The multifaceted role of the inferior colliculus in sensory prediction, reward processing, and decision-making
Figures
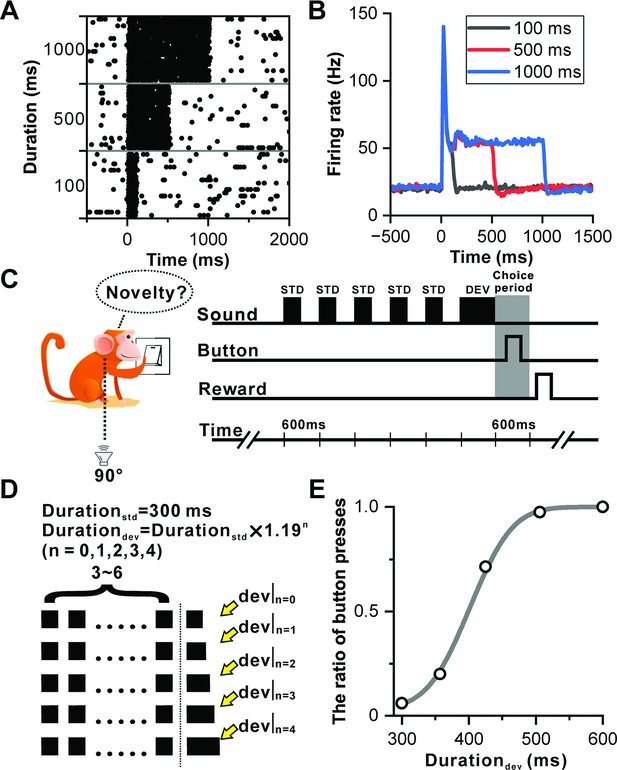
Sustained firing in IC and deviation detection paradigm based on sound duration.
(A) Raster plots of a representative neuron depicting the response profile to white noise stimuli with varying durations (100 ms, 500 ms, and 1000 ms). (B) PSTHs of neuronal population responses (n=104) to the stimuli described in (A) (black, 100 ms white noise; red, 500 ms white noise; blue, 1000 ms white noise). (C) Schematic display of deviation detection behavior. A button is positioned in front of the monkey, and a loudspeaker is placed contralateral to the recording site at the height of the monkey's ear. Within each experimental block, a series of repeated 300 ms white noise bursts (WNB) serves as the standard stimulus, accompanied by a rare-duration WNB serving as the deviant stimulus. Following the presentation of the deviant stimulus, the primate is required to press the designated button within a 600 ms timeframe to obtain a water reward. (D) Oddball stimulation paradigm employed in all experimental blocks. While the duration of the standard sound remains constant at 300 ms, the duration of the deviant sound deviates from the standard sound across five distinct levels. This deviation is determined by the formula: (where n=0, 1, 2, 3, 4; ). Furthermore, the number of standard stimuli within each block is randomly selected from a range of 3–6. Upon completion of the deviant sound, the primate is required to press the designated button within a 600 ms window to receive the reward. In the absence of a deviant sound (control condition), the reward is granted if the primate refrains from pressing the button within 600 ms after the onset of the final sound. (E) Cumulative Gaussian fits of psychophysical data in one example session. The duration of the deviant sound is plotted along the abscissa, while the ratio of button presses is plotted along the ordinate.
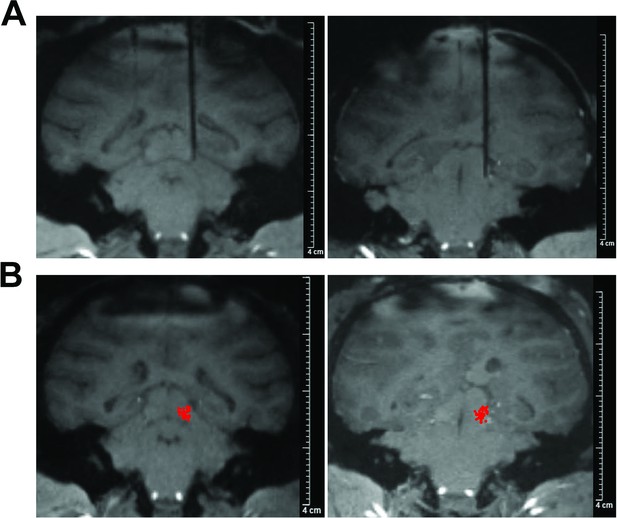
Neurons in the inferior colliculus with sustained response.
