Complete suspension culture of human induced pluripotent stem cells supplemented with suppressors of spontaneous differentiation
Figures
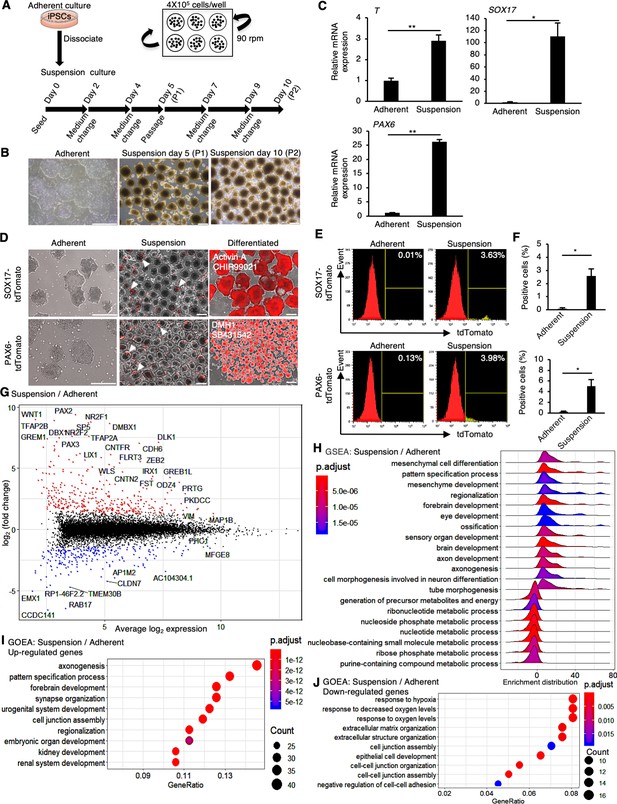
Human induced pluripotent stem cells (hiPSCs) maintained under suspension conditions undergo spontaneous differentiation.
(A) Schematics representing hiPSCs in suspension conditions. (B) Phase-contrast images of adherent- or suspension-cultured hiPSCs on day 5 (passage 1 [P1]) and day 10 (passage 2 [P2]). Scale bars: 400 µm. (C) Gene expression in hiPSCs cultured under adherent or suspension conditions on P2. Bar graphs show the mean ± SE (n = 3). p-values were statistically analyzed with Student’s t-test. (D) Phase-contrast and fluorescent images of adherent or suspension-cultured reporter hiPSCs on P2. White arrowheads indicate spontaneous expression of SOX17 and PAX6 in suspension conditions. Scale bars: 400 µm. (E) Quantification of hiPSCs spontaneous differentiation with flow cytometry. (F) Averaged tdTomato-positive cell ratio (%) from flow cytometry data (mean ± SE from n = 3). p-values were statistically analyzed with Student’s t-test. (G) An MA plot (log2 fold change versus mean average expression) comparing transcriptomes between suspension and adherent conditions from RNA-seq data. The representative gene name is shown in the plot. (H) Gene Set Enrichment Analysis (GSEA) on the gene sets of suspension-cultured hiPSCs to adherent cultures. Adjusted p-values are shown as blue to red from low to high values. (I, J) Gene Ontology Enrichment Analysis (GOEA) on the gene sets of suspension-cultured hiPSCs to adherent culture. Results are ranked by significance (p-adjusted value) and/or counted gene numbers. * or ** in the graphs indicate p<0.05 or p<0.01, respectively.
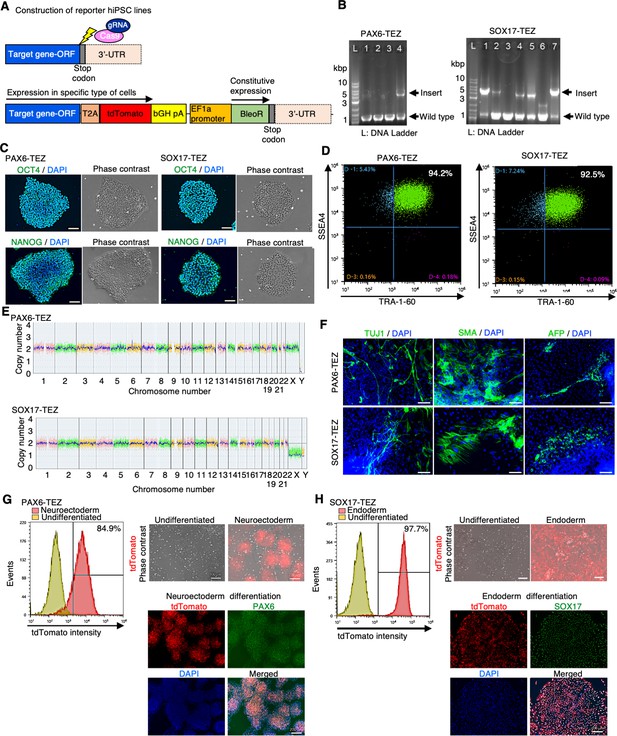
Characterization of PAX6-tdTomato and SOX17-tdTomato reporter lines.
(A) Schematic of construction of reporter human induced pluripotent stem cell (hiPSC) lines. CRISPR/Cas9 was employed to knock-in the 2A-tdTomato gene and EF1a promoter-driven Zeocin (Bleomycin) resistance gene sequence (TEZ) to the 3' terminal of SOX17 or PAX6 open reading frame (ORF), respectively. (B) Genotyping of selected clones by genomic PCR. Arrows indicate alleles of wild type or insertion in the PAX6 (left panel) or SOX17 (right panel) genes. (C) Phase-contrast images of representative colonies and immunocytochemistry of pluripotency markers: OCT4 and NANOG. Left panels: PAX6-TEZ. Right panels: SOX17-TEZ. (D) Flow cytometry of cell surface markers: TRA-1-60 and SSEA4. Left: PAX6-TEZ. Right: SOX17-TEZ. (E) Chromosomal copy numbers detected with copy number variation (CNV) array analysis of PAX6-TEZ (top) and SOX17-TEZ (bottom). (F) Immunocytochemistry of differentiated cells in embryoid bodies (EBs). Anti-TUJ1, -SMA, and -AFP antibodies were used. Scale bars: 100 µm. (G) Differentiation potency of PAX6-TEZ in the neural ectoderm. Left: flow cytometry of tdTomato using PAX6-TEZ cultured in neuroectodermal differentiation medium for 7 days. Right upper: overlay images of phase-contrast and tdTomato fluorescence of PAX6-TEZ cultured in maintenance medium (left) or neuroectodermal differentiation medium (right). Scale bars: 200 µm. Right lower: images of tdTomato fluorescence and endogenous PAX6 protein detected with immunocytochemistry with anti-PAX6 antibody. Nuclear staining was performed with DAPI. Scale bars: 200 µm. (H) Differentiation potency of SOX17-TEZ in the endoderm. Left: flow cytometry of tdTomato using SOX17-TEZ cultured in endodermal differentiation medium for 7 days. Right upper: overlay images of phase-contrast and tdTomato fluorescence of SOX17-TEZ cultured in maintenance medium (left) or endoderm differentiation medium (right). Right lower: images of tdTomato and endogenous SOX17 protein detected by immunocytochemistry with anti-SOX17 antibody. Nuclear staining was performed with DAPI. Scale bars: 200 µm.
-
Figure 1—figure supplement 1—source data 1
File containing original gel images of agarose gel electrophoresis for Figure 1—figure supplement 1B, indicating the relevant bands from genotyping PCR bands.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig1-figsupp1-data1-v1.zip
-
Figure 1—figure supplement 1—source data 2
Original files gel images of agarose gel electrophoresis for Figure 1—figure supplement 1B.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig1-figsupp1-data2-v1.zip
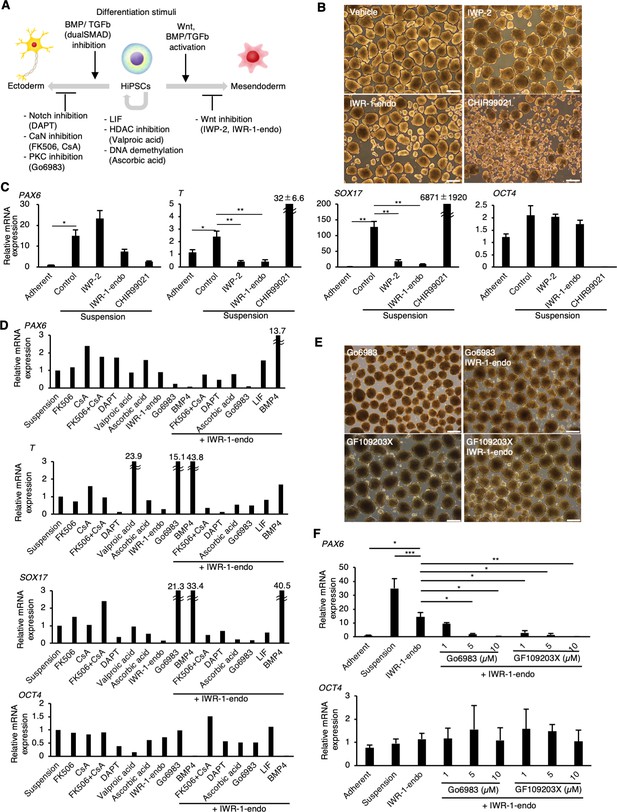
PKC inhibitors suppress spontaneous differentiation of human induced pluripotent stem cells (hiPSCs) into neural ectoderm in suspension conditions.
