Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission
Abstract
Plasmodium vivax relapse infections occur following activation of latent liver-stages parasites (hypnozoites) causing new blood-stage infections weeks to months after the initial infection. We develop a within-host mathematical model of liver-stage hypnozoites, and validate it against data from tropical strains of P. vivax. The within-host model is embedded in a P. vivax transmission model to demonstrate the build-up of the hypnozoite reservoir following new infections and its depletion through hypnozoite activation and death. The hypnozoite reservoir is predicted to be over-dispersed with many individuals having few or no hypnozoites, and some having intensely infected livers. Individuals with more hypnozoites are predicted to experience more relapses and contribute more to onwards P. vivax transmission. Incorporating hypnozoite killing drugs such as primaquine into first-line treatment regimens is predicted to cause substantial reductions in P. vivax transmission as individuals with the most hypnozoites are more likely to relapse and be targeted for treatment.
https://doi.org/10.7554/eLife.04692.001eLife digest
Malaria is one of the world's most deadly infections, causing 100s of 1000s of deaths each year despite being both preventable and curable. Malaria is caused by Plasmodium parasites, which are transmitted between humans by mosquitoes. When a mosquito bites a human, Plasmodium is injected into the bloodstream with the mosquito's saliva. The parasite then travels through the bloodstream to the liver, infects liver cells and multiplies within those cells without causing any noticeable symptoms. After remaining silent in the liver for weeks or months, the now abundant parasite ruptures the host liver cell, re-enters the bloodstream, and begins infecting red blood cells. If another mosquito bites the infected individual and takes a blood meal, the parasite moves into the mosquito and the cycle of transmission continues.
There are several species of Plasmodium that are known to cause malaria. The most widely studied species is P. falciparum, which also causes one of the deadliest types of malaria. However, another Plasmodium species called P. vivax is the most widely distributed species and, despite being less virulent than P. falciparum, is particularly dangerous because it causes recurring malaria.
In contrast to P. falciparum, P. vivax has the ability to form hypnozoites: a dormant form of the parasite that can remain inside liver cells for long periods of time, sometimes for years. The reservoir of P. vivax hypnozoites can regularly populate the bloodstream with the infectious form of the parasite, triggering relapses of malaria. Even if an individual suffering a relapse receives prompt treatment to clear parasites in the blood, more parasites may emerge from the liver and cause new blood-stage infections.
White et al. developed a mathematical model to help understand how P. vivax is transmitted. Unlike many of the established models of malaria transmission, the new model accounts for the reservoir of P. vivax hypnozoites in the liver, and assumes that hypnozoites in the reservoir either die, or are activated and enter the bloodstream, at a constant rate. This produces patterns that closely match how often relapses occur in patients. White et al. go on to predict that although many infected people have few or no hypnozoites in their liver, some have many hypnozoites, and these people are more likely to suffer from malaria relapses. This suggests that if the initial treatments given to malaria sufferers incorporate additional drugs that kill the hypnozoites in the liver, then it may be possible to substantially reduce the extent of P. vivax transmission.
https://doi.org/10.7554/eLife.04692.002Introduction
The study of the transmission dynamics of vector-borne diseases such as Plasmodium falciparum malaria has a rich history, with a theoretical foundation based on the Ross-Macdonald models (malERA Consultative Group on Modeling, 2011; Smith et al., 2012; Reiner et al., 2013; Smith et al., 2014), a class of mathematical models describing the transmission of a pathogen between human and vector hosts. In the case of P. falciparum, the parasite has a reservoir in both the human host and the Anopheles mosquito, with transmission occurring when a mosquito takes a blood meal from a human. Ross-Macdonald models have provided insights into the dynamics of P. falciparum transmission resulting in valuable guidance for historical and contemporary malaria control programmes, most notably the large reductions in transmission that are achievable if the lifespan of the mosquito is reduced through vector control (Macdonald, 1952a; Macdonald, 1952b).
In contrast to the extensive theory of the mathematical epidemiology of P. falciparum malaria (Smith et al., 2012), P. vivax malaria has been comparatively neglected. This is in spite of P. vivax being the geographically most widely distributed species of malaria in the world, causing in the region of 80–300 million clinical episodes every year (Mueller et al., 2009a; Gething et al., 2012). P. falciparum models are not applicable to P. vivax as they fail to account for the reservoir of dormant liver stages (hypnozoites) which give rise to relapsing infections—one of the defining characteristics of the biology and epidemiology of P. vivax (Mueller et al., 2009a). In natural transmission settings relapses are often undistinguishable from re-infections from new mosquito bites or recrudescences of existing blood-stage infections. When the origin of renewed parasitemia following primary P. vivax infection is unknown, it can be classified as a recurrent infection (Battle et al., 2014).
Most existing models of malaria transmission do not account for the additional reservoir of parasites in the liver, but the hypnozoite reservoir has been incorporated into some P. vivax transmission models as a state to denote hypnozoite infection (Ishikawa et al., 2003; Chamchod and Beier, 2013; Roy et al., 2013) or as up to two broods of hypnozoites (Dezoysa et al., 1991). Relapse patterns and their implications for transmission have also been investigated using statistical distributions for the time to first relapse (Lover et al., 2014). Here we advance on existing work by considering how the number of hypnozoites in the liver contributes to patterns of relapse infections and the epidemiology and control of P. vivax.
When a P. vivax infected mosquito takes a blood meal from a human, sporozoites are injected into the skin and migrate to the liver, where they invade hepatocytes and develop into either actively dividing schizonts or dormant hypnozoites. The development of actively dividing schizonts may lead to a primary blood-stage infection and potentially clinical malaria (Mueller et al., 2013). Hypnozoites will lie dormant in the liver for weeks to years before activating to initiate new blood-stage infections. The biological mechanisms regulating the activation of hypnozoites remain unknown (Mueller et al., 2009a), although a number of triggers for relapses have been proposed, including fever caused by other pathogens such as P. falciparum (Shanks and White, 2013) and exposure to Anopheles-specific proteins (Hulden and Hulden, 2011).
There is considerable geographical variation in the timing and frequency of P. vivax relapse infections, with strains from tropical areas having an average time to first relapse of 3–6 weeks and long-latency strains from temperate areas relapsing within 6–9 months (Lover and Coker, 2013; Battle et al., 2014). Beyond the first relapse, periodic patterns in multiple relapses from a single mosquito bite have been observed (White, 2011). For example, following a single infection with a tropical strain of P. vivax the time until next relapse has been observed to increase with each successive relapse (Berliner et al., 1948; White, 2011). In contrast, temperate strains are associated with a long latency period until first relapse (of the order of 6 months) followed by short intervals between successive relapses (Coatney et al., 1950; Hankey et al., 1953; White, 2011). A descriptive epidemiology of P. vivax relapses will thus require estimation of three key quantities: (i) the time to first relapse, (ii) the number of relapses per primary infection, and (iii) the duration of hypnozoite carriage.
In this manuscript, a within-host model of hypnozoites in liver hepatocytes is developed to demonstrate that many of the epidemiological patterns of relapse infections can be explained by making the assumption that hypnozoites activate and die at a constant rate. This model is integrated into the existing theory of Ross-Macdonald models to account for the relapse infections characteristic of P. vivax malaria. We use this model to provide qualitative insights into the relative contribution of relapses to P. vivax transmission and illustrate the consequences for controlling P. vivax with vector control and anti-malarial drugs.