(A) 7T MRI with recording electrode: High-resolution 7T MRI scans displaying the recording electrode in place within the brains of two monkeys (left, monkey J; right, monkey B). (B) Localization of recording sites on fMRI image. The red dots overlaid on the fMRI image indicate the locations of the recording sites within the inferior colliculus. The fMRI image was acquired at a plane positioned 1 mm posterior to the ear bar zero (EBZ). The red dots represent the projection of the recording sites onto this specific plane.
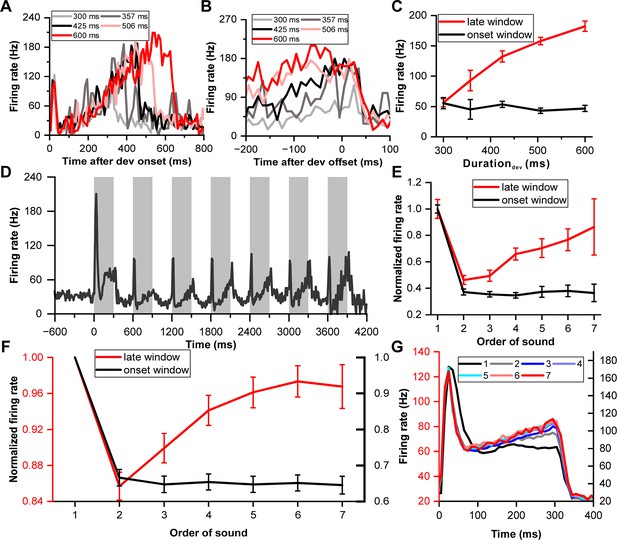
Neuronal climbing effect in deviation detection behavior.
(A) Peri-stimulus time histograms (PSTHs) depicting example neuronal responses to different deviant sounds aligned to deviant onset (light gray, 300 ms deviant sound; gray, 357 ms deviant sound; black, 425 ms deviant sound; pink, 506 ms deviant sound; red, 600 ms deviant sound). (B) The same neuronal responses as in (A), but this time aligned to the offset of the deviant stimulus. The PSTHs represent the neural activity before the offset of each deviant sound. The color scheme remains consistent with (A) to indicate the different deviant durations. (C) Firing rate to deviant stimuli in two windows: the late window ([–100 0] ms relative to offset time) and the onset window ([0 60] ms relative to onset time) (red, late window; black, onset window). (D) PSTH showing neuronal responses to standard sounds (1-7) from the neuron described in (A). (E) Normalized firing rate of the neuron described in (A) to standard sounds in two temporal windows (late window: [–100 0] ms relative to offset time; onset window: [0 60] ms relative to onset time) plotted as a function of the order of the sounds (red, late window; black, onset window). (F) Average normalized responses to standard sounds in the onset window and offset window (red, late window; black, onset window. n=99). The left axis represents the scale for responses in the late window, and the right axis represents the scale for responses in the onset window. (G) PSTHs depicting the responses of the neuronal population to the 300 ms sounds, ranging from the first to the seventh order in the block (black, first; gray, second; blue, third; light blue, fourth; cyan, fifth; pink, sixth; red, seventh). The left axis represents the response scale for sounds from the second to the seventh, and the right axis represents the response scale for the first sound.
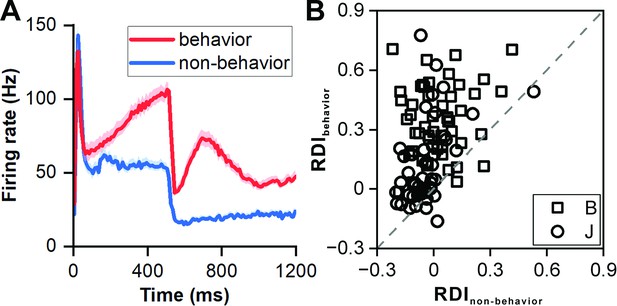
Comparison of neuronal responses between behavior protocol and non-behavior protocol.
