (PC-100, Narishige) from soda lime glass (King’s Precision Glass R-6) wrapped in paraffin to reduce pipette capacitance. Internal solution contained (in mM) 135 KCl, 0.5 MgCl2, 3 MgATP, 5 4-(2-hydroxyethyl)piperazine-1-ethane-sulfonic acid (HEPES), 5 ethylene glycol tetraacetic acid (EGTA), 0.1 CaCl2, 0.1 Na-cAMP, 0.1 LiGTP, 5 Na2CreatinePO4 adjusted to pH 7.25 and ~280 mmol/kg by adding ~30 mM KOH. External solution was Liebowitz-15 media supplemented with 10 mM HEPES (pH 7.40, 310 ± 10 mmol/kg). Recording temperature was 22–25°C. Pipette capacitance and membrane capacitance transients were subtracted during recordings with Patchmaster software. Series resistance (8–12 MΩ) was measured after rupture and compensated 60–80% with the amplifier, to final values of ~2 MΩ. Potentials are corrected for remaining (uncompensated) series resistance and liquid junction potential of ~+4 mV, calculated with LJPCalc software (Marino et al., 2014).
Kcna10–/– HCs appeared healthy in that cells had resting potentials negative to –50 mV, cells lasted a long time (20–30 min) in ruptured patch recordings, membranes were not fragile, and extensive blebbing was not seen. Type I HCs with gK,L were transiently hyperpolarized to ~–90 mV to close gK,L enough to increase Rinput above 100 MΩ, as needed to estimate series resistance and cell capacitance. The average resting potential, Vrest, was –87 mV ±1 (41), similar to the calculated EK of –86.1 mV, which is not surprising given the large K+ conductance of these cells. Vrest is likely more positive in vivo, where lower endolymphatic Ca2+ increases standing inward current through MET channels.
Voltage protocols to characterize KV currents differed slightly for type I and II HCs. In standard protocols, the cell is held at a voltage near resting potential (–74 mV in type I and –64 mV in type II), then jumped to –124 mV for 200 ms in type I HCs in order to fully deactivate gK,L and 50 ms in type II HCs in order to remove baseline inactivation of gA. The subsequent iterated step depolarizations lasted 500 ms in type I HCs because gK,L activates slowly (Wong et al., 2004) and 200 ms in type II HCs, where KV conductances activate faster. The 50 ms tail voltage was near the reversal potential of HCN channels (–44 mV in mouse utricular HCs, Rüsch et al., 1998) to avoid HCN current contamination.
G–V (activation) parameters for control type I cells may be expected to vary across experiments on semi-intact (as here), organotypically cultured and denervated (Rüsch et al., 1998), or dissociated-cell preparations, reflecting variation in retention of the calyx (Discussion) and voltage step durations (Wong et al., 2004) which elevate K+ concentration around the HC. Nevertheless, the values we obtained for type I and II HCs resemble values recorded elsewhere, including experiments in which extra care was taken to avoid extracellular K+ accumulation (Spaiardi et al., 2017; Spaiardi et al., 2020). The effects of K+ accumulation on gK,L’s steady-state activation curves are small because the operating range is centered on E and can be characterized with relatively small currents (Figure 1A).
Pharmacology
Request a detailed protocolDrug-containing solutions were locally with BASI Bee Hive syringes at a final flow rate of 20 μl/min and a dead time of ~30 s. Global bath perfusion was paused during drug perfusion and recording, and only one cell was used per utricle. Aliquots of test agents in solution were prepared, stored at –20°C, and thawed and added to external solution on the recording day (see Key Resources Table).
Analysis
Data analysis was performed with software from OriginLab (Northampton, MA) and custom MATLAB scripts using MATLAB fitting algorithms.
Fitting voltage dependence and time course of conductances
Request a detailed protocolG–V curves. Current was converted to conductance (G) by dividing by driving force (V – EK; EK calculated from solutions). For type I HCs, tail G–V curves were generated from current 1 ms after the end of the iterated voltage test step. For type II HCs, peak G–V curves were generated from peak current during the step and steady-state G–V curves were generated from current 1 ms before the end of a 200-ms step. We fit G–V curves to the first-order Boltzmann equation (Equation 1) using a custom MATLAB function (fitzmann.m, Source code 2).