Results
Within-host relapse model
Figure 1 shows the best fit within-host relapse model to data on time to first relapse infection from three ecological zones with tropical strains of P. vivax: South America, South East Asia and Melanesia (Battle et al., 2014). In each ecological zone, the number of hypnozoites N and the hypnozoite activation rate α were correlated (see Figure 1—figure supplement 1). For example, a short time to relapse could be explained by a single fast activating hypnozoite or a large number of slow activating hypnozoites. Longitudinal data where multiple relapses are observed in individuals would allow better estimation of the number of hypnozoites in the liver and the duration of hypnozoite carriage.
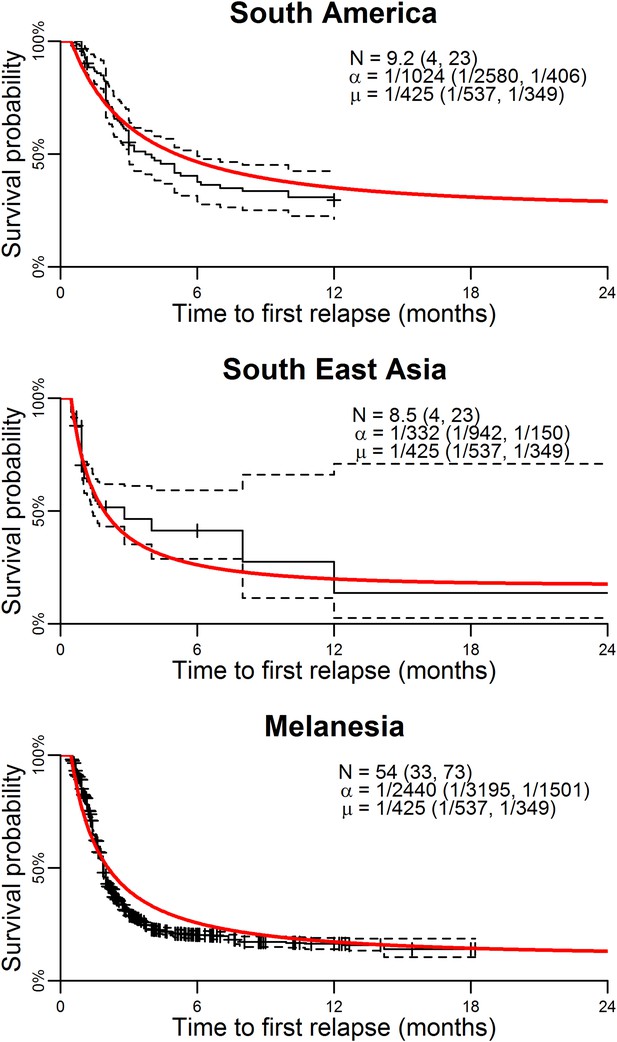
Model parameterisation.
Time to first relapse infection from the within-host model fitted to data from three ecological zones with tropical strains of P. vivax (Battle et al., 2014). The red curves show the model fits with estimated posterior median parameters.
The within-host model can be used to simulate beyond the first relapse infection. Figure 2 shows some sample relapse patterns from the within-host models for tropical and temperate strains of P. vivax. This model predicts notable dose-dependency with increased numbers of hypnozoites associated with a greater number of relapses and shorter time to first relapse. Following the long latency to first relapse in temperate strains, the interval between subsequent relapses is considerably shorter. The within-host model assumes that hypnozoites act independently of each other, and hence the time to next relapsing hypnozoite is exponentially distributed. In particular we do not predict periodicity between relapsing hypnozoites (in the absence of external triggers [White, 2011; Shanks and White, 2013]). If the simulated data are censored such that relapses occurring within 14 days of a previous relapse remain undetected (due to either prophylaxis by blood-stage anti-malarials or the presence of parasites from an existing infection) then there is an apparent periodicity in detected relapses. The observed periodicity of relapses will be determined by the duration of prophylactic protection and not via the biological mechanisms considered here. The periodicity in detected relapses is most evident for large numbers of hypnozoites with the period being determined by the assumed duration of prophylactic protection (Figure 2—figure supplement 1). However, as has been previously argued, periodicity in relapses could also be attributable to a cycle of fevers initiating hypnozoite activation which in turn cause new blood-stage infections and malaria-associated fevers (White, 2011).
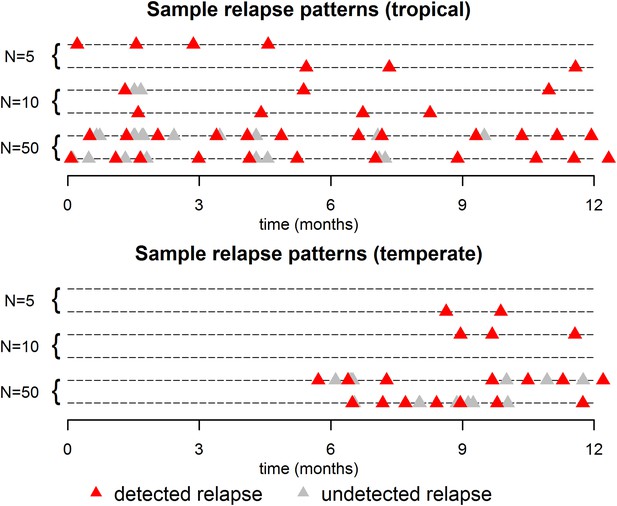
Sample relapse patterns for tropical and temperate strains of P. vivax.
A relapse is assumed to be undetected if it occurs within 14 days of a detected relapse. Both tropical and temperate phenotypes exhibit dose dependency, with a larger number of hypnozoites giving rise to a greater number of relapses and shorter times to first relapse. For larger numbers of hypnozoites (N = 50), periodicity in detected relapses is observed. The appearance of this periodicity is due to the undetected relapses.
Figure 3 shows the predicted number of relapsing hypnozoites in a population exposed to P. vivax in the absence of new infections. For tropical strains the mean number of hypnozoites in the liver is expected to decrease exponentially, but the proportion of individuals carrying hypnozoites is expected to decrease at a slower rate as an individual can relapse even if they have just one hypnozoite (Figure 3A). For temperate strains the mean number of hypnozoites in the liver decreases slowly, as hypnozoites remain in the long-latency phase for approximately 6 months (Figure 3B). The model allows estimation of time to second, third and consecutive relapses in addition to estimates of time to first relapse obtainable via survival analysis of patient data (Lover and Coker, 2013; Battle et al., 2014) (Figure 3C,D). The expected number of relapsing hypnozoites per individual is expected to follow an approximately exponential distribution (Figure 3E,F) in agreement with empirical observations (Horing, 1947; White, 2011).