(A) PSTHs comparing neuronal responses in non-behavior and behavior protocols (red: responses to 506 ms deviant sound in behavior protocol; blue: responses to 500 ms sound in non-behavior protocol; n=99). The colored area indicates the error band (standard error). (B) Scatterplots of RDI in non-behavior protocol versus behavior protocol (square, monkey B; circle, monkey J). Each data point represents the RDI value of an individual neuron, allowing for a comparison between the two protocols.
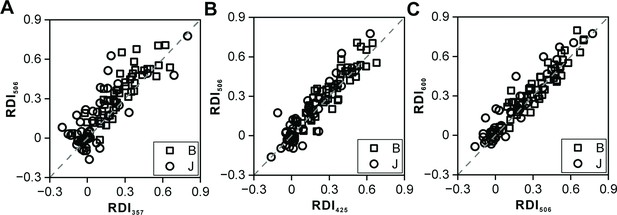
Response dynamic index (RDI) of different deviant sounds.
(A) Scatterplots of RDI for 506 ms deviant sound versus RDI for 357 ms deviant sound (n=99). (B) Scatterplots of RDI for 506 ms deviant sound versus RDI for 425 ms deviant sound. (C) Scatterplots of RDI for 506 ms deviant sound versus RDI for 600 ms deviant sound.
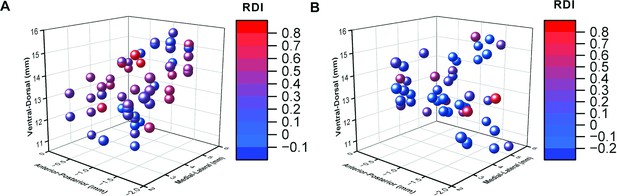
Three-dimensional representation of recorded neurons based on response dynamic index (RDI).
(A) Three-dimensional representation of the recorded neurons obtained from monkey B relative to EBZ. Each neuron is assigned a distinct color based on its RDI. (B) Three-dimensional representation of the recorded neurons obtained from monkey J relative to EBZ. Each neuron is color-coded according to its RDI.
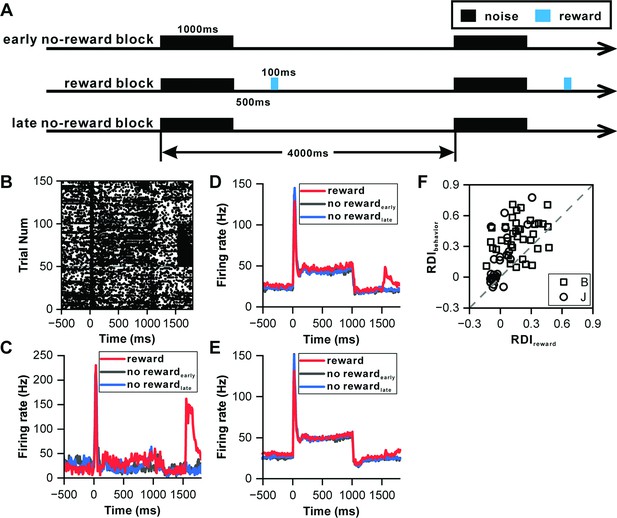
Influence of reward on inferior colliculus neurons.
(A) Schematic representation of the reward protocol. The protocol involves the presentation of a 1000 ms white noise stimulus at 60 dB SPL, repeated 150 times with a 4 s interstimulus interval. The experimental design comprises three blocks: an initial 'early no-reward' block consisting of 50 trials without reward, a 'reward' block of 50 trials with water reward administered 500 ms after sound offset, and a final 'late no-reward' block of 50 trials without reward. (B) Raster plots of a representative neuron depicting the response patterns during the reward protocol. (C) PSTHs of the neuron in (B) representing the neuronal responses during the reward block, early no-reward block, and late no-reward block (red, reward block; black, early no-reward block; blue, late no-reward block). (D) PSTHs of the neuronal population exhibiting significant reward responses (n=24) representing the neuronal responses during the reward block, early no-reward block, and late no-reward block (red, reward block; black, early no-reward block; blue, late no-reward block). (E) Equivalent responses as in (D) but from neurons with nonsignificant reward responses (n=35). (F) Scatterplots of RDI in reward block versus RDI in behavior protocol (square, monkey B; circle, monkey J; n=63).