(A) Schematics of the factors related to the self-renewal and early differentiation of hiPSCs. (B) Phase-contrast pictures of suspension-cultured hiPSCs in the presence of Wnt signaling inhibitors (IWP-2 and IWR-1-endo) or activator (CHIR99021). Scale bars: 400 µm. (C) Gene expression of hiPSCs in suspension conditions with or without IWP2, IWR-1-endo, or CHIR99021. RT-qPCR was performed on day 10 samples (P2). Gene expressions were normalized to GAPDH and displayed as relative fold increase to adherent-cultured samples. Bar graphs show the mean ± SE. p-values were statistically analyzed using Dunnett’s multiple comparisons test. (D) Screening of inhibitory activity of candidate molecules on neuroectoderm differentiation in suspension-cultured hiPSCs. Candidate molecules were added in combination as shown. Results are displayed as relative fold increase to suspension-cultured samples without pharmacological treatment. n = 1. (E) Phase-contrast images of suspension-cultured hiPSCs on P2 in the presence of PKC inhibitors (Gö6983 or GF109203X) alone, or in combination with IWR-1-endo. Scale bars: 400µm. (F) The gene expression in suspension-cultured hiPSCs in the presence of IWR-1-endo or with combined IWR-1-endo and different doses of PKC inhibitors. Results are displayed as relative fold increase to adherent-culture. Data are presented as mean ± SE (n = 3). *, **, or *** in the graphs indicate p<0.05, p<0.01, or p<0.001, respectively.
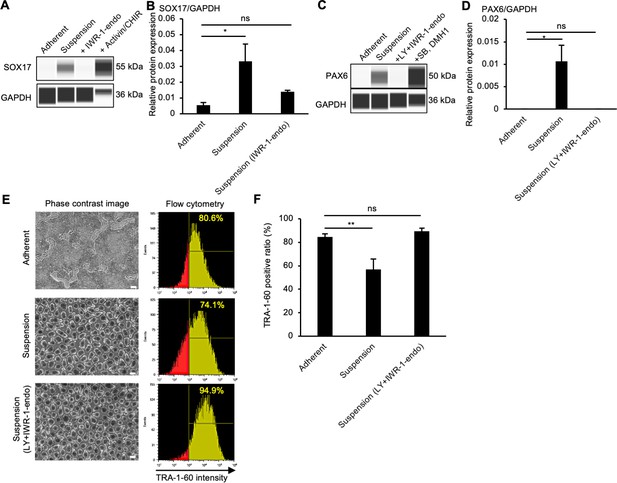
Effects of PKCβ and IWR-1-endo on suspension-cultured human induced pluripotent stem cells (hiPSCs) at protein levels.
(A) Simple western blotting of adherent- or suspension-cultured hiPSCs (SOX17-TEZ) in the presence or absence of IWR-1-endo on day 10 (passage 2). SOX17-TEZ cultured in endoderm differentiation medium (Activin A + CHIR99021) was used as a positive control for SOX17 expression. (B) The quantification of SOX17 expression. The protein expression was normalized to GAPDH. Data are presented as mean ± SE (n = 3). (C) Simple western blotting of adherent- or suspension-cultured hiPSCs (PAX6-TEZ) in the presence or absence of IWR-1-endo and LY333531 on day 10 (passage 2). PAX6-TEZ cultured in neuroectoderm differentiation medium (SB431542 + DMH1) was used as the positive control for PAX6 expression. (D) The quantification of expression of PAX6. The protein expression was normalized to GAPDH. Data are presented as mean ± SE (n = 3). (E) Phase-contrast images and representative flow cytometry data of pluripotency marker, TRA-1-60, in adherent- or suspension-cultured hiPSCs (1383D6 line) on day10 (passage 2). Suspension culture was performed in the presence or absence of IWR-1-end and LY333531. The percentage of TRA-1-60-positive cells is shown in the right corner. Scale bars: 400 µm. (F) Quantified data for TRA-1-60-positive ratio (%). The percentage of TRA-1-60-positive cells is shown as mean ± SE (n = 3). * or ** in the graphs indicate p<0.05 or p<0.01, respectively.
-
Figure 2—figure supplement 1—source data 1
File containing original automatic capillary western blots (Simple Western assays) for Figure 2—figure supplement 1A, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig2-figsupp1-data1-v1.zip
-
Figure 2—figure supplement 1—source data 2
Original files of capillary images of automatic capillary western blots (Simple Western assays) for Figure 2—figure supplement 1A.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig2-figsupp1-data2-v1.zip
-
Figure 2—figure supplement 1—source data 3
File containing original automatic capillary western blots (Simple Western assays) for Figure 2—figure supplement 1C, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig2-figsupp1-data3-v1.zip
-
Figure 2—figure supplement 1—source data 4
Original files of capillary images of automatic capillary western blots (Simple Western assays) for Figure 2—figure supplement 1C.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig2-figsupp1-data4-v1.zip
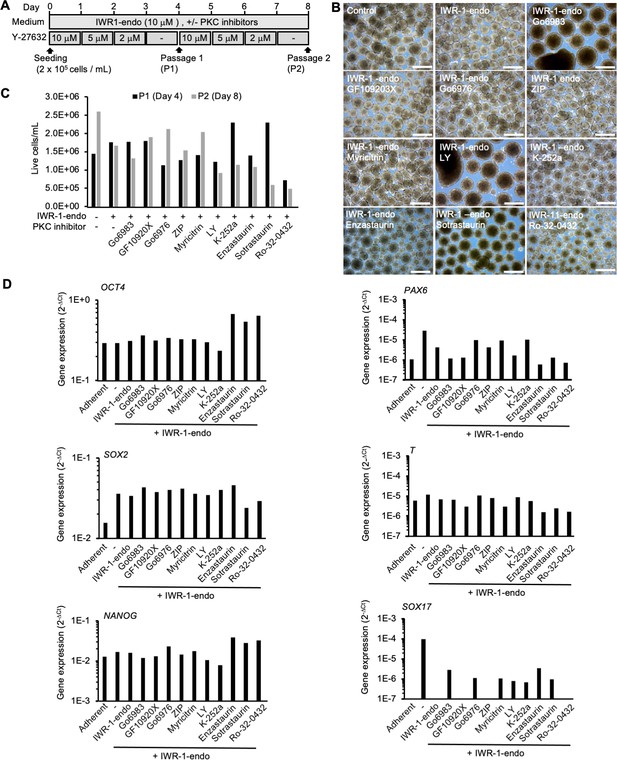
Activity of different type of PKC inhibitors on spontaneous differentiation of suspension-cultured human induced pluripotent stem cells (hiPSCs).
(A) Schematics representing suspension culture of hiPSCs supplemented with PKC inhibitors. Healthy donor-derived hiPSCs (201B7 line) were grown under suspension conditions in the presence of Y-27632, IWR-1-endo, and PKC inhibitors. Passage was performed every 4 days. (B) Phase-contrast images of suspension-cultured hiPSCs on day 8 (P2) in the absence or presence of IWR-1-endo and each PKC inhibitors. Scale bars: 500 µm. (C) Cell growth of hiPSCs (201B7). Graph shows live cell numbers counted on days 4 and day 8 in suspension conditions (n = 1). The PKC inhibitors added are labeled in the graphs. (D) Gene expression in hiPSCs cultured in adherent or suspension conditions with each PKC inhibitor. RT-qPCR was performed on cDNAs prepared from day 8 (passage 2) samples (n = 1). qPCR was performed for pluripotency (OCT4, SOX2, and NANOG) and early differentiation markers (PAX6, T, and SOX17). Gene expression was normalized to beta-ACTIN.
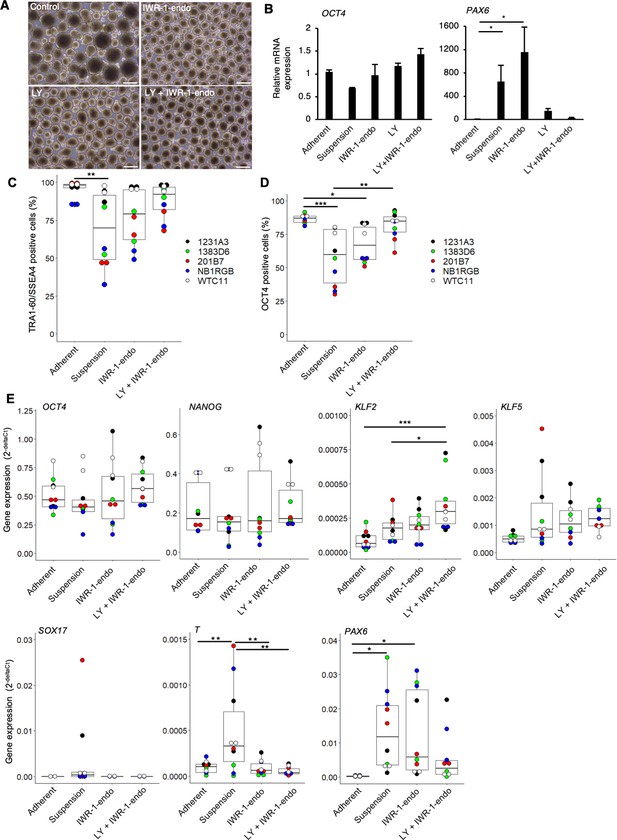
The inhibitors of Wnt and PKCβ efficiently maintain the self-renewal of human induced pluripotent stem cells (hiPSCs) in suspension conditions.