Vhalf is the midpoint, and S is the slope factor, inversely related to curve steepness near activation threshold.
Activation time course of type II HCs. We fit current traces using a custom MATLAB function (fitkin.m, Source code 1). For type II HCs lacking fast inactivation, outward current activation was fit with Equation 2.
ISS is steady-state current; is activation time constant (referred to elsewhere as ); n is the state factor related to the number of closed states (typically constrained to 3); and Io is baseline current.
To measure activation and inactivation time course of gA, we used Equation 3 to fit outward K+ currents evoked by steps from –125 mV to above –50 mV (Rothman and Manis, 2003).
Z is total steady-state inactivation (0 ≤ Z < 1 means incomplete inactivation, which allows the equation to fit non-inactivating delayed rectifier currents); f is the fraction of fast inactivation relative to total inactivation; Imax is maximal current; (referred to elsewhere as ) and are the fast and slow inactivation time constants. We chose to compare fit parameters at 30 ± 2 mV (91), where fast and slow inactivation were consistently separable and gA was maximized. In most Kcna10–/– and some striolar Kcna10+/+,+/– cells, where fast inactivation was absent and adjusted R2 did not improve on a single-exponential fit by >0.01, we constrained f in Equation 3 to 0 to avoid overfitting.
For Peak G–V relations, peak conductance was taken from fitted curves (Equations 2 and 3). To construct ‘Steady-state’ G–V relations, we used current at 200 ms, which was only 6 ± 1% (94) greater than steady-state estimated from fits to Equation 3 (Figure 3C, D).
Percent inactivation was calculated at 30 mV with Equation 4:
IPeak is maximal current, and ISS is current at the end of a 200-ms voltage step.
The electrical resonance of type II HCs was quantified by fitting voltage responses to current injection steps (Songer and Eatock, 2013). We fit Equation 5, a damped sinusoid, to the voltage trace from half-maximum of the initial depolarizing peak until the end of the current step.
VSS is steady-state voltage; Vp is the voltage of the peak response; is the decay time constant; fe is the fundamental frequency; and θ is the phase angle shift.
Quality factor, Qe, was calculated with Equation 6 (Crawford and Fettiplace, 1981).
Statistics
We give means ± SEM for normally distributed data, and otherwise, median and range. Data normality was assessed with the Shapiro–Wilk test for n < 50 and the Kolmogorov–Smirnov test for n > 50. To assess homogeneity of variance we used Levene’s test. With homogeneous variance, we used two-way ANOVA for genotype and zone with the post hoc Tukey’s test. When variance was non-homogeneous, we used one-way Welch ANOVA with the posthoc Games–Howell test. For data that were not normally distributed, we used the non-parametric one-way Kruskal–Wallis ANOVA (KWA) with posthoc Dunn’s test. Effect size is Hedge’s g (g). For age dependence, we used partial correlation coefficients controlling for genotype and zone. Statistical groups may have different median ages, but all have overlapping age ranges. In figures, asterisks represent p-value ranges as follows: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Immunohistochemistry
Request a detailed protocolMice were anesthetized with Nembutal (80 mg/kg), then perfused transcardially with 40 ml of physiological saline containing heparin (400 IU), followed by 2 ml/g body weight fixative (4% paraformaldehyde, 1% picric acid, and 5% sucrose in 0.1 M phosphate buffer at pH 7.4, sometimes with 1% acrolein). Vestibular epithelia were dissected in phosphate buffer, and tissues were cryoprotected in 30% sucrose-phosphate buffer overnight at 4°C. Otoconia were dissolved with Cal-Ex (Fisher Scientific) for 10 min. Frozen sections (35 μm) were cut with a sliding microtome. Immunohistochemistry was performed on free-floating sections. Tissues were first permeabilized with 4% Triton X-100 in phosphate-buffered saline (PBS) for 1 hr at room temperature, then incubated with 0.5% Triton X-100 in a blocking solution of 0.5% fish gelatin and 1% bovine serum albumin for 1 hr at room temperature. Sections were incubated with two to three primary antibodies for 72 hr at 4°C and with two to three secondary antibodies. Sections were rinsed with PBS between and after incubations and mounted on slides in Mowiol (Calbiochem).