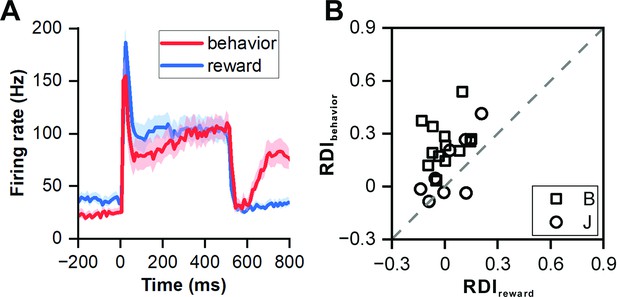
Influence of reward on neuronal responses.
(A) PSTHs showing neuronal population responses (n=22) in reward protocol with 500 ms sound (blue) and behavior protocol (red). (B) Scatterplots of RDI in reward block with 500 ms sound versus RDI in behavior protocol (square, monkey B; circle, monkey J).
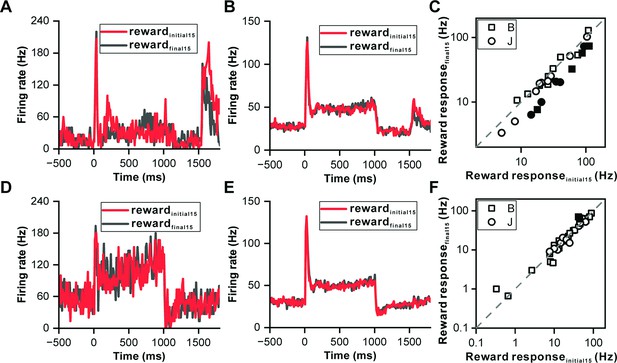
Reward prediction error in inferior colliculus neurons.
(A) PSTHs of the representative neuron in Figure 3B depicting its response profiles during the initial 15 reward trials (red) and the final 15 reward trials (black). (B) PSTHs of the neuronal population with significant reward responses (n=28) demonstrating the firing activity of these neurons during the initial 15 reward trials (red) and the final 15 reward trials (black). (C) Scatterplots of reward responses in initial 15 reward trials versus final 15 reward trials from neurons in (B). The data points are color-coded, with solid circles representing neurons that exhibit a significant difference in reward responses between the initial and final reward trials. Conversely, open circles represent neurons wherein the difference in reward responses between the two trial sets is nonsignificant. The square and circle symbols correspond to monkey B and monkey J, respectively. (D) PSTHs of a neuron with nonsignificant reward responses demonstrating the response patterns of the neuron, similar to those presented in (A). (E–F) Equivalent PSTHs and scatterplots as in (B–C) but from neurons with nonsignificant reward responses (n=35).
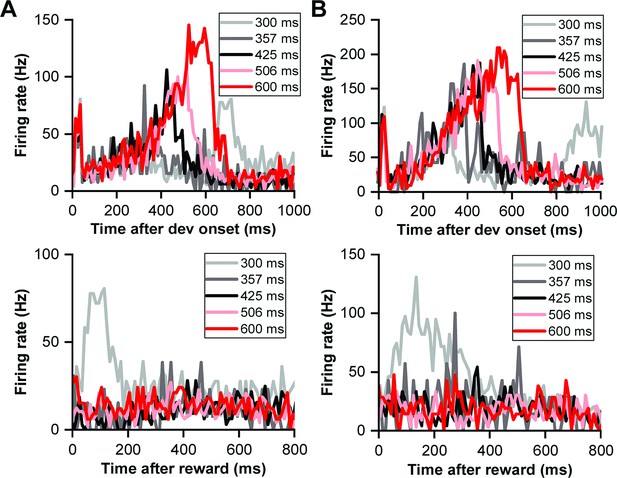
Reward prediction error in deviation detection behavior.