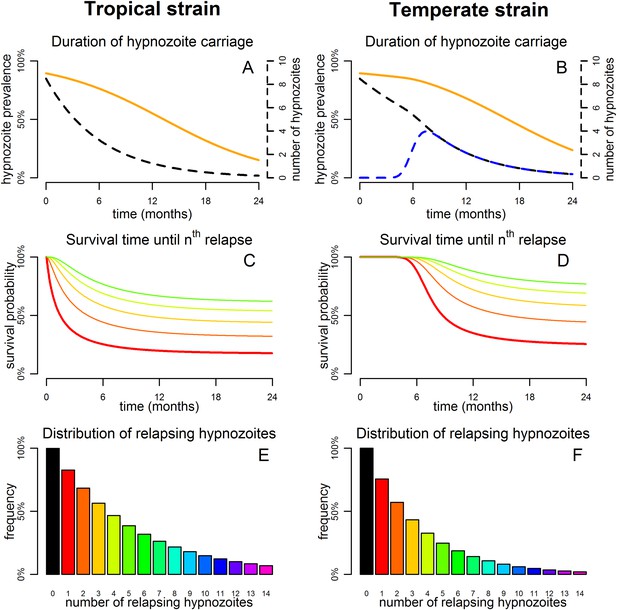
Predicted relapse infections following primary P. vivax infection.
(A and B) Duration of hypnozoite carriage (orange) and expected number of hypnozoites in the liver (dashed). For the temperate strain, the dashed blue line shows the number of hypnozoites in the relapsing phase. (C and D) Survival time until nth relapsing hypnozoite. The red curve is equivalent to the Kaplan–Meier curve for time to first blood-stage infection that would be observed in the absence of new infections from mosquito bites. Only the curves for the first five relapses are shown. (E and F) Proportion of individuals with at least n relapsing hypnozoites following primary infection.
Dynamics and steady states of P. vivax transmission
Figure 4A shows the predicted steady states (the equilibrium blood-stage prevalence in the absence of seasonally varying transmission) as a function of entomological inoculation rate (EIR). EIR is a measurement of the number of infectious bites per person per year. The proportion of people infected with hypnozoites is predicted to be higher than the proportion infected with P. vivax blood-stage parasites. For a given EIR, P. vivax blood-stage prevalence is predicted to be higher than P. falciparum prevalence as a single mosquito bite can give rise to multiple blood-stage infections. However this does not account for the longer duration of P. falciparum infections as a consequence of antigenic switching (Molineaux et al., 2001), and the important role of heterogeneity in exposure (Smith et al., 2005). With the exception of the P. vivax hypnozoite rate, these quantities can be measured in epidemiological field studies (Smith et al., 2005; Kelly-Hope and McKenzie, 2009).
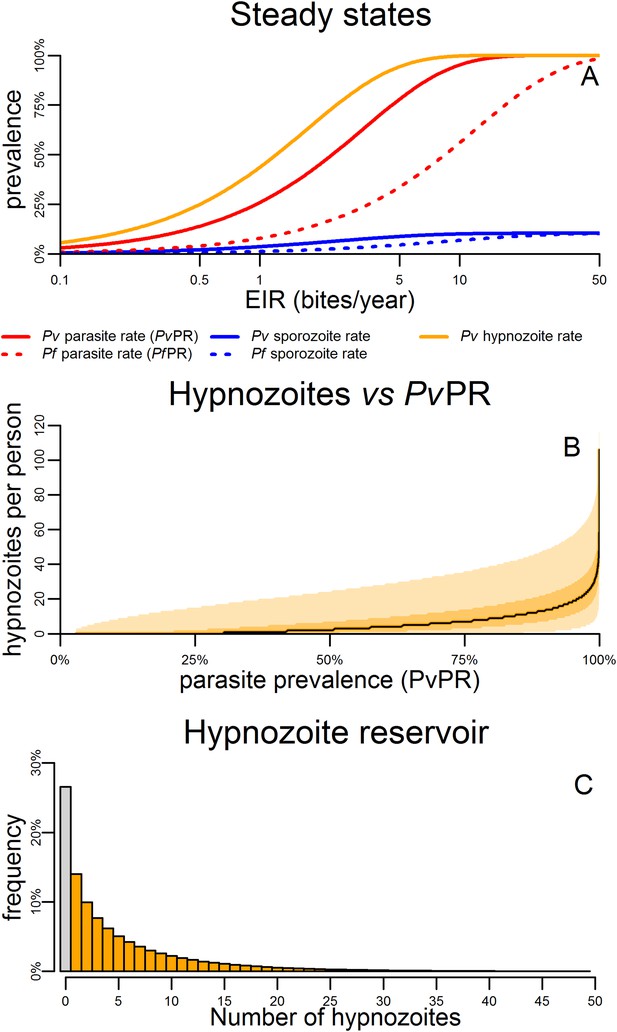
Within-host model for tropical relapses embedded in a P. vivax transmission model.
(A) The statics (estimated equilibrium prevalence) of P. vivax and P. falciparum transmission for different values of the entomological inoculation rate (EIR). EIR was varied by changing the number of mosquitoes per person m. (B) The number of hypnozoites per person is expected to increase with transmission intensity. The black line denotes the median number of hypnozoites, and the shaded areas denote the 50% and 95% ranges. (C) The distribution of the hypnozoite reservoir when PvPR = 50%. The grey bar represents individuals with zero hypnozoites.
Figure 4B shows how the median number of hypnozoites increases with increasing P. vivax transmission. Figure 4C shows the distribution in the number of hypnozoites when PvPR = 50%. The number of hypnozoites per individual is predicted to be over-dispersed following a negative binomial distribution. Thus some individuals will harbour a large number of hypnozoites while some will have none. This phenomenon will be further amplified if there is heterogeneity in exposure where some individuals receive a large number of mosquito bites.
Control of P. vivax
The impact of malaria control interventions will depend on how effectively the parasite is targeted in each of the reservoirs in the mosquito, the blood and the liver. Figure 5 shows the qualitative effects of malaria control on the transmission dynamics of P. falciparum and P. vivax. Vector control with insecticide treated nets (ITNs) or indoor residual spraying (IRS) is assumed to increase mosquito mortality. The introduction of vector control is expected to cause a rapid decline in P. falciparum parasite rate (PfPR), and a smaller and slower decline in P. vivax (Figure 5A).
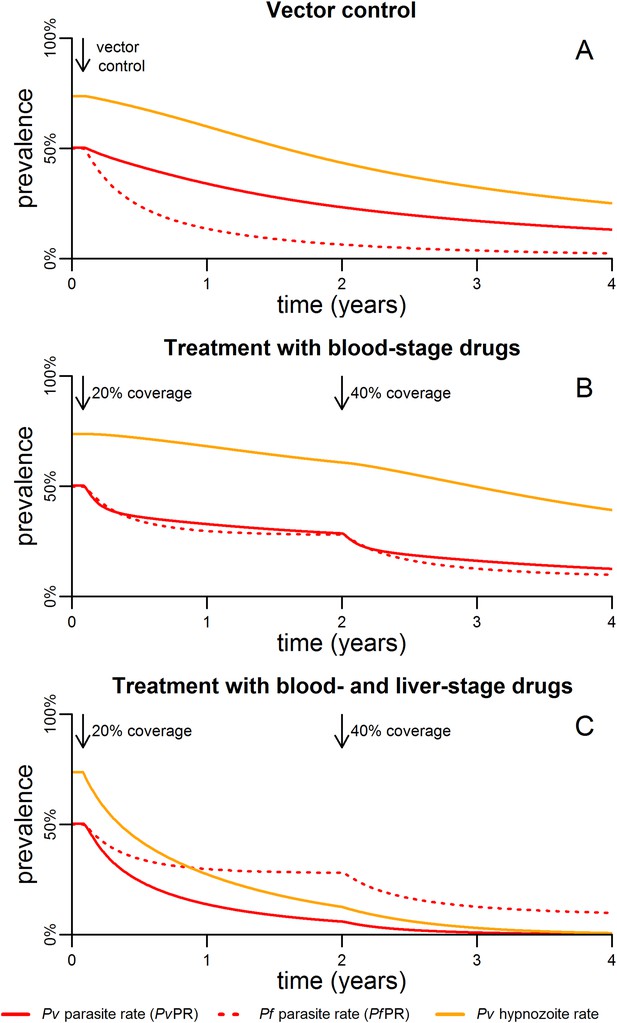
Timelines for malaria control.
