Linking rattiness, geography and environmental degradation to spillover Leptospira infections in marginalised urban settings: An eco-epidemiological community-based cohort study in Brazil
Abstract
Background:
Zoonotic spillover from animal reservoirs is responsible for a significant global public health burden, but the processes that promote spillover events are poorly understood in complex urban settings. Endemic transmission of Leptospira, the agent of leptospirosis, in marginalised urban communities occurs through human exposure to an environment contaminated by bacteria shed in the urine of the rat reservoir. However, it is unclear to what extent transmission is driven by variation in the distribution of rats or by the dispersal of bacteria in rainwater runoff and overflow from open sewer systems.
Methods:
We conducted an eco-epidemiological study in a high-risk community in Salvador, Brazil, by prospectively following a cohort of 1401 residents to ascertain serological evidence for leptospiral infections. A concurrent rat ecology study was used to collect information on the fine-scale spatial distribution of ‘rattiness’, our proxy for rat abundance and exposure of interest. We developed and applied a novel geostatistical framework for joint spatial modelling of multiple indices of disease reservoir abundance and human infection risk.
Results:
The estimated infection rate was 51.4 (95%CI 40.4, 64.2) infections per 1000 follow-up events. Infection risk increased with age until 30 years of age and was associated with male gender. Rattiness was positively associated with infection risk for residents across the entire study area, but this effect was stronger in higher elevation areas (OR 3.27 95% CI 1.68, 19.07) than in lower elevation areas (OR 1.14 95% CI 1.05, 1.53).
Conclusions:
These findings suggest that, while frequent flooding events may disperse bacteria in regions of low elevation, environmental risk in higher elevation areas is more localised and directly driven by the distribution of local rat populations. The modelling framework developed may have broad applications in delineating complex animal-environment-human interactions during zoonotic spillover and identifying opportunities for public health intervention.
Funding:
This work was supported by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health, the National Institutes of Health of the United States (grant numbers F31 AI114245, R01 AI052473, U01 AI088752, R01 TW009504 and R25 TW009338); the Wellcome Trust (102330/Z/13/Z), and by the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB/JCB0020/2016). MTE was supported by a Medical Research UK doctorate studentship. FBS participated in this study under a FAPESB doctorate scholarship.
Editor's evaluation
In their work, the authors present a novel geostatistical framework allowing for modelling complex animal-environment-human interactions during zoonotic spillover. The presented case relates to zoonotic spillover of Leptospira infections in a marginalised urban setting in Salvador, Brazil. The outcomes of such applications could contribute to inform public health interventions. The methodological approach is to be applauded and can be of benefit beyond the study of zoonotic spillover.
https://doi.org/10.7554/eLife.73120.sa0Introduction
Zoonotic spillover, the transmission of pathogens from infected vertebrate animals to humans, is responsible for a significant public health burden globally. Understanding the processes that promote spillover transmission is essential for improving our ability to predict and prevent spillover events, but for many zoonoses, such as Leptospira interrogans, Escherichia coli O157 and Giardia spp., they are poorly understood (Plowright et al., 2017). This is due to the complex nature of the spillover system, in which the probability of transmission is governed by dynamic interactions in space and time between ecological, epidemiological, behavioural, and immunological factors that determine pathogen pressure, exposure and host susceptibility. Zoonotic spillover research must explore interactions between the environment, disease reservoirs and local epidemiology, presenting two central challenges: (i) the need for transdisciplinary studies at the animal-human disease interface (a One Health approach) that accurately collect data on multiple components of the spillover process at common temporal and spatial scales at which these events take place; (ii) the development of integrative approaches to jointly analyse these diverse datasets within a spatially and temporally explicit framework (Plowright et al., 2017; Becker et al., 2019; Dhewantara et al., 2019).
