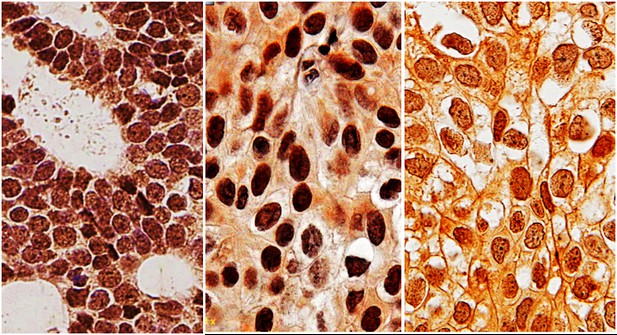
Immunohistochemistry images showing the calcium ion channel TRPV4 (stained in brown) in ductal carcinoma in situ lesions of increasing grade: low (left), intermediate (middle), and high (right). In high-grade ductal carcinoma in situ, TRPV4 localizes to the plasma membrane, indicating increased mechanotransduction capability. Image credit: Bu et al. (CC BY 4.0).
Ductal carcinoma in situ (known as DCIS) is an early form of breast cancer that develops in the milk ducts. It is non-invasive, which means that it does not spread into the surrounding breast tissue. Despite this, if left untreated, DCIS can develop into an invasive cancer that spreads to nearby tissues and has the potential to spread to other parts of the body.
It is difficult to predict which DCIS cases will develop into invasive breast cancers. Although DCIS cells can be graded according to how abnormal their appearance is, with high-grade being the most abnormal, this classification does not directly predict their invasive potential. Therefore, there is a need to develop new ways to better predict which DCIS cases are at risk of progressing to invasive cancers.
Bu et al. aimed to investigate whether cell crowding impacts how likely a DCIS cell is to become invasive. The rapid proliferation of cancer cells in confined spaces means that they often become crowded together, causing mechanical stress that can change their features and behaviour.
Bu et al. probed the effects of cell crowding on different types of breast cancer cells, including healthy breast cells, non-invasive DCIS cells of different grades and invasive breast cancer cells. The experiments revealed that while cell crowding does not drive low-grade DCIS and non-cancerous cells to become invasive, it can promote invasiveness in high-grade DCIS cells. This is due to inhibition of proteins that allow calcium ions to pass into the cells. In particular, this inhibition of a protein known as TRPV4 reduces the number of calcium ions inside the cells, which makes the cells smaller and able to move more efficiently. Cell crowding also caused TRPV4 to move from the centre of the high-grade DCIS cells to the cell membrane.
Taken together, the findings reveal that different types of cancer cells respond differently to mechanical stress and identify cell crowding as a factor that can cause high-grade DCIS cells to become invasive. While further investigation is needed, the findings also suggest the location of TRPV4 as a potential marker of DCIS cells that are more likely to become invasive. In the future, markers of potential invasiveness could be used to ensure that cancer treatments are targeted specifically at patients with a greater risk of developing invasive breast cancer, preventing overtreatment of lower risk cases.