(A) The introduction of vector control with ITNs or IRS (assumed to increase mosquito mortality by 30%) is predicted to cause substantial reductions in both PvPR and PfPR. (B) Simulated effect of expanding first-line treatment with blood-stage anti-malarial drugs (e.g., chloroquine or ACTs) so that 20% and 40% of new blood-stage infections are treated. (C) Simulated effect of first-line treatment with a combined regimen of blood-stage anti-malarials and primaquine to remove liver-stage hypnozoites.
Figure 5B shows the effect of targeting the parasite reservoir in the blood by providing first-line treatment for new blood-stage infections with anti-malarial drugs such as chloroquine or artemisinin combination therapies (ACTs). See details of how treatment was implemented in the model are provided in Figure 5—figure supplement 1,2. Increasing treatment coverage leads to reductions in blood-stage prevalence of both P. falciparum and P. vivax. Notably the reduction in the P. vivax hypnozoite rate is slow as the hypnozoite reservoir is not directly targeted.
The hypnozoite reservoir can be directly targeted using a drug such as primaquine that can eliminate hypnozoites from the liver (Wells et al., 2010). The inclusion of primaquine in first-line treatment regimens is predicted to cause substantial reductions in both the P. vivax parasite rate and hypnozoite rate (Figure 5C), as individuals being treated for blood-stage P. vivax infections will also have their hypnozoites removed. A consequence of this strategy is that the hypnozoite reservoir can be targeted efficiently, as individuals with the most hypnozoites are most likely to relapse and potentially be detected by health systems. Figure 6 shows how the inclusion of primaquine in first-line treatment regimens preferentially targets the most intense infections, with the greatest reductions observed in individuals with the most hypnozoites.
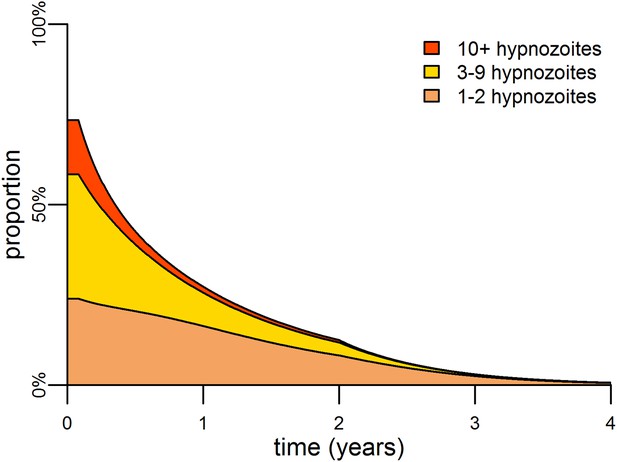
Targeting the hypnozoite reservoir.
Proportion of the population infected with 1–2, 3–9 or 10+ hypnozoites following the introduction of a first-line treatment regimen with blood-stage anti-malarial drugs and primaquine. Individuals with large numbers of hypnozoites are more likely to experience new blood-stage infections and hence become targeted for treatment and have their hypnozoites removed. This results in a selective targeting of the most intensely infected individuals.
Discussion
Relapse infections arising from the activation of hypnozoites in the human liver have important consequences for the transmission dynamics of P. vivax. Hypnozoites in the liver constitute a third malaria parasite reservoir, in addition to the reservoirs in the blood circulation and mosquito also present for P. falciparum. Relapses can be incorporated into Ross-Macdonald models of malaria transmission through the addition of a state to represent the hypnozoite reservoir (Roy et al., 2013), or as demonstrated here, through consideration of the number of hypnozoites in the liver. This allows the intensity of hypnozoite infection to be estimated which is crucial for understanding patterns of relapse infections (White, 2011) and evaluating the effect of interventions such as primaquine treatment that directly target the hypnozoite reservoir. Hypnozoites are assumed to be subjected to two processes: activation at constant rate α, and death at constant rate µ. By considering infection with batches of hypnozoites, these simple processes can explain many of the complex patterns observed in P. vivax relapses (see Box 1).
Properties of predicted P. vivax relapse patterns
Nicholas White (White, 2011) has argued that a theory seeking to explain the remarkable biology of P. vivax relapses must accommodate eight phenomena. The within-host relapse model proposed here accounts for six of these, and can be extended to accommodate the other two.
Relapses show remarkable periodicity. The assumption of constant hypnozoite activation and death does not allow for periodic relapses. However if we assume some relapses are undetected due to prophylaxis following treatment or the presence of existing blood-stage parasites, an apparent periodicity in detected relapses is predicted (Figure 2).
For tropical strains of P. vivax the highest incidence of relapses is predicted to be immediately after primary infection (Figure 1), thus the first relapses will be detected after the prophylactic effects of treatment have waned.
Not all P. vivax infections are followed by relapses. Individuals will avoid relapses if the number of initial hypnozoites is zero, or if hypnozoites die before relapsing (Figure 3D).
Multiple relapses are common. The within-host model predicts an approximately exponential distribution in the numbers of relapsing hypnozoites per person (Figure 3E,F). Dose-dependency in the number of relapses is predicted, with greater numbers of hypnozoites giving rise to greater numbers of relapses.
The time to first relapse for temperate strains is predicted to follow a gamma distribution (approximately Normal).
After the first relapse of a temperate strain, the intervals between consecutive relapses are predicted to be similar to those observed in tropical strains (Figure 2).
The model described here does not account for the varying genotypes arising from single or multiple mosquito bites, although it can be extended to account for the diversity of genotypes in the hypnozoite reservoir (Koepfli et al., 2011, 2013). The model represents a baseline scenario with constant hypnozoite activation and death in the absence of external triggers. The role of triggers such as fever can be tested for in detail with data on the timing and peak temperatures of episodes of febrile illness.
https://doi.org/10.7554/eLife.04692.013A key assumption in the proposed model is the constant activation of hypnozoites. This implies that relapses can occur immediately after primary P. vivax infection, in contrast to suggestions that the first relapse does not occur until 2–3 weeks later. The best evidence on early relapses comes from treatment efficacy studies where patients treated for P. vivax are followed for recurrent infection for 42 days (Douglas et al., 2010). Except in cases with documented chloroquine resistance (Price et al., 2014), recurrent infections are rarely observed prior to day 14, however the inclusion of long-lasting anti-malarials in treatment regimens provides prophylaxis during this period making detection of parasites unlikely. In a recent study of the slowly eliminated drug dihydroartemisinin-piperaquine (DP) Tarning et al. (2014) tested a model where relapses occur in bursts every 3 weeks, but it arguably provided no better fit to the data than a model of constant hypnozoite activation. Testing the hypothesis of constant activation would require follow up of patients treated with rapidly eliminated artemisinin monotherapy, a challenging proposition given the concerns over artemisinin resistant P. falciparum (Ashley et al., 2014).
