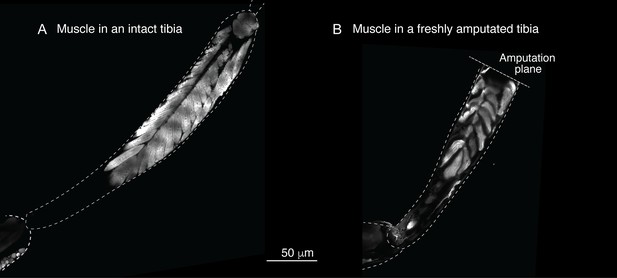
Response to comment on 'A conserved strategy for inducing appendage regeneration in moon jellyfish, Drosophila, and mice'
Figures
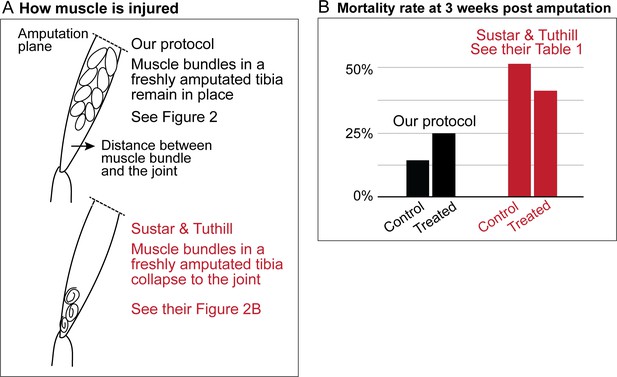
Sustar and Tuthill did not replicate our protocol.
(A) In our amputation method, muscle bundles in the residual tibia remain in place (see Figure 2). By contrast, in the method used by Sustar and Tuthil, muscle bundles collapsed (see their Figure 2B). (B) The lack of stress management in the protocol of Sustar and Tuthill is reflected in the much higher mortality rate in their experiment (as reported in their Table 1).
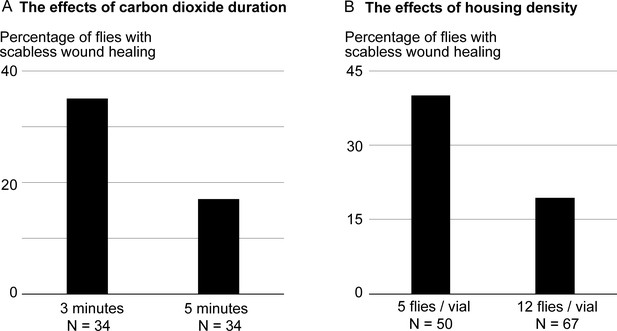
Duration of CO2 exposure and housing density can alter the experimental outcomes.
In these experiments, adult flies were amputated across the tibia, and placed on food supplemented with leucine, glutamine, and insulin. The effects of the treatment were assessed three days after amputation by the absence of scab formation over the amputation site. (A) To perform the amputation, we normally anesthetize the flies using the lowest possible CO2 level, and limit anesthesia duration to one to three minutes. Even at thissub-anesthetic CO2 level, increasing the CO2 exposure time to five minutes is enough to halving the frequency of flies responding to the treatment. (B) After amputation, we normally place up to 6 flies per vial. Increasing the housing density reduces the frequency of flies responding to the treatment.