(A) Phase-contrast images of suspension-cultured hiPSCs on day 10 (two passages) in the presence of IWR-1-endo, LY333531, or both. Scale bars: 400 µm. (B) Gene expression in suspension-cultured hiPSCs on P2 in the presence of IWR-1-endo, LY333531, or both. Data are presented as mean ± SE. p-values were statistically analyzed with Dunnett’s test. (C) After suspension culture of five hiPSC lines, WTC11, 1231A3, HiPS-NB1RGB (NB1RGB), 1383D6, and 201B7, for 10 days (two passages) in StemFit AK02N medium with or without IWR-1-endo and LY333531, flow cytometry, immunocytochemistry, and RT-qPCR were performed. Box plots of flow cytometry for TRA-1-60 and SSEA4 double-positive cells (%) detected with flow cytometry are shown. Used cell lines are indicated by the different colored circles shown on the right side of the graph (n = 2 × 5 cell lines). (D) Box plots of OCT4-positive cells (%) detected with immunocytochemistry are shown (n = 2 × 5 cell lines). (E) Box plots of RT-qPCR data are shown. Undifferentiated markers (OCT4, NANOG), naïve pluripotency markers (KLF2, KLF5), and differentiation markers (SOX17, T, PAX6), were assessed. Statistical analysis was performed using one-way ANOVA and Tukey’s test for all graphs. p-values <0.05 were considered statistically significant. *, **, or *** in the graphs indicate p<0.05, p<0.01, or p<0.001, respectively (n = 2 × 5 cell lines).
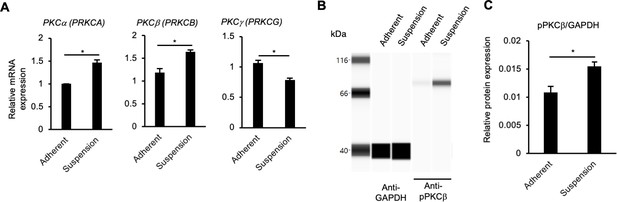
Upregulation of PKC genes in suspension-cultured human induced pluripotent stem cells (hiPSCs).
(A) RNA expression of conventional PKC isoform genes in hiPSCs (WTC11 line) as detected by RT-qPCR. cDNA was prepared from adherent- or suspension-cultured hiPSCs on day 5. The gene expression was normalized to GAPDH. Results are displayed as relative fold increase to adherent culture. Data are presented as mean ± SE (n = 3). p-values were statistically analyzed with Student’s t-test. (B) Detection of phosphorylated PKCβ protein (pPKCβ) in suspension-cultured hiPSCs on day 5 using an automated capillary western blot (Simple Western) assay. Adherent-cultured hiPSCs were used as a control. (C) Quantification of pPKCβ expression. The protein expression was normalized to GAPDH. Data are shown as mean ± SE (n = 3). p-value was statistically analyzed using Student’s t-test. * in the graphs indicate p<0.05.
-
Figure 3—figure supplement 1—source data 1
File containing original automatic capillary western blots (Simple Western assays) for Figure 3—figure supplement 1B, indicating the relevant bands and culture conditions.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig3-figsupp1-data1-v1.zip
-
Figure 3—figure supplement 1—source data 2
Original files of capillary images of automatic capillary western blots (Simple Western assays) for Figure 3—figure supplement 1B.
- https://cdn.elifesciences.org/articles/89724/elife-89724-fig3-figsupp1-data2-v1.zip
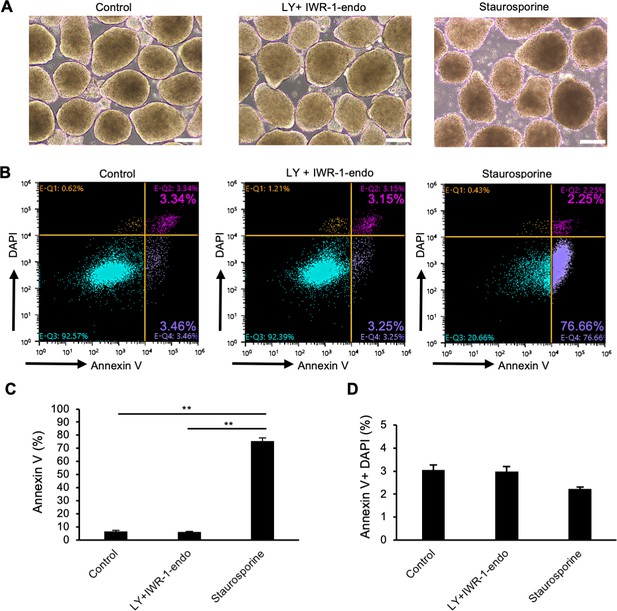
Cell death of suspension-cultured human induced pluripotent stem cells (hiPSCs) after the treatment with LY333531 and IWR-1-endo.
(A) Phase-contrast images of suspension-cultured hiPSCs (WTC11 line) on day 10 (passage 2). Cells were culture in StemFit AK02N medium with no supplement (left), with LY333531 + IWR-1-endo (middle) for 10 days, with Staurosporine for 2 hours. Scale bars: 200 µm. (B) Evaluation of apoptotic cells by flow cytometry using Annexin V (Alexa Fluor 680) conjugates. (C) Bar graph indicating Annexin V-positive cells (%) in each culture conditions. (D) Bar graph indicating double positive cells for Annexin V and DAPI (%) in each culture conditions. Data are presented as mean ± SE (n = 3). Statistical analysis was performed using by one-way ANOVA and Tukey’s tests for all graphs. p-values <0.05 were considered statistically significant. ** in the graphs indicate p<0.01.
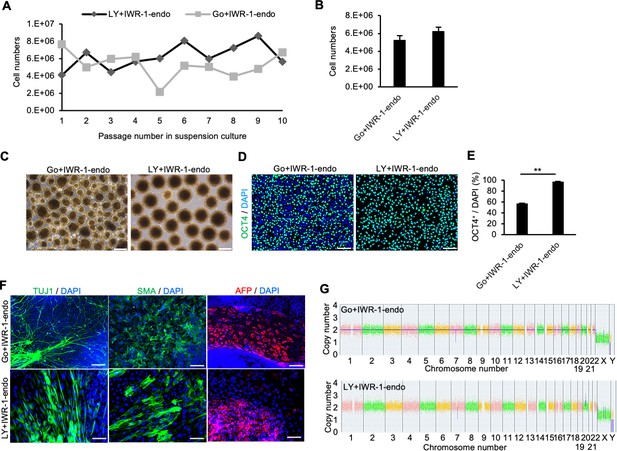
Long-term suspension culture of human induced pluripotent stem cells (hiPSCs) is maintained by simultaneous suppression of PKCβ and Wnt signals.
(A) The number of hiPSCs (WTC11 line) counted at each passage (every 5 days) during long-term suspension culture. Cell culture was performed in the presence of IWR-1-endo plus Gö6983 or LY333531. (B) Bar graph indicating average cell numbers for 10 passages (mean ± SE). (C) Phase-contrast images of suspension-cultured hiPSCs on passage 10. Scale bars: 400 µm. (D) Immunocytochemistry of OCT4 on passage 10 samples. Scale bars: 100 µm. (E) Bar graph showing the percentages of OCT4-positive cells. Values were calculated from randomly selected three regions from immunofluorescence images. Data are presented as mean ± SE. p-value was statistically analyzed with Student’s t-test. (F) Immunocytochemistry of differentiated cells in embryoid bodies (EBs) from suspension-cultured hiPSCs in the presence of IWR-1-endo, and Gö6983 or LY333531. Anti-TUJ1, -SMA, and -AFP antibodies were used to detect ectoderm, mesoderm, and endoderm differentiation, respectively. Scale bars: 100 µm. (G) Chromosomal copy numbers detected with copy number variation (CNV) array analysis (Karyostat assay) of suspension-cultured hiPSCs in the presence of IWR-1-endo and Gö6983 (upper panel) or LY333531 (lower panel) at passage 10. ** in the graphs indicate p<0.01.