Although the model captures the key drivers of the dynamics of P. vivax transmission, it is a simplified representation subject to a number of limiting assumptions. The potential role of triggers of hypnozoite activation such as febrile illness (Shanks and White, 2013) are not accounted for. There is no heterogeneity or seasonality in transmission, and no age structure. Incorporation of the acquisition of natural immunity into the model will be particularly important for settings with high transmission intensity where immunity has a role in regulating blood-stage infections (Mueller et al., 2013). It is assumed that all individuals infected with blood-stage parasites are capable of transmitting to mosquitoes. Similar to the corresponding P. falciparum models, incorporation of these factors would change the quantitative predictions of the model, but not its qualitative behaviour.
P. falciparum and P. vivax parasite prevalence (PfPR and PvPR) are the most widely reported and best validated metrics of malaria transmission from epidemiological studies (Gething et al., 2011a; Gething et al., 2012) providing measurements of the proportion of individuals with detectable blood-stage parasites. In settings with similar levels of P. vivax and P. falciparum transmission, the model predicts PvPR to be greater than PfPR due to the additional blood-stage infections arising from relapses. However, this does not agree with empirical observations which find PfPR to be similar to or greater than PvPR (Snounou and White, 2004; Mueller et al., 2009b). This is most likely explained by the rapid acquisition of immunity to P. vivax (Koepfli et al., 2013; Mueller et al., 2013) the low detectability of P. vivax blood-stage infections (Harris et al., 2010), and the longer durations of P. falciparum blood-stage infection (Molineaux et al., 2001) not captured in the model. Furthermore, the additional P. vivax parasite reservoir in the liver means that PfPR and PvPR are not directly comparable metrics. Thus if a parasitological survey indicates similar parasite prevalence, a greater control effort will be required to reduce P. vivax transmission than to reduce P. falciparum transmission because of the additional infections emerging from the hypnozoite reservoir. The model described here allows the proportion of individuals harbouring hypnozoites to be estimated given metrics such as PvPR. The number of hypnozoites per person is predicted to be over-dispersed with some individuals with intensely infected livers and most carrying few or no hypnozoites (Figure 4C). Estimates of the prevalence and intensity of hypnozoite infection will be dependent on both the uncertainty in the measurable data and the model assumptions.
Vector control with ITNs or IRS, and treatment with effective anti-malarial drugs are the cornerstones of malaria control efforts targeting the parasite in the vector and the human host, however they are predicted to have different effects on P. vivax and P. falciparum transmission. Vector control interventions that increase mosquito mortality are expected to cause greater reductions in PfPR than PvPR (Figure 5A), as higher levels of P. vivax transmission can be maintained with fewer mosquito bites. This has been observed in both Thailand (Sattabongkot et al., 2004) and Brazil (Coura et al., 2006) where increased vector control has caused greater reductions in P. falciparum than P. vivax.
First-line treatment of new blood-stage infections with anti-malarial drugs such as chloroquine or ACTs is predicted to cause moderate reductions in blood-stage prevalence of both P. falciparum and P. vivax (Figure 5B). The addition of primaquine to first-line treatment regimens is expected to cause large reductions in P. vivax blood-stage prevalence, as individuals with the most intense hypnozoite infections are more likely to relapse and be targeted for treatment and hence have their hypnozoites eliminated. The potential to simultaneously target parasite reservoirs in the blood and liver may turn the cause of P. vivax parasites' robust transmission into its Achilles' heel.
In P. vivax and P. falciparum co-endemic regions, heterogeneity in exposure to mosquito bites may cause associations between P. falciparum fevers and the risk of future P. vivax relapses (Douglas et al., 2011). Thus the inclusion of primaquine in first-line treatment for P. falciparum may also reduce P. vivax transmission. In addition to inclusion in first-line treatment regimens, primaquine can also be administered as part of mass drug administration (MDA) programmes. In treatment-reinfection studies of Papua New Guinean children (Betuela et al., 2012; Robinson et al., unpublished), mass administration of drugs such as chloroquine or artemether-lumefantrine successfully cleared P. vivax blood-stage infections but rapid recurrence of infection was observed during follow-up—most likely due to relapses. The addition of primaquine to the treatment regimen caused large reductions in the rate of recurrent infections.
Although primaquine treatment clears the hypnozoite reservoir, it requires a difficult 14 day treatment regimen, and is not without risk due to vulnerability to haemolytic toxicity among glucose-6-phosphate dehydrogenase (G6PD) deficient patients (Howes et al., 2013). Individuals should thus be tested for G6PD deficiency (Kim et al., 2011) before the administration of primaquine. A primaquine analogue, tafenoquine, is currently undergoing phase three trials and is likely to be licensed for use by 2017 (Llanos-Cuentas et al., 2014). Tafenoquine requires a single dose alongside a 3 day chloroquine regimen, but is subject to the same risks in G6PD deficient patients. A quantitative model of P. vivax transmission will allow for the benefits of primaquine treatment to be weighed against the risks of G6PD deficiency and the costs of G6PD testing.
Mathematical models of malaria transmission that account for P. vivax relapses can provide valuable insights into the impact of malaria control interventions on the parasite's reservoirs in the vector, the blood and the liver. In the absence of effective diagnostics for detecting liver-stage parasites (malERA Consultative Group on Diagnoses and Diagnostics, 2011), models will play a crucial role in estimating and predicting the effectiveness of interventions that target the hypnozoite reservoir, either indirectly via vector control and blood-stage anti-malarials or directly via primaquine treatment.
Materials and methods
Within-host model for tropical relapses
Request a detailed protocolFollowing infection with a tropical strain of P. vivax, the population dynamics of hypnozoites in the liver can be described by a within-host model where each hypnozoite is subject to two processes: (i) activation leading to relapse infection; and (ii) death, either of the hypnozoite itself or the host hepatocyte (Malato et al., 2011). Constant activation (α) and death (µ) rates are assumed implying hypnozoite residence time in the liver is exponentially distributed. The long latency of temperate strains before first relapse can be accounted for by assuming a period of dormancy during which hypnozoites must wait before they can activate. A schematic representation of the within-host model is presented in Figure 7.
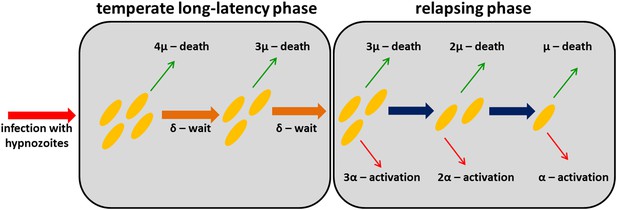
Within-host model schematic of relapsing hypnozoites in the liver.