Data availability
Data generated and analyzed in this study are available on Dryad (https://doi.org/10.5061/dryad.37pvmcvrw). Dryad hosts downloadable spreadsheets that are organized as follows: F1_sourcedata, numerical data for Figure 1; F1-fs1_sourcedata, numerical data for Figure 1-figure supplement 1; F2_sourcedata, numerical data for Figure 2; F3_sourcedata, numerical data for Figure 3; F3-fs1_sourcedata, numerical data for Figure 3—figure supplement 1; F3-fs2_sourcedata, numerical data for Figure 3—figure supplement 2; F3-fs3_sourcedata, numerical data for Figure 3—figure supplement 3; F4_sourcedata, numerical data for Figure 4; F6_sourcedata, numerical data for Figure 6; F7_sourcedata, numerical data for Figure 7; F7-fs1_sourcedata, numerical data for Figure 7—figure supplement 1; F7-fs2_sourcedata, numerical data for Figure 7—figure supplement 2.
-
Dryad Digital RepositoryThe potassium channel subunit Kv1.8 (Kcna10) is essential for the distinctive outwardly rectifying conductances of type I and II vestibular hair cells.https://doi.org/10.5061/dryad.37pvmcvrw
References
-
The concise guide to pharmacology 2019/20: ion channelsBritish Journal of Pharmacology 176 Suppl 1:S142–S228.https://doi.org/10.1111/bph.14749
-
Position-dependent attenuation by Kv1.6 of N-type inactivation of Kv1.4-containing channelsThe Biochemical Journal 438:389–396.https://doi.org/10.1042/BJ20102169
-
Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat cristaJournal of Neurophysiology 90:155–164.https://doi.org/10.1152/jn.00244.2003
-
Some pharmacological properties of neural KCNQ channelsNeurophysiology 34:91–94.https://doi.org/10.1023/A:1020768914645
-
Specific expression of Kcna10, Pxn and Odf2 in the organ of CortiGene Expression Patterns 12:172–179.https://doi.org/10.1016/j.gep.2012.03.001
-
Filtering properties of vestibular hair cells: an updateAnnals of the New York Academy of Sciences 781:138–149.https://doi.org/10.1111/j.1749-6632.1996.tb15698.x
-
An electrical tuning mechanism in turtle cochlear hair cellsThe Journal of Physiology 312:377–412.https://doi.org/10.1113/jphysiol.1981.sp013634
-
Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculaeJournal of Neurophysiology 93:251–266.https://doi.org/10.1152/jn.00746.2003
-
Vestibular hair cells and afferents: two channels for head motion signalsAnnual Review of Neuroscience 34:501–534.https://doi.org/10.1146/annurev-neuro-061010-113710
-
Electrical tuning of hair cells in the inner earTrends in Neurosciences 10:421–425.https://doi.org/10.1016/0166-2236(87)90013-0
-
Afferent diversity and the organization of central vestibular pathwaysExperimental Brain Research 130:277–297.https://doi.org/10.1007/s002210050033
-
The differentiation status of hair cells that regenerate naturally in the vestibular inner ear of the adult mouseThe Journal of Neuroscience 41:7779–7796.https://doi.org/10.1523/JNEUROSCI.3127-20.2021
-
Stimulus processing by type II hair cells in the mouse utricleAnnals of the New York Academy of Sciences 871:15–26.https://doi.org/10.1111/j.1749-6632.1999.tb09172.x
-
Dominant-negative inhibition of M-like potassium conductances in hair cells of the mouse inner earThe Journal of Neuroscience 27:8940–8951.https://doi.org/10.1523/JNEUROSCI.2085-07.2007
-
HCN channels expressed in the inner ear are necessary for normal balance functionThe Journal of Neuroscience 31:16814–16825.https://doi.org/10.1523/JNEUROSCI.3064-11.2011
-
M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricleThe Journal of Neuroscience 26:10253–10269.https://doi.org/10.1523/JNEUROSCI.2596-06.2006
-
Inactivation as a new regulatory mechanism for neuronal Kv7 channelsBiophysical Journal 92:2747–2756.https://doi.org/10.1529/biophysj.106.101287
-
KCNA10: A novel ion channel functionally related to both voltage-gated potassium and CNG cation channelsAmerican Journal of Physiology-Renal Physiology 278:F1013–F1021.https://doi.org/10.1152/ajprenal.2000.278.6.F1013
-
The function and molecular identity of inward rectifier channels in vestibular hair cells of the mouse inner earJournal of Neurophysiology 108:175–186.https://doi.org/10.1152/jn.00098.2012
-
Tuning in the bullfrog earBiophysical Journal 53:441–447.https://doi.org/10.1016/S0006-3495(88)83120-5
-
Potassium accumulation between type I hair cells and calyx terminals in mouse cristaExperimental Brain Research 210:607–621.https://doi.org/10.1007/s00221-011-2592-4
-
Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channelsReceptors & Channels 1:61–71.