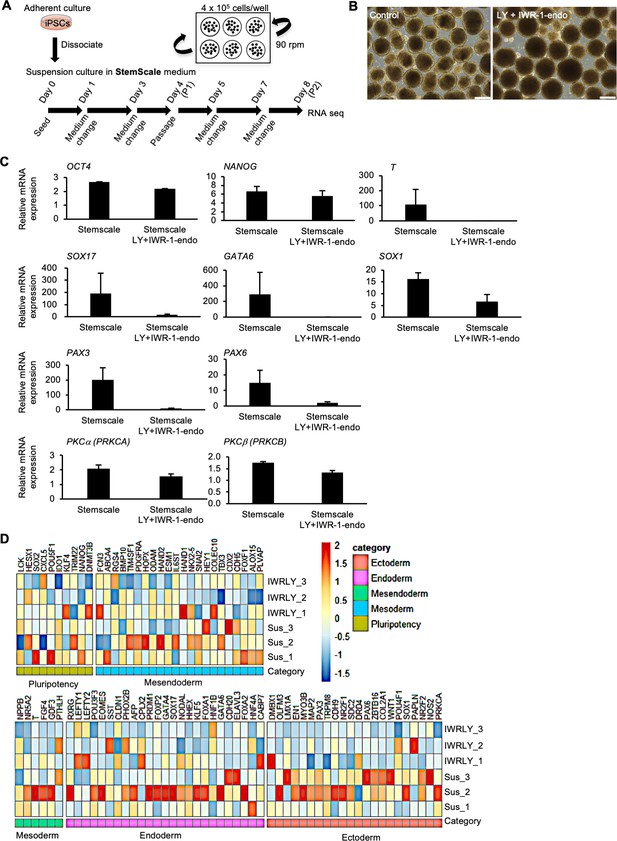
Generality of the inhibitory effects of IWR-1-endo and LY333531 on spontaneous differentiation of human induced pluripotent stem cells (hiPSCs) cultured in suspension condition.
(A) Schematic representing suspension culture of hiPSCs using StemScale medium (Thermo Fisher Scientific) as the basic culture medium. hiPSCs were grown in suspension conditions with continuous rotation (90 rpm) in the presence or absence of IWR-1-endo and LY333531. Passage was performed every 4 days according to the manufacturer’s protocol. cDNAs were prepared from day 8 (P2) and used for RT-qPCR and RNA-seq. (B) Phase-contrast images of suspension-cultured hiPSCs on day 8 in the presence or absence of IWR-1-endo and LY333531. Scale bars: 400 µm. (C) RNA expression of pluripotency and early differentiated genes in hiPSCs, as detected by RT-qPCR. cDNA was prepared from suspension-cultured hiPSCs on day 8 in the presence or absence of IWR-1-endo and LY33353. The gene expression was normalized to GAPDH. Data are presented as mean ± SE (n = 3). (D) Heat map of marker genes for pluripotency and differentiation to three germ layers from RNA-seq data in suspension-cultured iPSCs without compounds (Sus_1, Sus_2, and Sus_3) or with IWR-1-endo and LY333531 (IWRLY_1, IWRLY_2, and IWRLY_3). The listed genes were classified as pluripotent, mesendoderm, mesoderm, endoderm, and ectoderm, as defined in the Scorecard Assay (Thermo Fisher Scientific).
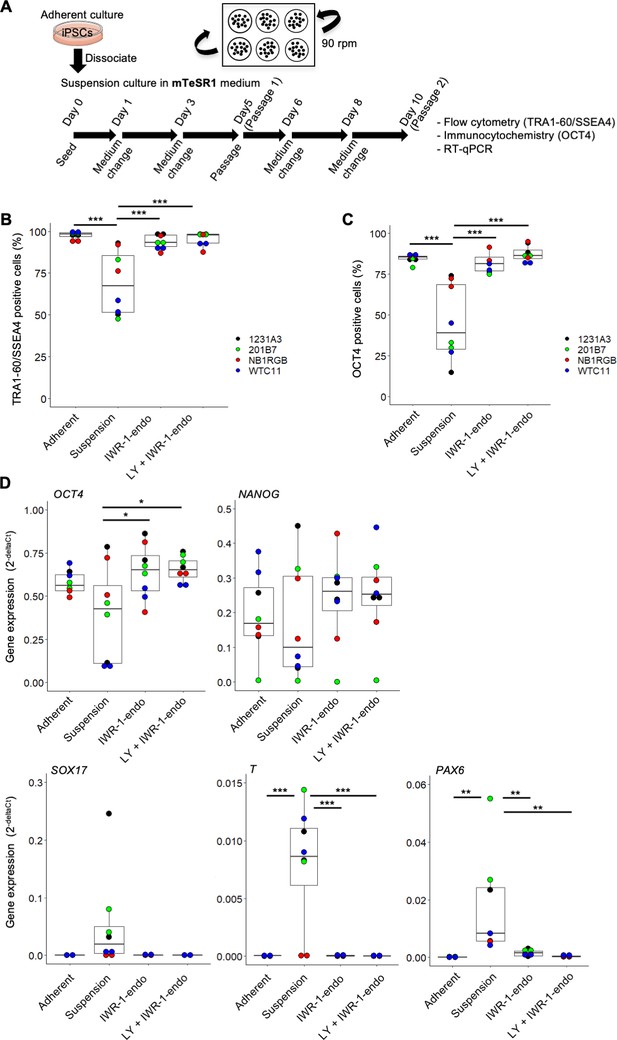
Suspension culture of multiple human induced pluripotent stem cell (hiPSC) lines in mTeSR1 medium supplemented with or without IWR-1-endo and LY333531.
(A) Schematics of suspension culture of WTC11, 1231A3, HiPS-NB1RGB (NB1RGB), and 201B7 hiPSC lines. After suspension culture of these four hiPSC lines in mTeSR1 medium with or without IWR-1-endo and LY333531, characteristic analysis was performed on day10 (passage 2). Suspension culture was performed twice in each cell lines (n = 2 each from four cell lines). (B) Box plots of flow cytometry for TRA-1-60 and SSEA4 double-positive cells (%). Used cell lines are indicated by the different colored circles shown on the right side of the graph. (C) Box plots of OCT4-positive cells (%). (D) Box plots of RT-qPCR. Undifferentiated markers (OCT4 and NANOG) and differentiation markers (SOX17, T, and PAX6) were assessed. Statistical analysis was performed using one-way ANOVA and Tukey’s test for all graphs. p-values <0.05 were considered statistically significant. *, **, or *** in the graphs indicate p<0.05, p<0.01, or p<0.001, respectively.
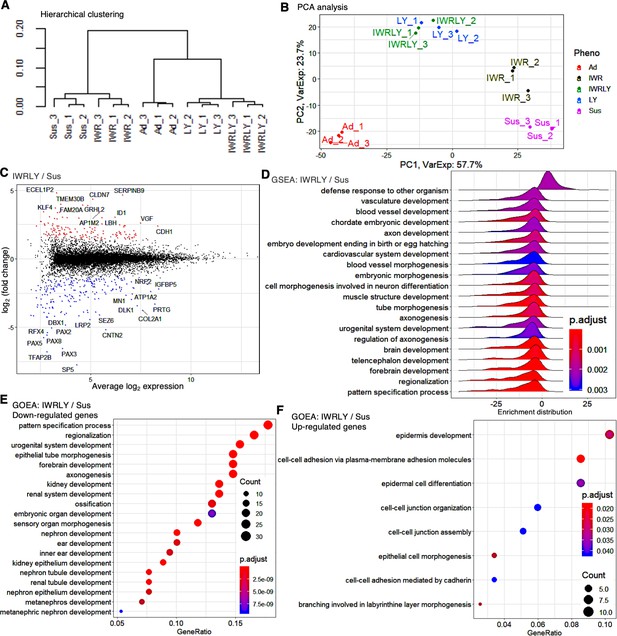
The inhibitors of Wnt and PKCβ in suspension conditions efficiently suppress differentiated gene marker expression in transcriptome analysis.
(A) Hierarchical clustering of adherent and suspension-cultured human induced pluripotent stem cells (hiPSCs) (WTC11 line) on P2 using Ward’s method from RNA-seq data (n = 3 in each condition). Ad, adherent; Sus, suspension; IWR, IWR-1-endo; LY, LY333531; IWRLY, IWR-1-endo, and LY333531. (B) Principal component analysis (PCA) plot showing clusters of samples based on similarity. Gene expression variance are displayed as PC1 and PC2. (C) MA plot (log2 fold change versus mean average expression) comparing transcriptomes between IWRLY and Sus conditions. (D) Gene Set Enrichment Analysis (GSEA) on the gene sets of IWRLY to Sus from these RNA-seq data. Statistically significant enrichment is shown. p-values are represented in blue to red from low to high values. (E, F) Gene Ontology Enrichment Analysis (GOEA) for the gene sets of IWRLY to Sus from these RNA-seq data. Analysis was performed on downregulated genes in (E) and upregulated genes in (F).
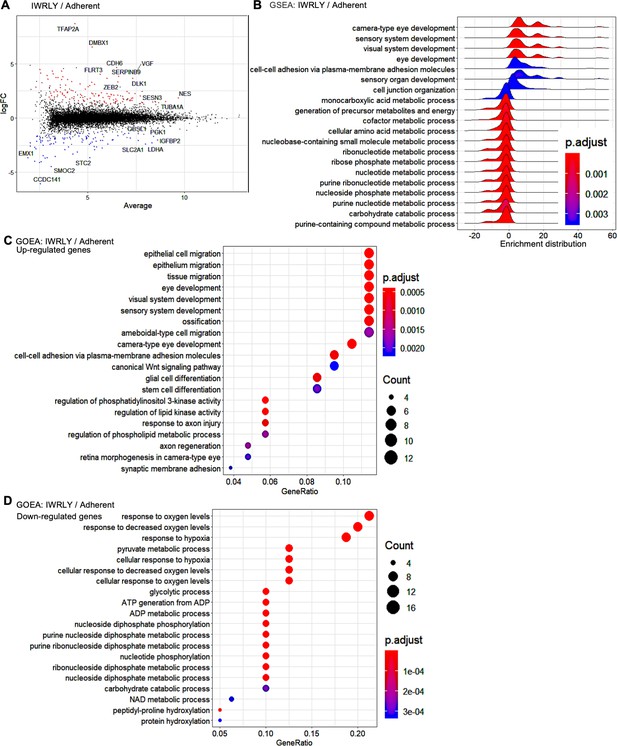
Global gene expression of suspension-cultured human induced pluripotent stem cells (hiPSCs) with IWR-1-endo and LY333531 in comparison to adherent-cultured hiPSCs.