Hypnozoites from tropical strains of P. vivax will progress to the relapsing phase where they are subject to two processes: death and activation leading to relapse. Hypnozoites from temperate strains will begin in a temperate long-latency phase where they must wait before progressing to the relapsing phase.
The tropical relapse model is assumed to begin with an initial population of N hypnozoites, each of which can either activate at rate α, or die at rate µ, independently of each other. The number of hypnozoites in the liver will decay exponentially with an expected Ne−(µ+α)t hypnozoites at time t. Let denote the probability that i of N hypnozoites remain in the liver after time t. The hypnozoite population dynamics can be described by the following set of equations:
Equation 1 can be solved analytically to give:
Define to be the probability that j relapses have occurred by time t. This can be calculated as follows: if i hypnozoites remain in the liver, then N − i have either activated or died. The probability of each hypnozoite activating is . The probability that j of N − i hypnozoites have activated can thus be calculated from a binomial distribution. Summing over the allowable number of hypnozoites (at least j hypnozoites must have activated or died for j relapses to be observed) gives:
Equations 1–3 describe the population dynamics of hypnozoites in a single individual with N hypnozoites in the absence of exposure to new infections. In a population of individuals, we would expect substantial variation in the numbers of hypnozoites due to heterogeneity in exposure and the variation in sporozoite inoculum from each infectious mosquito bite (Beier et al., 1991; Medica and Sinnis, 2005; White et al., 2013). Based on evidence that the number of sporozoites injected with a mosquito bite approximately follows a geometric distribution (Beier et al., 1991), we assume that the number of hypnozoites following a primary infection is also geometrically distributed. If the mean number of hypnozoites is N, then the probability of k hypnozoites is . Assuming a geometrically distributed number of hypnozoites, the three quantities describing the epidemiology of relapses can be estimated in terms of the within-host parameters. The expected number of relapsing hypnozoites is:
The mean duration of hypnozoite carriage is:
The expected time to first relapse is:
The within-host relapse model describes a baseline scenario in the absence of potential external triggers for relapse such as fever (Shanks and White, 2013). Underlying assumptions of this model are: (i) each hypnozoite acts independently of other hypnozoites, for example, hypnozoites will not activate in batches due to mechanisms such as quorum sensing; and, (ii) hypnozoite death occurs at a constant rate, due to either death of the hypnozoite within the hepatocyte or death of the hepatocyte itself (Malato et al., 2011). The activation of a hypnozoite may not directly correspond to a detected relapse. For example, an infection arising from two hypnozoites activating within a day of each other is likely to be classified as a single relapse.
Within-host model for temperate relapses
Request a detailed protocolThe within-host model can be extended to account for temperate strains of P. vivax. We assume that before a hypnozoite is capable of activating, it must undergo a long-latency phase of duration d. During this period hypnozoites are subject to death at rate µ. In particular, we assume that the time spent in the temperate long-latency phase can be described by a gamma distribution with mean d and variance d2/M. This gamma distribution can be simulated by M successive compartments with exponential waiting times 1/δ = d/M. Increasing the number of compartments M reduces the variance in the duration of the dormancy period (Wearing et al., 2005). Following a primary infection where N hypnozoites of a temperate phenotype develop in the liver, we define as the probability that i of N hypnozoites are waiting in long-latency compartment number j, then the number of dormant and potentially active hypnozoites can be described by the following system of differential equations.
The equations are presented schematically in Figure 7—figure supplement 1. Equation 7 cannot be solved analytically and must be computed numerically to calculate and . A greater deal of uncertainty surrounds the biological processes accounting for the initial long-latency phase observed in temperate strains of P. vivax. In the model implemented here, it is assumed that all hypnozoites in an infection must undergo some waiting period before any of them can activate, and that during the long-latency phase hypnozoites are at risk of death due to natural hepatocyte death.
P. vivax transmission model
Request a detailed protocolWe next embedded the within-host model for tropical relapses in a model for the transmission of P. vivax between humans and mosquitoes (Figure 8). The transmission dynamics are driven by two processes: (i) transmission of parasites through mosquito bites; and (ii) relapsing of liver-stage hypnozoites to cause new blood-stage infections. As per the standard Ross-Macdonald theory, the force of blood-stage infections in humans can be calculated as the product of the number of mosquitoes per human m, the rate at which each mosquito bites a human host a, the probability of transmission from mosquito to human following an infectious bite b, and the proportion of mosquitoes that are infectious IM, to give λ = mabIM. Parameter values are provided in Table 1. The force of infection on mosquitoes can be calculated in a similar manner. We assume that people can be susceptible (Si) or infected with blood-stage parasites (Ii), where i denotes the number of hypnozoites in the liver.
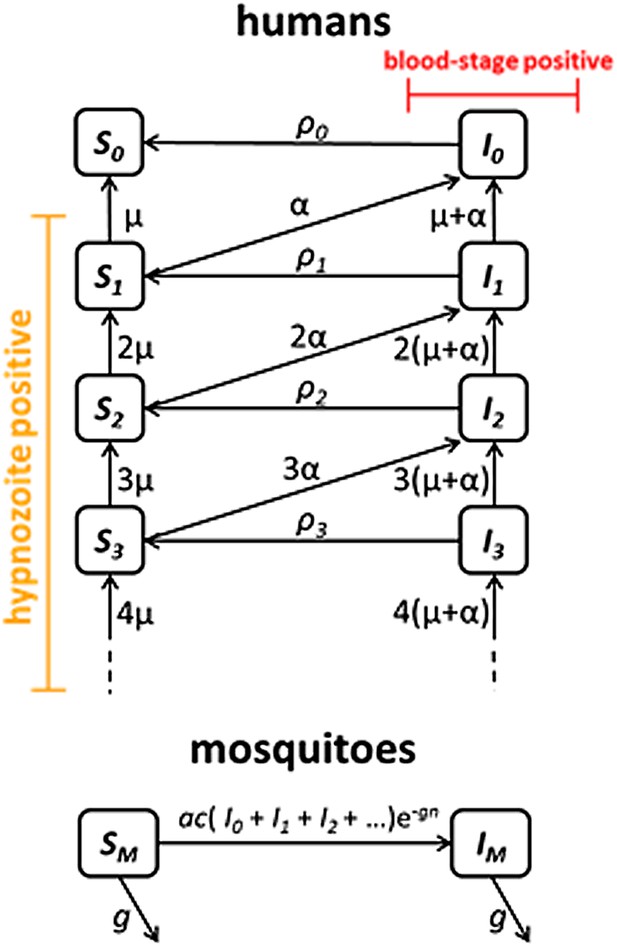
Transmission model schematic.
Within-host model for tropical relapses embedded in a transmission model. Si denotes the proportion of humans susceptible to blood-stage infection with i hypnozoites. Ii denotes the proportion of humans with blood-stage infections carrying i hypnozoites. Individuals in all compartments are exposed to primary infections at rate λ, following which they will move down the flow diagram to a compartment representing blood-stage infection and carrying a greater number of hypnozoites.