Leptospirosis, a neglected zoonotic disease caused by pathogenic bacteria from the genus Leptospira, is an important example of zoonotic spillover. Globally, it is estimated to cause more than one million cases and over 58,000 deaths each year (Costa et al., 2015a), with an annual global burden of 2.9 million disability-adjusted life years (DALYs) (Torgerson et al., 2015). This burden falls heavily on marginalised urban populations in low- and middle-income countries who live in areas characterised by high population density, poor quality housing and inadequate provision of healthcare, sanitation, and waste management services. In these settings, leptospiral infection occurs through contact with water or soil contaminated with leptospires shed in the urine of the principal reservoir, the Norway rat (Rattus norvegicus; Bierque et al., 2020). These areas produce the socio-ecological conditions that allow rodent populations to proliferate and leptospires to persist for long periods in the environment (Goarant, 2016). Residents consequently have frequent, intense and largely unavoidable exposure to the contaminated environment, often exacerbated by their geographical vulnerability to flooding events (Lau et al., 2010). In response, the World Health Organisation (WHO) has convened the Leptospirosis Burden Epidemiology Reference Group (LERG) which has recommended ‘Targeted intervention based on the improved knowledge of disease ecology’ (WHO, 2010), highlighting the current knowledge gap for Leptospira transmission mechanisms and target points for effective intervention.
Multiple studies have helped to elucidate key aspects of the Leptospira transmission cycle in urban settings, identifying socioeconomic vulnerability, household environment and behavioural exposures as important determinants of infection risk (Reis et al., 2008; Felzemburgh et al., 2014; Hagan et al., 2016; Khalil et al., 2021; Barcellos and Sabroza, 2000; Barcellos and Sabroza, 2001; Mwachui et al., 2015; Keenan et al., 2010; Goarant, 2016; Prabhakaran et al., 2014; Briskin et al., 2019). However, these variables have been unable to explain fine-scale spatial variation in risk (Reis et al., 2008; Hagan et al., 2016). This is likely to be driven by the high spatial and temporal heterogeneity in environmental risk, observed in recent studies of Leptospira in soil, and surface and sewage waters (Schneider et al., 2018; Casanovas-Massana et al., 2018; Bierque et al., 2020). These findings lead to two key questions: (i) to what extent does environmental contamination by localised rat shedding drive infection risk, rather than exposure to leptospires that have been dispersed by rainwater runoff and overflowing sewer systems; and (ii) how does this change across the geography of a community, for example at different elevation levels?
Establishing a dynamic link between rats, the environment and Leptospira transmission is complicated by the difficulty of measuring and modelling the rat contamination process. However, urban Norway rats have been found to have high Leptospira prevalence and shedding rates worldwide (Pellizzaro et al., 2019; Costa et al., 2014a; Boey et al., 2019; Yusof et al., 2019; Krøjgaard et al., 2009; Costa et al., 2015b; de Faria et al., 2008). This suggests that rat abundance may be predictive of environmental risk, and could be used as a proxy for this shedding process. While several studies have identified associations between infection risk and household rat sightings and infestation (Reis et al., 2008; Costa et al., 2014b; Hagan et al., 2016; Costa et al., 2021; Pellizzaro et al., 2019; Bhardwaj et al., 2008), their ability to explore fine-scale spatial variation in risk was limited by a reliance on household infestation surveys or aggregation of incidence and abundance indices to a common coarse spatial scale. All modelled abundance as a regression covariate, thereby not accounting for uncertainty in its measurement. The absence of methods applied to formally integrate abundance and spillover infection data is an issue for rodent-borne zoonoses more widely (Bordes et al., 2015; Dhewantara et al., 2019).
There is no gold-standard index of abundance and field teams use a range of imperfect indices, such as traps, infestation surveys and track plates. In our previous work, we developed a multivariate generalized linear geostatistical model for joint spatial modelling of multiple imperfect abundance indices (Eyre et al., 2020). We use the term ‘abundance’ here to denote all ecological processes that are associated with animal abundance and measured by abundance indices, for example animal presence, density and activity, and that may be useful to quantify exposure to a zoonotic disease of interest. This methodology was then used to model the spatial distribution of ‘rattiness’, our proxy for rat abundance, at a fine scale within a community in Salvador, Brazil (Eyre et al., 2020). The spatial distribution of rattiness was highly heterogeneous, suggesting that it could be a driver of micro-heterogeneity in infection risk.