(A) MA plot (log2 fold change versus mean average expression) comparing the transcriptomes between adherent- and suspension-cultured hiPSCs treated with IWR-1-endo and LY33353 (IWRLY). Transcripts with log2 fold change ≧ 2 or ≦ –2 (false discovery rate [FDR] <0.01) are highlighted with red or blue dots, respectively. The names of the representative genes are shown in the plot. (B) Gene Set Enrichment Analysis (GSEA) of the gene sets of suspension-cultured hiPSCs with IWRLY compared to adherent-cultured hiPSCs. Analysis was performed on both upregulated and downregulated genes. Statistically significant enrichment is shown. Adjusted p-values are illustrated from blue to red as low to high. (C, D) Gene Ontology Enrichment Analysis (GOEA) of the gene sets of suspension-cultured hiPSCs with IWRLY compared to adherent-cultured hiPSCs. Analysis was performed on upregulated genes in (C) and downregulated genes in (D), respectively. Results are ranked by significance (adjusted p-values) and/or counted gene numbers.
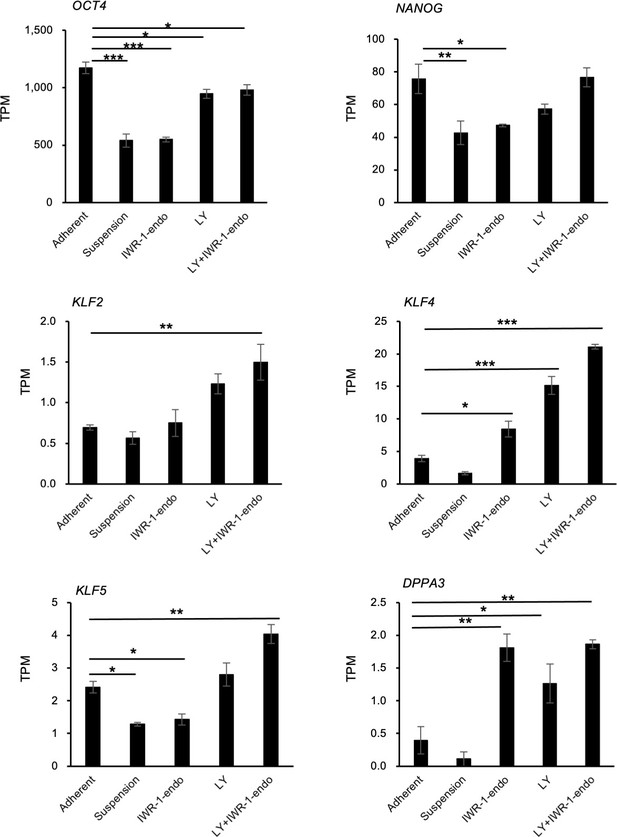
Comparison of expression on naïve pluripotency markers between adherent and suspension-cultured human induced pluripotent stem cells (hiPSCs).
hiPSCs (WTC11 line) were cultured in continuous suspension conditions in StemFit AK02N in the presence of IWR-1-endo, LY333531, or both. RNA-seq was performed using day 10 samples. The gene expression of naïve pluripotency markers (OCT4, NANOG, KLF2, KLF4, KLF5, and DPPA3) was extracted based on the TPM (transcripts per million) values. Bar graphs show the mean ± SE (n = 3). p-values were statistically analyzed with Dunnett’s test to the samples of adherent conditions. p-values<0.05 were considered statistically significant. *, **, or *** in the graphs indicate p<0.05, p<0.01, or p<0.001, respectively.
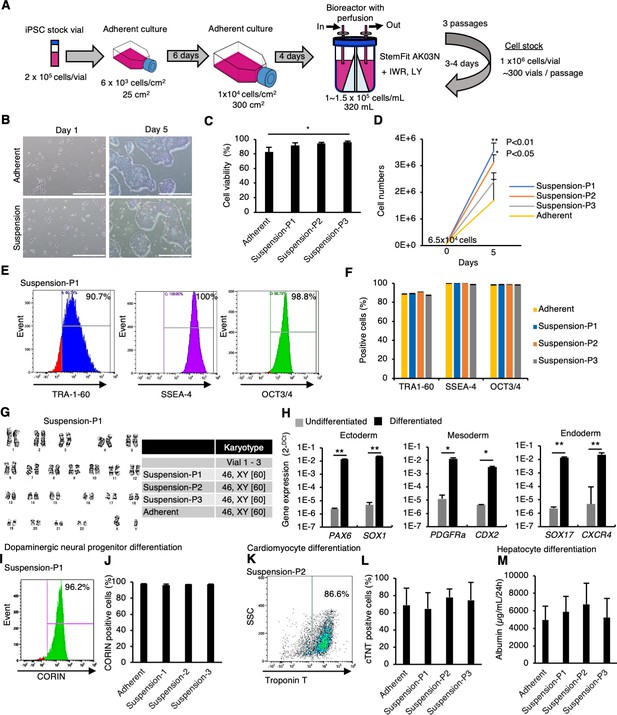
Mass suspension culture of clinical-grade human induced pluripotent stem cells (hiPSCs) in the presence of PKCβ and Wnt signaling inhibitors.
(A) Schematics of mass suspension culture using a bioreactor. (B) Representative phase-contrast images of hiPSCs after seeding from frozen vials. Scale bar: 1 mm. (C) Cell viability at seeding. Data are presented as mean ± SE (n = 3). p-values were statistically analyzed with Dunnett’s multiple comparisons test. (D) Total cell numbers were counted on day 5 after thawing. Data were presented as mean ± SE (n = 3). p-values were statistically analyzed with Dunnett’s test. (E) Representative flow cytometry data of pluripotent markers in these hiPSCs. (F) Quantification of flow cytometry data for the pluripotent markers. Data are presented as mean ±SE (n = 3). (G) Karyotypes of these hiPSCs. Left: representative pictures of G-band analysis. Right: table of karyotype results (n = 3). The numbers in brackets indicate the cell numbers examined. (H) In vitro differentiation into early phases of three germ layers from hiPSCs was assessed using RT-qPCR (mean ± SE) (n = 3). p-value was statistically analyzed with Student’s t-test. (I) Representative flow cytometry data for CORIN in the cells in the dopaminergic neural progenitors differentiated from hiPSCs. (J) Quantification of CORIN-positive cells (mean ± SE, n = 3). (K) Representative flow cytometry data for cardiac Troponin T (cTnT) in the cardiomyocytes differentiated from hiPSCs. (L) Quantification of cTnT-positive cells (mean ± SE, n = 3). (M) Albumin secretion levels of hepatocytes differentiated from hiPSCs (mean ± SE, n = 3). * or ** in the graphs indicate p<0.05 or p<0.01, respectively.
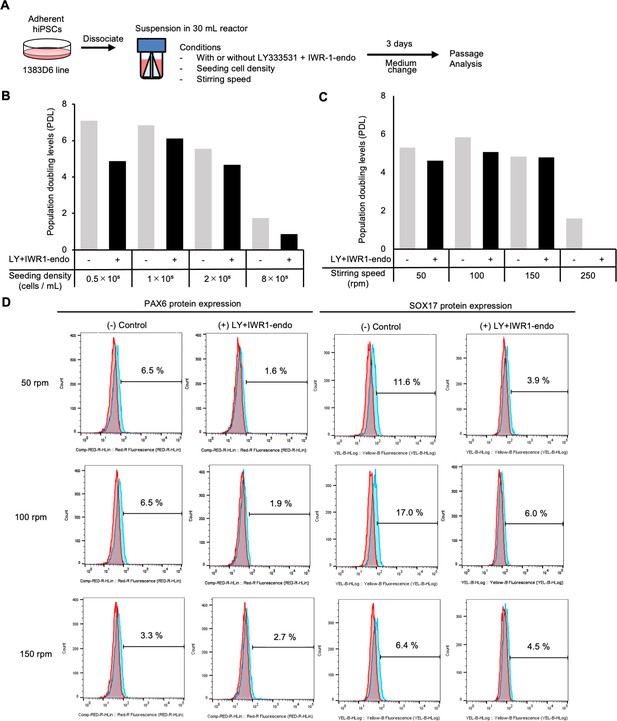
Suspension culture of human induced pluripotent stem cells (hiPSCs) supplemented with IWR-1-endo and LY333531 using bioreactors with continuous agitation.
(A) Schematic representing suspension culture of hiPSCs in a 30 mL bioreactor. Healthy donor-derived hiPSCs (1383D6 lines) were grown with continuous agitation in the presence or absence of IWR-1-endo and LY333531. Passages were performed every 3–4 days. (B, C) Graphs showing population doubling levels from the seeding cell numbers on day 3. (D) Flow cytometric analysis of differentiation marker proteins, PAX6 and SOX17. Positive cells (%) are shown.