-
BookMorphophysiology of the vestibular peripheryIn: Highstein SM, Fay RR, Popper AN, editors. The Vestibular System. New York: Springer. pp. 57–152.https://doi.org/10.1007/0-387-21567-0_3
-
Molecular microdomains in a sensory terminal, the vestibular calyx endingThe Journal of Neuroscience 31:10101–10114.https://doi.org/10.1523/JNEUROSCI.0521-11.2011
-
Evidence that ultrafast nonquantal transmission underlies synchronized vestibular action potential generationThe Journal of Neuroscience 43:7149–7157.https://doi.org/10.1523/JNEUROSCI.1417-23.2023
-
Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organsThe Journal of Comparative Neurology 522:3141–3159.https://doi.org/10.1002/cne.23625
-
A kinetic map of the homomeric voltage-gated potassium channel (Kv) familyFrontiers in Cellular Neuroscience 13:358.https://doi.org/10.3389/fncel.2019.00358
-
Potassium currents in mammalian and avian isolated type I semicircular canal hair cellsJournal of Neurophysiology 71:317–329.https://doi.org/10.1152/jn.1994.71.1.317
-
Effects of KCNQ channel blockers on K+ currents in vestibular hair cellsAmerican Journal of Physiology-Cell Physiology 280:C473–C480.https://doi.org/10.1152/ajpcell.2001.280.3.C473
-
The delayed rectifier, IKΙ, is the major conductance in type I vestibular hair cells across vestibular end organsPflügers Archiv - European Journal of Physiology 432:34–42.https://doi.org/10.1007/s004240050102
-
Kinetic analyses of three distinct potassium conductances in ventral cochlear nucleus neuronsJournal of Neurophysiology 89:3083–3096.https://doi.org/10.1152/jn.00126.2002
-
A delayed rectifier conductance in type I hair cells of the mouse utricleJournal of Neurophysiology 76:995–1004.https://doi.org/10.1152/jn.1996.76.2.995
-
Voltage responses of mouse utricular hair cells to injected currentsAnnals of the New York Academy of Sciences 781:71–84.https://doi.org/10.1111/j.1749-6632.1996.tb15694.x
-
Gene expression by mouse inner ear hair cells during developmentThe Journal of Neuroscience 35:6366–6380.https://doi.org/10.1523/JNEUROSCI.5126-14.2015
-
Cell-type identity of the avian utricleCell Reports 40:111432.https://doi.org/10.1016/j.celrep.2022.111432
-
KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currentsJournal of Biological Chemistry 275:24089–24095.https://doi.org/10.1074/jbc.M003245200
-
Distribution of high-conductance calcium-activated potassium channels in rat vestibular epitheliaThe Journal of Comparative Neurology 517:134–145.https://doi.org/10.1002/cne.22148
-
Genes linked to species diversity in a sexually dimorphic communication signal in electric fishJournal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 204:93–112.https://doi.org/10.1007/s00359-017-1223-3
-
Tuning and timing in mammalian type I hair cells and calyceal synapsesThe Journal of Neuroscience 33:3706–3724.https://doi.org/10.1523/JNEUROSCI.4067-12.2013
-
An allosteric gating model recapitulates the biophysical properties of IK,L expressed in mouse vestibular type I hair cellsThe Journal of Physiology 595:6735–6750.https://doi.org/10.1113/JP274202
-
Vestibular role of KCNQ4 and KCNQ5 K+ channels revealed by mouse modelsJournal of Biological Chemistry 288:9334–9344.https://doi.org/10.1074/jbc.M112.433383
-
Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigationActa Oto-Laryngologica. Supplementum 126:1–85.