(A) PSTHs illustrating neuronal responses aligned to deviant onset (top) and reward time (bottom). (B) PSTHs demonstrating neuronal responses aligned to deviant onset (top) and reward time (bottom) in another neuron.
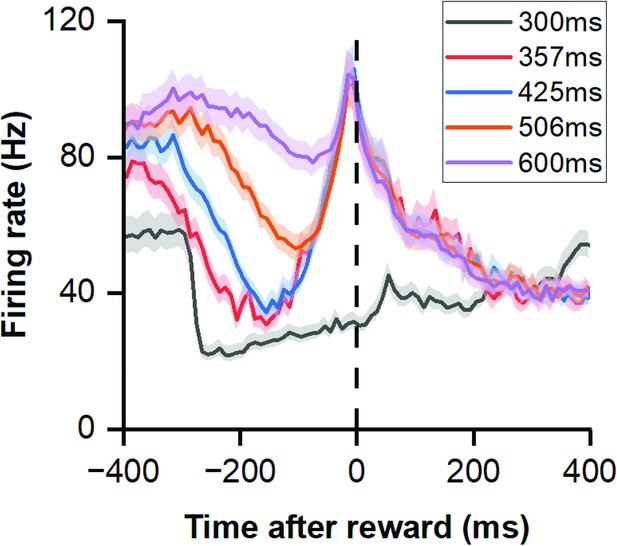
Responses to the reward in deviation detection behavior.
PSTHs showing neuronal population responses (n=99) aligned to the time of button pressing, with the dashed line indicating the exact moment of the button press.
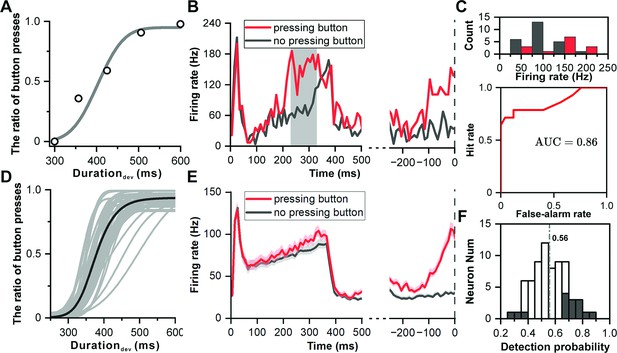
Neuronal responses of decision making in deviation detection behavior.
(A) Cumulative Gaussian fits of psychophysical data in the example recording session. The duration of the deviant sound is plotted along the abscissa, while the ratio of button presses is plotted along the ordinate. (B) PSTHs showing example neuronal responses to deviant sound with 357 ms duration (red, pressing button after deviant sound; black, no pressing button after deviant sound). The left side of the subfigure shows the PSTHs aligned to the onset of the deviant sound, while the right side shows the PSTHs aligned to the time of button presses. (C) Top: Distribution of neuronal responses to 357 ms deviant sound categorized based on the behavioral choice made by the subject (red, pressing the button; black, no pressing the button). Bottom, receiver operating characteristic (ROC) analysis of comparison between two distributions. (D) Cumulative Gaussian fits to psychophysical data in population (n=69). (E) PSTHs showing neuronal responses to deviant sound with 357 ms duration in population (red, pressing button after deviant sound; black, no pressing button after deviant sound; left, aligned to deviant onset; right, aligned to time of pressing button). (F) Distribution of detection probability: This panel displays the distribution of detection probability (DP), with significant DP indicated by black bars.