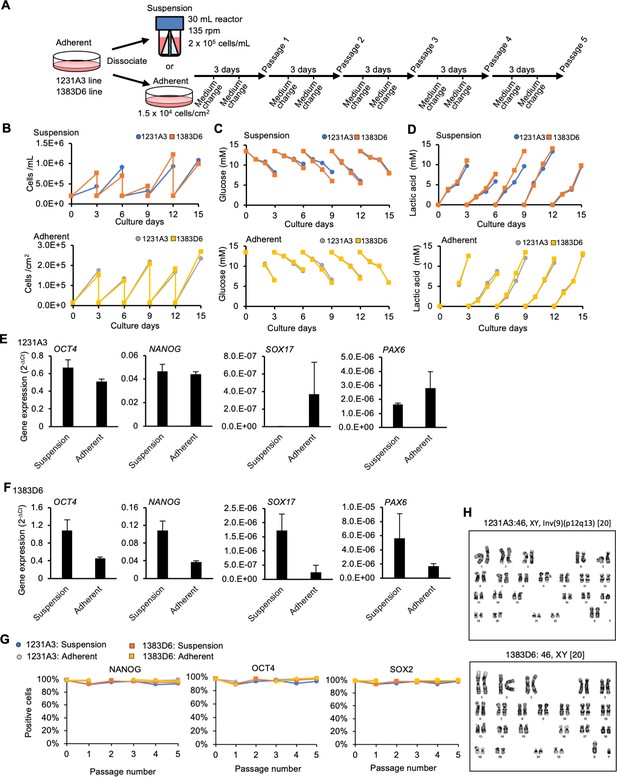
Suspension culture of human induced pluripotent stem cells (hiPSCs) supplemented with IWR-1-endo and LY333531 using bioreactors with continuous agitation.
(A) Schematic representing suspension culture of hiPSCs in a 30 mL bioreactor. Healthy donor-derived hiPSCs (1231A3 and 1383D6 lines) were grown with continuous agitation in the presence or absence of IWR-1-endo and LY333531. Passages were performed every 3 days. (B) Graphs showing cell numbers at each passage (n = 1). Upper: suspension culture of 1231A3 and 1383D6 lines. Lower: adherent culture of 1231A3 and 1383D6 lines. (C) Graphs showing glucose concentration in the culture medium at each culture day. Upper: suspension culture of 1231A3 and 1383D6 lines. Lower: adherent culture of 1231A3 and 1383D6 lines. (D) Graphs showing lactic acid concentration in the culture medium at each culture day. Upper: suspension culture of 1231A3 and 1383D6 lines. Lower: adherent culture of 1231A3 and 1383D6 lines. (E, F) RT-qPCR analysis of hiPSCs cultured in 30 mL bioreactor or adherent conditions. cDNA samples were prepared from passages 3 and 5 of the 1231A3 in (E) and 1383D6 in (F). qPCR was performed for OCT4 and NANOG (pluripotent), SOX17 (endoderm), and PAX6 (neuroectoderm). The gene expression was normalized to β-ACTIN. Bar graph showing the average gene expressions of passages 3 and 5 (mean ± SE). (G) Flow cytometric analysis of adherent or suspension-cultured 1231A3 and 1383D6 lines. Positive cells (%) of pluripotency marker proteins: NANOG, OCT4, and SOX2 are shown. (H) Karyotyping of hiPSCs cultured in 30 mL bioreactor. Cell samples were prepared on passage 5 and used for G-band karyotyping analysis. A short deletion on chromosome 9 of 1231A3 (indicated with arrows) was derived from the original line.
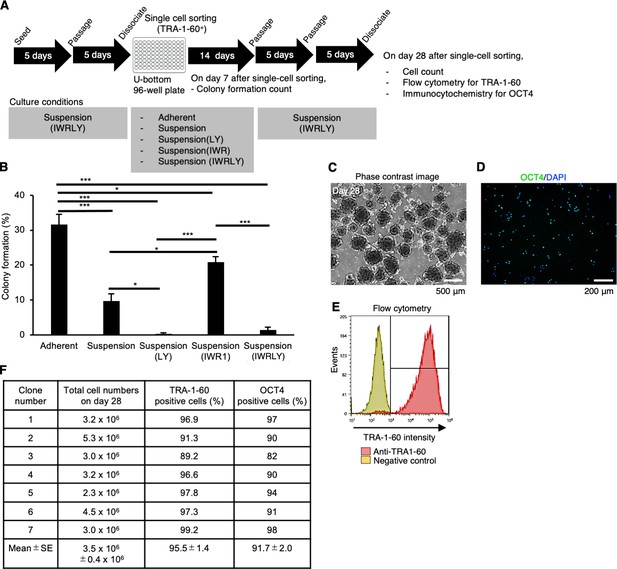
Establishment of single-cell-sorted human induced pluripotent stem cell (hiPSC) subclones cultured in suspension conditions supplemented with IWR-1-endo and LY3333531.
(A) Schematics representing the establishment of single-cell-derived hiPSC subclones from 201B7 line. Single-cell-sorted cells were expanded in the culture medium supplemented with IWR-1-endo and LY333531 (IWRLY). Formed colonies were picked on day 14, and expanded by repeating passage every 4–5 days under suspension conditions. Characteristic analysis was performed on day 28 after single-cell sorting. (B) On day 7 after single-cell sorting, formed colonies were counted per well in the 96-well plate. The ratio (%) of the colony formation is shown in a bar graph (mean ± SE) (n = 3). Statistical analysis was performed using one-way ANOVA and Tukey’s tests for all graphs. p-values <0.05 were considered statistically significant. *, **, or *** in the graphs indicate p<0.05, p<0.01,or p<0.001, respectively. (C) Phase-contrast images of Clone #2 on day 28 after single-cell sorting. Scale bars: 500 µm. (D) Represented immunocytochemistry of OCT4 (Clone #2). Scale bars: 200 µm. (E) Represented flow cytometry of TRA-1-60 (Clone #2). (F) Summary table of the characterization of single cell-sorted clones. Total cell numbers on day 28, the ratio of TRA-1-60-positive cells (%), and the ratio of OCT4-positive cells (%) are shown (mean ± SE) (n = 7).
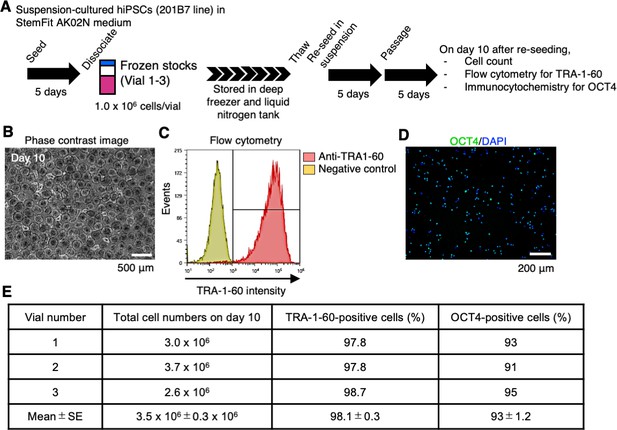
Direct re-suspension culture from frozen stocks of human induced pluripotent stem cells (hiPSCs) in suspension conditions supplemented with IWR-1-endo and LY3333531.
(A) Schematics of direct re-suspension culture of frozen stocks of single-cell-derived 201B7 clone (generated in Figure 7; Vials 1-3). (B) Represented phase-contrast images on day 10 (Vial 3). Scale bars: 500 µm. (C) Represented flow cytometry of TRA-1-60 (Vial 3). (D) Represented immunocytochemistry of OCT4 (Vial 3). Scale bars: 200 µm. (E) Summary table of the characterization of re-suspension-cultured hiPSCs from frozen stocks. Total cell numbers on day 10, the ratio of TRA-1-60-positive cells (%), and the ratio of OCT4-positive cells (%) are shown (mean ± SE) (n = 3).
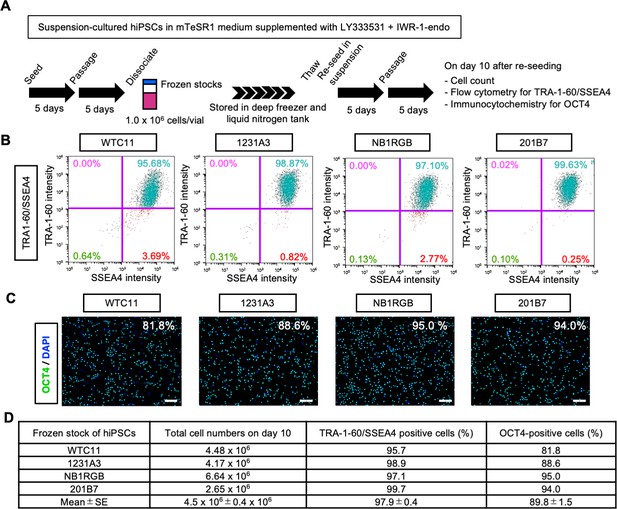
Direct re-suspension from frozen stocks of human induced pluripotent stem cells (hiPSCs) under suspension conditions supplemented with IWR-1-endo and LY333531 in mTeSR1 medium.
