Patch-walking, a coordinated multi-pipette patch clamp for efficiently finding synaptic connections
Figures
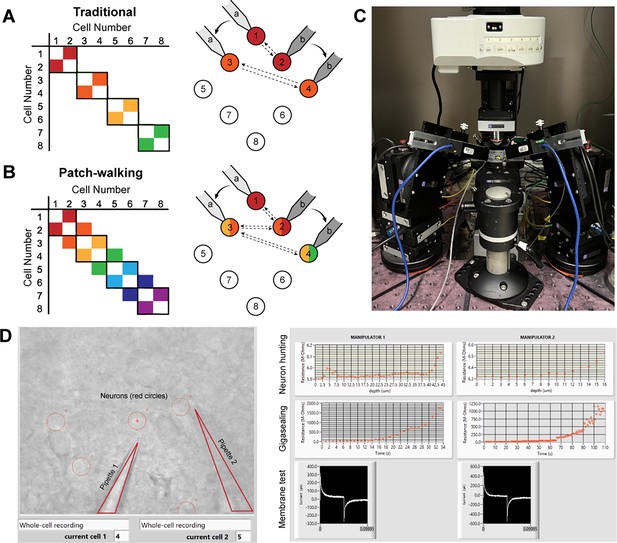
Patch-walk methodology and apparatus.
(A-B) Schematically, for patch clamp apparatus with two pipettes in search of synaptically connected neurons to record; colored squares represent connections that can be probed using the traditional approach (A) as compared to patch-walking (B). In this schematic, n=8 cells were patched by P=2 pipettes, either in groups of two (A), which yields two possible connections, or by walking across the tissue (B), which yields almost double the number of possible connections. (C) A multi-patching apparatus with two pipettes was built with automated pressure control and manipulator movement. (D) The software interface used for patch-walking. On the left is the view of the brain slice under the microscope, with the two pipettes highlighted by triangles and user selected cell locations indicated by red circles. On the right are plots used to monitor each step of the patch clamp process: neuron hunting, gigasealing, and membrane test waveform (to monitor break-in state).
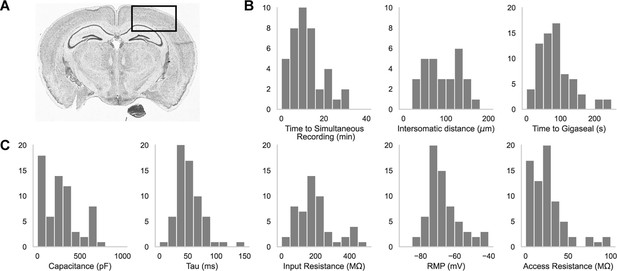
Dual-patching throughput and quality metrics.
(A) An image of a brain slice with a box highlighting the brain region used for experiments: the somatosensory and visual cortices. (B) Histograms of patch clamp metrics: time to achieve simultaneous recording (n=44), distance between neurons during paired recordings (n=44), and amount of time to achieve gigaseal after a neuron is detected by the pipette (n=71), and (C) Membrane capacitance, time constant (tau), input resistance, resting membrane potential, and access resistance of all cells recorded during patch-walking experiments (n=71).
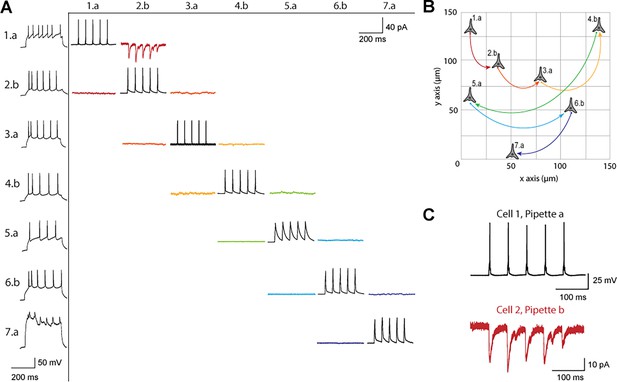
Connectivity matrix and recordings using patch-walking.
(A) Matrix of voltage and current traces from seven neurons in one acute brain slice recorded using the patch-walking algorithm for the robot. Left column shows the firing pattern of the recorded neurons. Cells are numbered such that the number represents the cell and the letter represents the manipulator (a or b). Scale bars: Horizontal 200ms for firing pattern and connection screening. Vertical 40 mV for action potentials, 50 pA for postsynaptic traces. (B) Patch-walking scheme of all neurons from the experiment matrix in (A). The curved lines between neurons represent probed connections in the matrix in (A). (C) The probed connection from the connectivity matrix in (A). The stimulus was sent to cell 1 (black) and the response from cell 2 (red) was recorded and averaged over three sweeps.
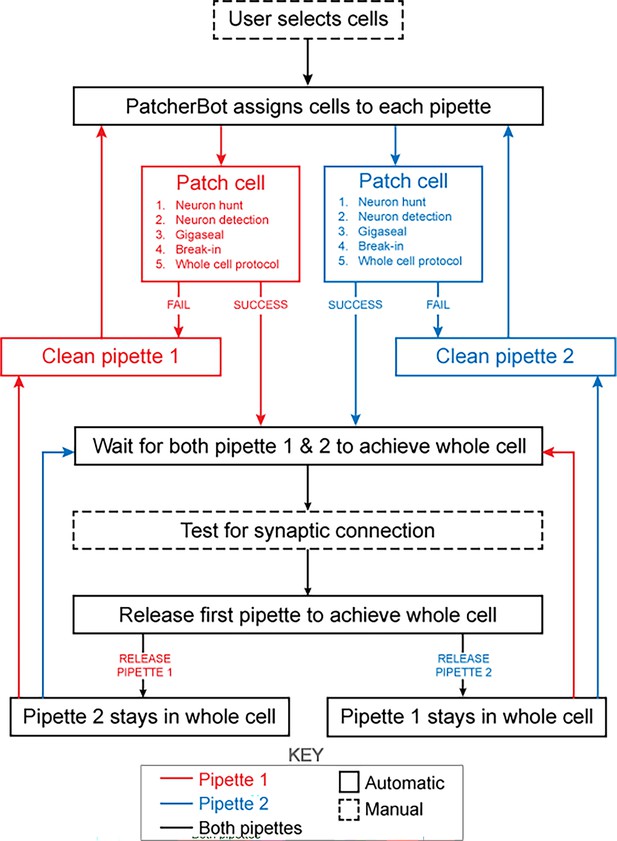
Schematic of the patch-walking experimental workflow.
The patch-walking process begins with selection of cells by the user. The Patcherbot then assigns cells to each pipette based on their distance to the pipettes' home positions. Each pipette works in parallel, only working independently during steps which require the camera and stage (ie neuron hunting, neuron detection). Once a pipette has achieved these steps successfully, the stage and camera are designated to the other pipette. If the pipette failed the patch attempt, it is cleaned and reused. Once both pipettes achieved whole cell configuration, they are tested for synaptic connectivity. In order to ‘'patch-walk,’ the first pipette to achieve whole cell is released to clean and obtain a new whole cell recording.