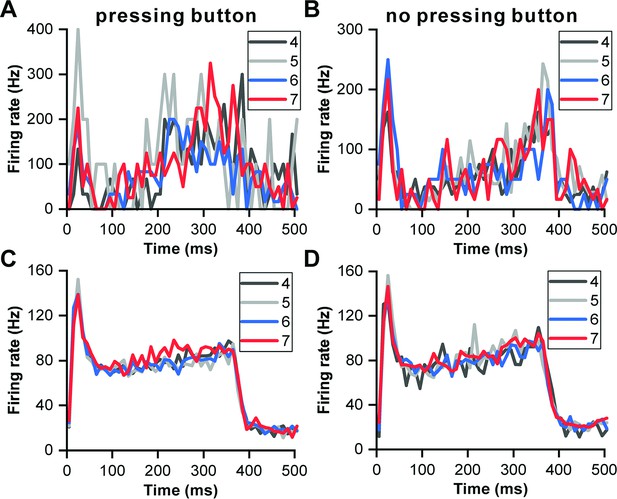
Influence of sound numbers on neuronal responses to 357 ms deviant sound.
(A) PSTHs illustrating the neuronal responses to 357 ms deviant sounds with different orders in the block when the monkey pressed the button. The PSTHs are color-coded, with grey representing the fourth order, light gray representing the fifth order, blue representing the sixth order, and red representing the seventh order. These responses are obtained from the neuron depicted in Figure 6B. (B) PSTHs illustrating the neuronal responses to 357 ms deviant sounds with different orders in the block when the monkey did not press the button. (C) Populational neuronal responses when the monkey pressed the button (n=49). (D) Populational neuronal responses when the monkey did not press the button (n=49).
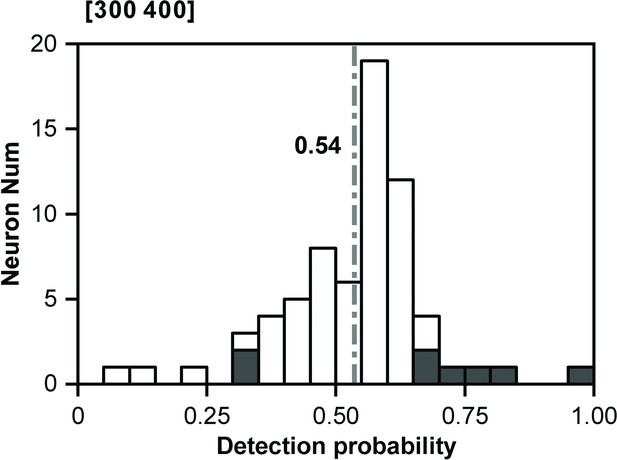
Distribution of detection probability (DP) for 425 ms deviant sounds.
Within the distribution, significant DP values are indicated by the presence of black bars.

(A) PSTH of the neuron from Figure 5A during a key press trial under control condition.The number in the parentheses in the legend represents the number of trials for control condition. (B) PSTHs of the neuron from Figure 5A during non-key press trials under experimental conditions. The numbers in the parentheses in the legend represent the number of trials for experimental conditions. (C-D) Equivalent PSTHs as in A-B but from the neuron in Figure 5B.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample (Macaca mulatta, male) | Monkey J;Monkey B | Hubei Topgene Biotechnology Co., Ltd. | http://en.topgenebio.com | |
| Software, algorithm | MATLAB R2022a | MathWorks | RRID:SCR_001622 | |
| Other | Auditory Workstation RZ6 | Tucker-Davis Technologies, TDT, Alachua, FL | https://www.tdt.com/component/rz6-multi-i-o-processor/ | |
| Other | Speaker, LS50 | KEF, UK | https://uk.kef.com/products/ls50-meta | |
| Other | ¼´´condenser microphone, 4954 | Brüel & Kjær, Nærum, Denmark | https://www.hbkworld.com/en | |
| Other | PHOTON/RT analyzer | Brüel & Kjær, Nærum, Denmark | https://www.hbkworld.com/en | |
| Other | Epoxy-coated tungsten microelectrodes | FHC Inc. | RRID:SCR_018426 | |
| Other | Remote-controlled microdrive | FHC Inc. | https://www.fh-co.com/product-category/star/ | |
| Other | 26-gauge transdural guide tubes | other | Self-made by Yu lab. For more information, please contact yuxiongj@gmail.com. | |
| Other | Plastic head-restraint ring | other | Self-made by Yu lab. For more information, please contact yuxiongj@gmail.com. | |
| Other | Recording grid | other | Self-made by Yu lab. For more information, please contact yuxiongj@gmail.com. |