(A) Schematics of direct re-suspension culture of frozen stocks of WTC11, 1231A3, HiPS-NB1RGB (NB1RGB), and 201B7 hiPSC lines. After suspension culture in mTeSR1 medium supplemented with IWR-1-endo and LY333531, expanded hiPSCs were cryopreserved. These frozen stocks were thawed and seeded in the mTeSR1 medium supplemented with IWR-1-endo and LY333531 by repeating passage every 5 days. Characteristic analysis was performed on day 10. (B) Representative flow cytometry of TRA-1-60 and SSEA4. (C) Representative immunocytochemistry of OCT4. DAPI indicates nuclear staining. Scale bars: 400 µm. (D) Summary of the characterization of re-suspension-cultured hiPSCs from frozen stocks. Total cell numbers on day 10, the ratio of double-positive cells for TRA-1-60 and SSEA4 (%), and the ratio of OCT4-positive cells (%) are shown (mean ± SE; n = 4).
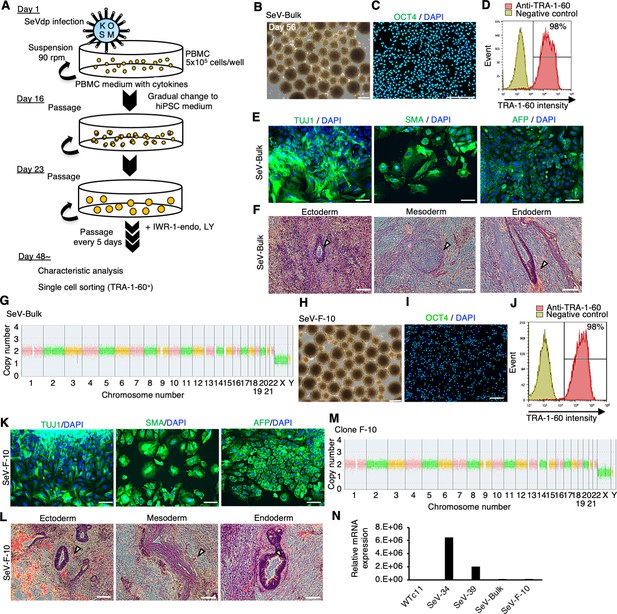
Establishment of human induced pluripotent stem cells (hiPSCs) in complete suspension conditions using SeVdp.
(A) Schematics of hiPSC generation in suspension conditions. (B) Phase-contrast images of peripheral blood mononuclear cells (PBMCs) on day 56 after infection. Scale bars: 400 µm. (C) Immunocytochemistry of OCT4 on bulk-hiPSCs on day 56. Scale bars: 200 µm. (D) Flow cytometry of TRA-1-60 in bulk-hiPSCs on day 61. (E) Immunocytochemistry of TUJ1, SMA, and AFP on differentiated cells in embryoid bodies (EBs) from bulk-hiPSCs on day 56. Scale bars: 100 µm. (F) HE staining of teratoma sections derived from bulk-hiPSCs. White arrowheads indicate representative tissue structures derived from ectoderm, mesoderm, and endoderm. Scale bars: 100 µm. (G) Chromosomal copy numbers detected with copy number variation (CNV) array analysis on bulk-hiPSCs. (H) Phase-contrast image of an established hiPSC clone at passage 7. Scale bars: 400 µm. (I) Immunocytochemistry of established clone, F-10, with anti-OCT4 antibody. Scale bars: 200 µm. (J) The percentage of TRA-1-60-positive cells with flow cytometry on established clone F-10 line. (K) HE staining of teratoma sections derived from F-10 clone line. The details are the same as in (F). (L) Immunocytochemistry in EBs from established F-10 clone. Scale bars: 100 µm. (M) Chromosomal copy numbers of F-10 clone line. (N) Residual SeVdp genomic RNA in established hiPSCs with RT-qPCR.
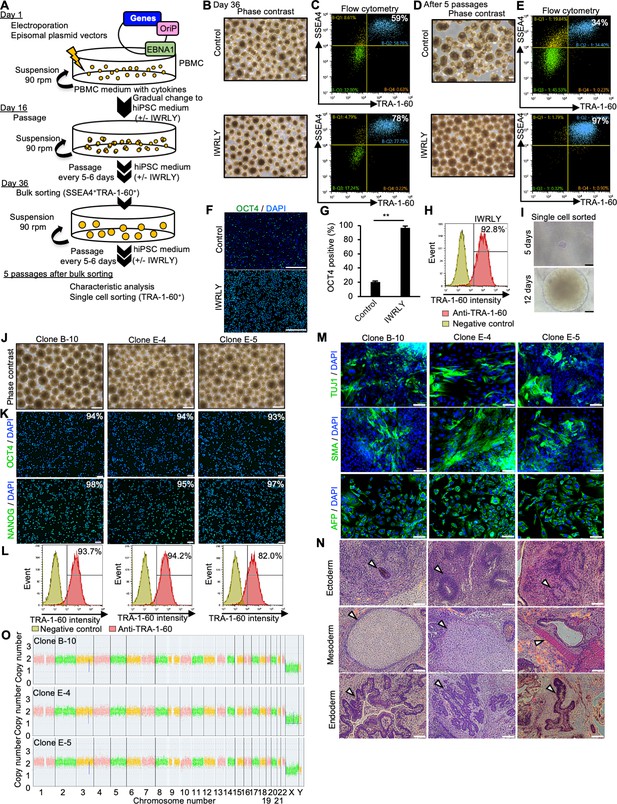
Establishment of human induced pluripotent stem cells (hiPSCs) in completed suspension conditions using episomal vectors.
(A) Flow chart representing the establishment of hiPSCs from peripheral blood mononuclear cells (PBMCs) by electroporation of episomal vectors. (B) Phase-contrast images of transfected cells on day 36 after electroporation of episomal vectors in the absence or presence of IWR-1-endo and LY333531. Scale bars: 400 µm. (C) Flow cytometry of TRA-1-60 and SSEA4. TRA-1-60 and SSEA4-psotive cells were sorted as the bulk fraction and further suspended in the absence or presence of IWR-1-endo and LY333531. The percentages of TRA-1-60 and SSEA4-psotive cells are shown in the right corner. (D) Phase-contrast images of suspension-cultured cell aggregates after bulk sorting on passage 5. Scale bars: 400 µm. (E) Flow cytometry of TRA-1-60 and SSEA4. The percentage of TRA-1-60- and SSEA4-psotive cells is shown in the right corner. (F) Immunocytochemistry of OCT4 on sorted cells cultured in the absence (top) or presence (bottom) of IWR-1-endo and LY333531 for five passages. Scale bars: 400 µm. (G) Quantified data for OCT4-positive cells. Bar graph indicating average OCT4-positive cell percentages (mean ± SE) (n = 3). p-value was statistically analyzed with Student’s t-test. (H) Single-cell sorting and cloning of TRA-1-60 positive cells. Flow cytometry was performed for cell fraction that was cultured in the presence of IWR-1-endo and LY333531. TRA1-60-positive cells were collected as single cells in a 96-well plate and expanded in the culture medium supplemented with Y-27632, IWR-1-endo, and LY333531. (I) Representative phase-contrast images of single-sorted cell aggregates on days 5 and 12. Scale bars: 400 µm. (J) Phase-contrast images of established clones (B-10, E-4, and E-5). Scale bars: 400 µm. (K) Immunocytochemistry of OCT4 and NANOG in these clones. The percentage of positive cell is shown. Scale bars: 100 µm. (L) Flow cytometry of TRA-1-60 in these clones. TRA-1-60-positive percentages are shown in the right corner. (M) Immunocytochemistry of differentiated cells in embryoid bodies (EBs) from these clones. Anti-TUJ1, -SMA, and -AFP antibodies were used. Scale bars: 100 µm. (N) Hematoxylin-eosin staining of paraffin-embedded sections of teratoma derived from these clones. White arrowheads indicate representative tissue structures of the ectoderm, mesoderm, or endoderm. Scale bars: 100 µm. (O) Chromosomal copy numbers detected by CNV array analysis (Karyostat assay) of these clones. ** in the graphs indicate p<0.01.