Description of model parameters
| Parameter | Description | Value | Reference |
|---|---|---|---|
| Within-host | |||
| N | number of hypnozoites per infection | 8.5 | estimate* |
| α | rate of hypnozoite activation | 1/332 day−1 | estimate* |
| µ | rate of hypnozoite/hepatocyte death | 1/425 day−1 | estimate* |
| d | duration of temperate long-latency | 180 days | (Battle et al., 2014) |
| σd | standard deviation of temperate long-latency | 30 days | (Battle et al., 2014) |
| M | number of compartments for simulating long-latency: M = (d/σd)2 | 36 | |
| δ | rate of progression through long-latency compartments: δ = M/d | 0.2 day−1 | |
| Humans | |||
| b | transmission probability: mosquito to human | 0.5 | (Smith et al., 2010) |
| r | rate of clearance of blood-stage infections | 1/60 day−1 | (Collins et al., 2003) |
| f | relapse frequency (1/time to first relapse) | 1/76 day−1 | Equation 6 |
| h | expected number of relapses | 4.7 | Equation 4 |
| γ | rate of hypnozoite clearance | 1/420 day−1 | Equation 5 |
| Mosquitoes | |||
| a | mosquito biting frequency | 0.21 day−1 | (Garrett-Jones, 1964) |
| g | mosquito death rate (1/mosquito life expectancy) | 0.1 day−1 | (Gething et al., 2011b) |
| m | number of mosquitoes per human | calculated | |
| n | duration of sporogony in mosquito | 12 days | (Gething et al., 2011b) |
| c | transmission probability: human to mosquito | 0.23 | (Bharti et al., 2006) |
-
*
Based on estimates from South East Asian tropical strains.
The increase in hypnozoites in the liver is determined by the force of infection λ and the number of hypnozoites per infection N, and the decrease is due to hypnozoite activation α and death µ. The model depicted in Figure 8 can be described by the following set of equations:
where . In the absence of super-infection, the recovery from blood-stage infection is . Accounting for super-infections (Dietz and Molineaux, 1973; Smith et al., 2012) gives .
Model parameterisation
Request a detailed protocolThe within-host model for tropical relapses was fitted in a Bayesian framework to data on time to first relapse infection from three ecological zones with tropical strains of P. vivax: South America, South East Asia and Melanesia (see Source data 1). The data are described in detail by Battle et al. (2014). Individual-level data on time to first recurrence was collated from individuals exposed to P. vivax infection (either via natural exposure or artificial challenge) and mostly followed up in the absence of exposure to new infections (Battle et al., 2014). The likelihood of the tropical relapse model can be evaluated by applying the model to the data on time to first relapse infection. The first detected relapse will occur after clearance of parasites from the primary infection and after the period of prophylactic protection from anti-malarial drugs. Define QN(t) to be the probability that at least 1 of N hypnozoites has relapsed by time t.
Accounting for a geometrically distributed number of hypnozoites gives:
where G(N) denotes a geometric distribution.
An individual j followed up after a primary P. vivax infection will either relapse (Ij = 1) or avoid infection (Ij = 0). Denote τj to be the time of detection of infection, or if uninfected, the time until the end of follow up. The likelihood of the parameters θ = {N, α, µ} given the data Dj = {Ij, τj} is:
The log-likelihood (LL) for all j individuals is:
Data on time to first relapse were not sufficiently informative to estimate the three parameters simultaneously and hence prior distributions were assumed. N was assumed to have an informative gamma prior distribution with median 10 (95% credible interval (CrI): 1, 28) (Beier et al., 1991). µ was assumed to have an informative gamma prior distribution with median 1/200 (95% CrI: 1/309, 1/140) day−1 (Malato et al., 2011). α was assumed to have an uninformative uniform prior distribution U(0,1). The likelihood in equation (Ishikawa et al., 2003) was sampled using a Metropolis–Hastings Markov Chain Monte Carlo (MCMC) algorithm and the posterior parameter distributions estimated (Figure 1—figure supplement 1). The posterior median parameter estimates and 95% credible intervals are presented in Supplementary file 1.
References
-
Spread of artemisinin resistance in Plasmodium falciparum nalariaThe New England Journal of Medicine 371:411–423.https://doi.org/10.1056/NEJMoa1314981
-
Geographical variation in Plasmodium vivax relapseMalaria Journal 13:144.https://doi.org/10.1186/1475-2875-13-144
-
Quantitation of Plasmodium-falciparum sporozoites transmitted invitro by experimentally infected Anopheles-gambiae and Anopheles-stephensiThe American Journal of Tropical Medicine and Hygiene 44:564–570.
-
Studies on the chemotherapy of the human malarias. vii. the antimalarial activity of pamaquineThe Journal of Clinical Investigation 27:108–113.https://doi.org/10.1172/JCI101947
-
Relapses contribute significantly to the risk of P. vivax infection and disease in Papua New Guinean children 1-5 years of ageThe Journal of Infectious Diseases 206:1771–1780.https://doi.org/10.1093/infdis/jis580
-
Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian AmazonThe American Journal of Tropical Medicine and Hygiene 75:610–616.
-
Modeling Plasmodium vivax: relapses, treatment, seasonality, and G6PD deficiencyJournal of Theoretical Biology 7:25–34.https://doi.org/10.1016/j.jtbi.2012.08.024
-
Studies in human malaria. XXX. A summary of 204 sporozoite-induced infections with the Chesson strain of Plasmodium vivaxJournal of the National Malaria Society 9:381–396.
-
A retrospective examination of anemia during infection of humans with Plasmodium vivaxThe American Journal of Tropical Medicine and Hygiene 68:410–412.
-
A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection - a reviewMemorias Do Instituto Oswaldo Cruz 101:229–237.
-
A mathematical model for Plasmodium vivax malaria transmission - estimation of the impact of transmission-blocking immunity in an endemic areaBulletin of the World Health Organization 69:725–734.
-
A malaria model tested in the African savannahNinth International Congress on Tropical Medicine and Malaria Athens Volume 1 Abstracts of Invited Papers 297.
-
Artemisinin combination therapy for vivax malariaThe Lancet Infectious Diseases 10:405–416.https://doi.org/10.1016/S1473-3099(10)70079-7
-
Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kineticsClinical Infectious Diseases 52:612–620.https://doi.org/10.1093/cid/ciq249
-
The human blood index of malaria vectors in relation to epidemiological assessmentBulletin of the World Health Organization 30:241–261.
-
A long neglected world malaria map: Plasmodium vivax endemicity in 2010PLOS Neglected Tropical Diseases 6:e1814.https://doi.org/10.1371/journal.pntd.0001814
-
Korean vivax malaria. I. Natural history and response to chloroquineThe American Journal of Tropical Medicine and Hygiene 2:958–969.
-
G6PD deficiency: global distribution, genetic variants and primaquine therapy133–201, Advances in Parasitology, 81, In: Hay SI, Price R, Baird JK, editors. Epidemiology of Plasmodium vivax: history, hiatus and hubris, Pt B, 10.1016/B978-0-12-407826-0.00004-7.