-
Differences between the negatively activating potassium conductances of mammalian cochlear and vestibular hair cellsJournal of the Association for Research in Otolaryngology 5:270–284.https://doi.org/10.1007/s10162-004-4051-4
-
Roles of alternative splicing in the functional properties of inner ear-specific KCNQ4 channelsJournal of Biological Chemistry 282:23899–23909.https://doi.org/10.1074/jbc.M702108200
-
Expression of KCNA10, a voltage-gated K channel, in glomerular endothelium and at the apical membrane of the renal proximal tubuleJournal of the American Society of Nephrology 13:2831–2839.https://doi.org/10.1097/01.ASN.0000036866.37886.C5
Article and author information
Author details
Funding
National Institute on Deafness and Other Communication Disorders (R01 DC012347)
- Anna Lysakowski
- Ruth Anne Eatock
National Science Foundation (Graduate Research Fellowship Program)
- Hannah R Martin
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
This study was supported by NIH grant R01 DC012347 to RAE and AL and an NSF Graduate Research Fellowship to HRM. We thank Drs. Thomas Friedman and Sherri Jones for the generous gift of the Kcna10–/– mouse line, and Drs. Zheng-Yi Chen and Deborah I Scheffer for bringing the expression of this subunit in mouse vestibular hair cells to our attention. We acknowledge Dr. Vicente Lumbreras for insights from his prior experiments on gA in mouse utricular hair cells, and thank him for helpful further discussions. We acknowledge Steven D Price for his help with immunocytochemistry. We thank Drs. Rebecca Lim and Ebenezer Yamoah for their critical feedback on the manuscript, and Drs. Rob Raphael and Aravind Chenrayan Govindaraju for feedback and many helpful discussions. We thank Drs. Joe Burns, Gabi Pregernig, and Lars Becker (Decibel Therapeutics, Inc) for helpful discussions.
Ethics
All procedures for handling animals followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of the University of Chicago (Animal Care and Use Procedure #72360) and the Office of Animal Care and Institutional Biosafety at the University of Illinois Chicago (Protocol for Animal Use #17106).
Version history
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Reviewed Preprint version 3:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.94342. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Martin et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 491
- views
-
- 28
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Time estimation is an essential prerequisite underlying various cognitive functions. Previous studies identified ‘sequential firing’ and ‘activity ramps’ as the primary neuron activity patterns in the medial frontal cortex (mPFC) that could convey information regarding time. However, the relationship between these patterns and the timing behavior has not been fully understood. In this study, we utilized in vivo calcium imaging of mPFC in rats performing a timing task. We observed cells that showed selective activation at trial start, end, or during the timing interval. By aligning long-term time-lapse datasets, we discovered that sequential patterns of time coding were stable over weeks, while cells coding for trial start or end showed constant dynamism. Furthermore, with a novel behavior design that allowed the animal to determine individual trial interval, we were able to demonstrate that real-time adjustment in the sequence procession speed closely tracked the trial-to-trial interval variations. And errors in the rats’ timing behavior can be primarily attributed to the premature ending of the time sequence. Together, our data suggest that sequential activity maybe a stable neural substrate that represents time under physiological conditions. Furthermore, our results imply the existence of a unique cell type in the mPFC that participates in the time-related sequences. Future characterization of this cell type could provide important insights in the neural mechanism of timing and related cognitive functions.
-
- Neuroscience
Sour taste, which is elicited by low pH, may serve to help animals distinguish appetitive from potentially harmful food sources. In all species studied to date, the attractiveness of oral acids is contingent on concentration. Many carboxylic acids are attractive at ecologically relevant concentrations but become aversive beyond some maximal concentration. Recent work found that Drosophila ionotropic receptors IR25a and IR76b expressed by sweet-responsive gustatory receptor neurons (GRNs) in the labellum, a peripheral gustatory organ, mediate appetitive feeding behaviors toward dilute carboxylic acids. Here, we disclose the existence of pharyngeal sensors in Drosophila melanogaster that detect ingested carboxylic acids and are also involved in the appetitive responses to carboxylic acids. These pharyngeal sensors rely on IR51b, IR94a, and IR94h, together with IR25a and IR76b, to drive responses to carboxylic acids. We then demonstrate that optogenetic activation of either Ir94a+ or Ir94h+ GRNs promotes an appetitive feeding response, confirming their contributions to appetitive feeding behavior. Our discovery of internal pharyngeal sour taste receptors opens up new avenues for investigating the internal sensation of tastants in insects.