Tables
The list of published studies on scalable suspension culture without microcarriers for human pluripotent stem cells (hPSCs).
| Article | Medium | Additives | Cell lines tested | Scalability | Transcriptome | Single-cell cloning | Direct freeze and thaw | iPSC generation |
|---|---|---|---|---|---|---|---|---|
| Amit et al., 2010; Amit et al., 2011 | DMEM/F12 + KSR | Y-27632, LIF, bFGF | hESC (I3, Ie, I5, H9.2, H9, H7, H14) hiPSC (iF4, J1.2.3, C3, C2, KTN7, KTN13) | ~107 | No | No | No | No |
| Krawetz et al., 2010 | mTeSR1 | Y-27632, Rapamycin | hESC (H9) | ~108 | No | No | No | No |
| Singh et al., 2010; Zweigerdt et al., 2011; Olmer et al., 2012 | KnockOut DMEM + KSR, mTeSR1 | Y-27632, bFGF | hESC (hES2, hES3, and ESI049) hiPSC (hCBiPS2, hiPSOCT4eGFP) | ~108 | No | No | No | No |
| Steiner et al., 2010 | Neurobasal medium + KSR | Y-27632, bFGF, Activin A, Fibronectin, Gelatin, BDNF, NT3, NT4, Nutridoma-CS | hESC (HES1, HES2, H7) | ~106 | No | No | No | No (of note, three hESC lines derivation) |
| Wang et al., 2013 | TeSR-E8 | Y-27632 | hiPSC (BC1, TNC1) | ~108 | No | No | No | No |
| Hunt et al., 2014 | mTeSR1 | Y-27632 | hESC (H1, H9) | ~107 | No | No | No | No |
| Elanzew et al., 2015 | mTeSR1, TeSR-E8 | Y-27632 | hESC (H9) and hiPSC (iLB-C-31f-r1) | ~107 | No | No | No | No |
| Horiguchi and Sakai, 2016; Ibuki et al., 2019 | TeSR-E8, mTeSR1 | Y-27632, KSR, Albumax, LPA, SIP, | hiPSC (TkDN4-M, TkDA3-4, 201B7) | ~106 | No | No | No | No |
| Nath et al., 2017 | Dialyzed DMEM/F-12 | Y-27632, bFGF, TGF-b1 | hiPSC (Tic) | ~108 | No | No | No | No |
| Kwok et al., 2018 | mTeSR1, StemMACS | Y-27632 | hiPSC (AR1034ZIMA hiPSC clone1, FS hiPSC clone2) | ~109 | No | No | No | No |
| Lipsitz et al., 2018 | Nutristem, DMEM/F12+KSR | Y-27632, LIF, TGFβ1 FGF2, CHIR99021 SP600125, BIRB796, Gö6983 | hESC (H9, HES2, WIB) and hiPSC (C1.15) | ~107 | No | No | No | No |
| Rohani et al., 2020 | mTeSR1, RSeT | Y-27632, Rapamycin | hESC (H1, H9) | ~107 | Yes | No | No | No |
| This study | AK02N, AK03N, StemScale, mTeSR1 | Y-27632, IWR-1-endo, LY333531 | hiPSC (WTC11, 201B7, 1383D6, 1231A3, HiPS-NB1RGB, Ff-I14s04) | ~109 (in 3 passages) | Yes | Yes | Yes | Yes |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, male) | NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice, 5 weeks at purchase | The Jackson Laboratory | Strain#: 005557 RRID:IMSR_JAX:05557 | |
| Cell line (Homo sapiens) | WTC11 | Coriell Institute; Hayashi et al., 2016 | GM25256 RRID:CVCL_Y803 | |
| Cell line (H. sapiens) | 201B7 | RIKEN Cell Bank; Takahashi et al., 2007 | HPS0063 RRID:CVCL_A324 | |
| Cell line (H. sapiens) | 454E2 | RIKEN Cell Bank; Okita et al., 2011 | HPS0077 RRID:CVCL_T791 | |
| Cell line (H. sapiens) | 1383D6 | RIKEN Cell Bank; Okita et al., 2011 | HPS1006 RRID:CVCL_UP39 | |
| Cell line (H. sapiens) | 1231A3 | RIKEN Cell Bank; Okita et al., 2011 | HPS0381 RRID:CVCL_LJ39 | |
| Cell line (H. sapiens) | Ff-I14s04 | CiRA foundation, Kyoto University; Kitano et al., 2022 | ||
| Cell line (H. sapiens) | HiPS-NB1RGB | RIKEN Cell Bank; Borisova et al., 2022; Shimizu et al., 2022 | HPS5067 | |
| Cell line (H. sapiens) | PAX6-TEZ | This paper | deposited as HPS4903 in RIKEN Cell Bank | |
| Cell line (H. sapiens) | SOX17-TEZ | This paper | deposited as HPS4905 in RIKEN Cell Bank | |
| Biological sample (H. sapiens) | Healthy donor-derived PBMCs | Precision for Medicine | Cat#33000-10M | |
| Peptide, recombinant protein | iMatrix-511 silk | Matrixome | Cat#892021 | |
| Peptide, recombinant protein | iMatrix-511MG | Matrixome | Cat#892005 | |
| Peptide, recombinant protein | Vitronectin (VTN-N) Recombinant Human Protein, Truncated | Thermo Fisher Scientific | Cat#A14700 | |
| Peptide, recombinant protein | Accutase | Nacalai Tesque | Cat#12679-54 | |
| Peptide, recombinant protein | TrypLE Select | Thermo Fisher Scientific | Cat#A12859-01 | |
| Peptide, recombinant protein | IL-6 | FUJIFILM Wako Pure Chemical Corporation | Cat#091-07511 | (100 ng/mL) |
| Peptide, recombinant protein | IL-3 | FUJIFILM Wako Pure Chemical Corporation | Cat#092-04621 | (10 ng/mL) |
| Peptide, recombinant protein | SCF | FUJIFILM Wako Pure Chemical Corporation | Cat#195-19071 | (300 ng/mL) |
| Peptide, recombinant protein | TPO | FUJIFILM Wako Pure Chemical Corporation | Cat#200-16471 | (300 ng/mL) |
| Peptide, recombinant protein | FLT3 ligand | FUJIFILM Wako Pure Chemical Corporation | Cat#060-07083 | (300 ng/mL) |
| Peptide, recombinant protein | Activin A | FUJIFILM Wako Pure Chemical Corporation | Cat#014-23961 | (10 ng/mL) |
| Commercial assay or kit | StemFit AK02N medium | Ajinomoto | Cat#AK02N | |
| Commercial assay or kit | StemScale PSC suspension medium | Thermo Fisher Scientific | Cat#A4965001 | |
| Commercial assay or kit | mTeSR1 medium | STEMCELL Technologies | Cat#85850 | |
| Commercial assay or kit | StemFitAK03N | Ajinomoto | Cat#AK03N | |
| Commercial assay or kit | StemSpan-AOF | STEMCELL Technologies | Cat#ST100-0130 | |
| Commercial assay or kit | Human iPS cell Generation Episomal vector Mix | Takara Bio | Cat#3673 | |
| Commercial assay or kit | Amaxa Human CD34+ Cell Nucleofector kit | Lonza | Cat#VPA-1003 | |
| Commercial assay or kit | CytoTune EX-iPS | ID Pharma | Cat#69060-61 | |
| Commercial assay or kit | DMEM high Glucose | Nacalai Tesque | Cat#08458-16 | |
| Commercial assay or kit | 0.1% (w/v) Gelatin Solution | FUJIFILM Wako Pure Chemical Corporation | Cat#190-15805 | |
| Commercial assay or kit | fetal bovine serum | Biosera | Cat#515-99055 | |
| Commercial assay or kit | RPMI1640 medium | FUJIFILM Wako Pure Chemical Corporation | Cat#189-02025 | |
| Commercial assay or kit | B-27 supplement, minus insulin | Thermo Fisher Scientific | A1895601 | |
| Commercial assay or kit | Jes/Wes 12- to 230 kDa separation module for Wes, 8×25 capillary cartridges | ProteinSimple | Cat#SM-W004 | |
| Commercial assay or kit | Anti-Rabbit/Goat/Mouse Detection Module kit | ProteinSimple | Cat#DM-002/DM-001/DM/006 | |
| Commercial assay or kit | FastGene RNA premium kit | NIPPON Genetics | Cat#FG-81250 | |
| Commercial assay or kit | ReverTra Ace qPCR RT kit | TOYOBO | Cat#FSQ-101 | |
| Commercial assay or kit | THUNDERBIRD Probe qPCR Mix | TOYOBO | Cat#QPS-101 | |
| Commercial assay or kit | DNeasy Blood & Tissue Kit | QIAGEN | Cat#69504 | |
| Commercial assay or kit | Karyostat Assay arrays | Thermo Fisher Scientific | Cat#905403 | |
| Chemical compound, drug | Y-27632 | FUJIFILM Wako Pure Chemical Corporation | Cat# HY-10071 | |
| Chemical compound, drug | 0.5 M EDTA solution | Nacalai Tesque | Cat#06894-14 | |
| Chemical compound, drug | Annexin V (Alexa Fluor 680) conjugates | Thermo Fisher Scientific | Cat#A35109 | |
| Chemical compound, drug | DAPI solution | FUJIFILM Wako Pure Chemical Corporation | Cat#340-07971 | |
| Chemical compound, drug | 4% paraformaldehyde in phosphate buffer solution | Nacalai Tesque | Cat#09154-85 | |
| Chemical compound, drug | SB431542 | FUJIFILM Wako Pure Chemical Corporation | Cat#198-16543 | (10 µM) |
| Chemical compound, drug | DMH1 | FUJIFILM Wako Pure Chemical Corporation | Cat#041-33881 | (10 µM) |
Additional files
-
Supplementary file 1
The list of chemicals and cytokines used in the screening assay.
- https://cdn.elifesciences.org/articles/89724/elife-89724-supp1-v1.docx
-
Supplementary file 2
The list of TaqMan probes and primer sets used in this study.
- https://cdn.elifesciences.org/articles/89724/elife-89724-supp2-v1.docx
-
Supplementary file 3
The list of primary antibodies used in this study.
- https://cdn.elifesciences.org/articles/89724/elife-89724-supp3-v1.docx
-
Supplementary file 4
The list of secondary antibodies used in this study.
- https://cdn.elifesciences.org/articles/89724/elife-89724-supp4-v1.docx
-
Supplementary file 5
Software used in this study.
- https://cdn.elifesciences.org/articles/89724/elife-89724-supp5-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/89724/elife-89724-mdarchecklist1-v1.pdf