-
A mathematical model for the transmission of Plasmodium vivax malariaParasitology International 52:81–93.https://doi.org/10.1016/S1383-5769(02)00084-3
-
Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New GuineaPLOS Neglected Tropical Diseases 5:e1424.https://doi.org/10.1371/journal.pntd.0001424
-
Quantifying effect of geographic location on epidemiology of Plasmodium vivax malariaEmerging Infectious Diseases 19:1058–1065.https://doi.org/10.3201/eid1907.121674
-
Fate tracing of mature hepatocytes in mouse liver homeostasis and regenerationThe Journal of Clinical Investigation 121:4850–4860.https://doi.org/10.1172/JCI59261
-
A research agenda for malaria eradication: modelingPLOS Medicine 8:e1000403.https://doi.org/10.1371/journal.pmed.1000403
-
Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasiteThe Lancet Infectious Diseases 9:555–566.https://doi.org/10.1016/S1473-3099(09)70177-X
-
Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysisThe Lancet Infectious Diseases 14:982–991.https://doi.org/10.1016/S1473-3099
-
A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970-2010Journal of the Royal Society, Interface 10:20120921.https://doi.org/10.1098/rsif.2012.0921
-
Plasmodium vivax transmission: chances for control?Trends in Parasitology 20:192–198.https://doi.org/10.1016/j.pt.2004.02.001
-
The activation of vivax malaria hypnozoites by infectious diseasesThe Lancet Infectious Diseases 13:900–906.https://doi.org/10.1016/S1473-3099(13)70095-1
-
Recasting the theory of mosquito-borne pathogen transmission dynamics and controlTransactions of the Royal Society of Tropical Medicine and Hygiene 108:185–197.https://doi.org/10.1093/trstmh/tru026
-
The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in ThailandTrends in Parasitology 20:333–339.https://doi.org/10.1016/j.pt.2004.05.004
-
Population pharmacokinetics and antimalarial pharmacodynamics of piperaquine in patients with Plasmodium vivax malaria in ThailandCPT: Pharmacometrics & Systems Pharmacology 3:e132.https://doi.org/10.1038/psp.2014.29
Article and author information
Author details
Funding
Medical Research Council (Population Health Scientist Fellowship)
- Michael T White
National Health and Medical Research Council (Senior Research Fellowship)
- Ivo Mueller
National Health and Medical Research Council (Research Fellowship)
- Stephan Karl
Wellcome Trust (Senior Research Fellowship)
- Katherine E Battle
- Simon I Hay
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
Bob Verity is thanked for contributions to the mathematical methods. This work was supported by a fellowship to MTW from the MRC. SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#095066), which also supports KEB. ACG acknowledges support from the Bill and Melinda Gates Foundation and MRC Centre funding. IM is supported by an NHMRC Senior Research Fellowship (#1043345). SK was supported by an NHMRC Early Career Fellowship (#1052760). IM and SK acknowledge support from the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Copyright
© 2014, White et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,503
- views
-
- 593
- downloads
-
- 86
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
Background:
Biological aging exhibits heterogeneity across multi-organ systems. However, it remains unclear how is lifestyle associated with overall and organ-specific aging and which factors contribute most in Southwest China.
Methods:
This study involved 8396 participants who completed two surveys from the China Multi-Ethnic Cohort (CMEC) study. The healthy lifestyle index (HLI) was developed using five lifestyle factors: smoking, alcohol, diet, exercise, and sleep. The comprehensive and organ-specific biological ages (BAs) were calculated using the Klemera–Doubal method based on longitudinal clinical laboratory measurements, and validation were conducted to select BA reflecting related diseases. Fixed effects model was used to examine the associations between HLI or its components and the acceleration of validated BAs. We further evaluated the relative contribution of lifestyle components to comprehension and organ systems BAs using quantile G-computation.
Results:
About two-thirds of participants changed HLI scores between surveys. After validation, three organ-specific BAs (the cardiopulmonary, metabolic, and liver BAs) were identified as reflective of specific diseases and included in further analyses with the comprehensive BA. The health alterations in HLI showed a protective association with the acceleration of all BAs, with a mean shift of –0.19 (95% CI −0.34, –0.03) in the comprehensive BA acceleration. Diet and smoking were the major contributors to overall negative associations of five lifestyle factors, with the comprehensive BA and metabolic BA accounting for 24% and 55% respectively.
Conclusions:
Healthy lifestyle changes were inversely related to comprehensive and organ-specific biological aging in Southwest China, with diet and smoking contributing most to comprehensive and metabolic BA separately. Our findings highlight the potential of lifestyle interventions to decelerate aging and identify intervention targets to limit organ-specific aging in less-developed regions.
Funding:
This work was primarily supported by the National Natural Science Foundation of China (Grant No. 82273740) and Sichuan Science and Technology Program (Natural Science Foundation of Sichuan Province, Grant No. 2024NSFSC0552). The CMEC study was funded by the National Key Research and Development Program of China (Grant No. 2017YFC0907305, 2017YFC0907300). The sponsors had no role in the design, analysis, interpretation, or writing of this article.
-
- Epidemiology and Global Health
- Microbiology and Infectious Disease
Background:
In many settings, a large fraction of the population has both been vaccinated against and infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hence, quantifying the protection provided by post-infection vaccination has become critical for policy. We aimed to estimate the protective effect against SARS-CoV-2 reinfection of an additional vaccine dose after an initial Omicron variant infection.
Methods:
We report a retrospective, population-based cohort study performed in Shanghai, China, using electronic databases with information on SARS-CoV-2 infections and vaccination history. We compared reinfection incidence by post-infection vaccination status in individuals initially infected during the April–May 2022 Omicron variant surge in Shanghai and who had been vaccinated before that period. Cox models were fit to estimate adjusted hazard ratios (aHRs).
Results:
275,896 individuals were diagnosed with real-time polymerase chain reaction-confirmed SARS-CoV-2 infection in April–May 2022; 199,312/275,896 were included in analyses on the effect of a post-infection vaccine dose. Post-infection vaccination provided protection against reinfection (aHR 0.82; 95% confidence interval 0.79–0.85). For patients who had received one, two, or three vaccine doses before their first infection, hazard ratios for the post-infection vaccination effect were 0.84 (0.76–0.93), 0.87 (0.83–0.90), and 0.96 (0.74–1.23), respectively. Post-infection vaccination within 30 and 90 days before the second Omicron wave provided different degrees of protection (in aHR): 0.51 (0.44–0.58) and 0.67 (0.61–0.74), respectively. Moreover, for all vaccine types, but to different extents, a post-infection dose given to individuals who were fully vaccinated before first infection was protective.
Conclusions:
In previously vaccinated and infected individuals, an additional vaccine dose provided protection against Omicron variant reinfection. These observations will inform future policy decisions on COVID-19 vaccination in China and other countries.
Funding:
This study was funded the Key Discipline Program of Pudong New Area Health System (PWZxk2022-25), the Development and Application of Intelligent Epidemic Surveillance and AI Analysis System (21002411400), the Shanghai Public Health System Construction (GWVI-11.2-XD08), the Shanghai Health Commission Key Disciplines (GWVI-11.1-02), the Shanghai Health Commission Clinical Research Program (20214Y0020), the Shanghai Natural Science Foundation (22ZR1414600), and the Shanghai Young Health Talents Program (2022YQ076).