To analyse reservoir host abundance (as defined previously) and infection data at fine spatial scales, we propose that a framework should (i) account for spatial correlation in human and reservoir host data; (ii) jointly model multiple imperfect indices of abundance while accounting for the appropriate sampling distribution of each index; (iii) account for uncertainty in abundance indices, (iv) allow for the prediction of abundance and infection risk at all locations within the study area, and (v) quantify the uncertainty associated with those predictions. Several studies have attempted to model spatial associations between disease reservoir or vector abundance and human infection for leptospirosis (Hurd et al., 2017; Lau et al., 2016; Mayfield et al., 2018), tularemia (Rotejanaprasert et al., 2018) Lyme disease (Nicholson and Mather, 1996) West Nile Virus (Winters et al., 2008) dengue fever (Cromwell et al., 2017) and Lassa fever (Fichet-Calvet et al., 2007). However, none of the approaches used satisfy all five of the above conditions. The development of new tools for the joint spatial analysis of abundance and human infection may consequently be beneficial for the study of other zoonoses and vector-borne diseases (Eisen and Eisen, 2008).
The aim of this study was to develop a flexible modelling framework for zoonotic spillover to explore whether rattiness, acting as a proxy for local leptospiral contamination by Norway rats, can explain spatial heterogeneity in leptospiral transmission in a high-risk urban community in Brazil where 80% of rats are estimated to be actively shedding the bacteria (Costa et al., 2015b; de Faria et al., 2008). We extend the rattiness framework of Eyre et al., 2020 to include human infection risk. We describe findings from a transdisciplinary eco-epidemiological study which comprises a prospective community-based cohort study with two serosurveys and a fine-scale rat ecology study. The ecology study was used to collect information on the spatial distribution of rat abundance, our exposure of interest, in the period between the two surveys using multiple abundance indices. Then, we explore associations between infection risk, rattiness and a range of measured environmental and individual risk factors.
Materials and methods
Study design
Study area
Request a detailed protocolThe study was conducted in Pau da Lima community (13°32’53.47” S; 38°43’51.10” W), a marginalised informal settlement located in the city of Salvador, Northeast Brazil. The study site has an area of and is characterised by three connected valleys with large elevation gradients, high population density and a heterogeneous environment of vegetation, paved surfaces and exposed soil (Figure 1). There are significant gradients in socioeconomic status and infrastructure quality over small elevation increases - with the most marginalised members of the community living at lower elevations. The community suffers from low quality housing, poor provision of waste management services and inadequate drainage and sanitation systems (Hagan et al., 2016; Hacker et al., 2020). Residents are consequently often unable to avoid intense exposure with mud and floodwater. These factors result in abundant rat populations (Eyre et al., 2020) and a high estimated annual Leptospira infection rate of 35.4 (95% CI, 30.7, 40.6) infections per 1000 annual follow-up events (Hagan et al., 2016). For this reason, Pau da Lima has become an exemplar for investigating urban Leptospira transmission in Brazil over the last 15 years.
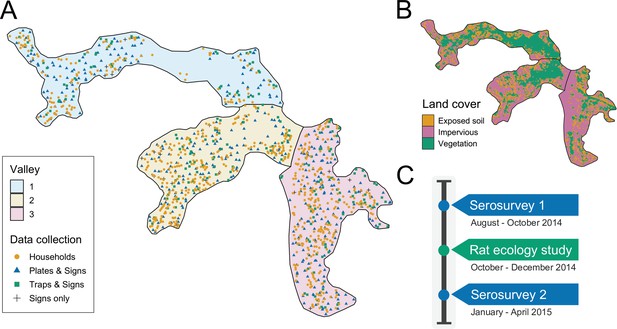
Study site and timeline.
(A) Map of the three valleys within the study site in Pau da Lima, with household locations for the serosurveys marked as orange circles. Locations sampled in the the rat ecology study are shown for each of the rat abundance indices as follows: Plates & Signs (track plates, burrows, faeces and trails), Traps & Signs (traps, burrows, faeces and trails) and Signs only (burrows, faeces, and trails); (B) Land cover classification map (impervious cover is defined as man-made structures e.g. pavement and buildings); (C) Study timeline for the two community serosurveys and rat ecology study.
Serosurveys
Request a detailed protocolWe conducted a prospective community cohort study with two serosurveys carried out in August-October 2014 and January-April 2015. After an initial census of the study site, all ground floor households were visited and inhabitants who met the eligibility criteria of ≥5 years of age who had slept ≥3 nights in the previous week in a study household were invited to join the study. This study focussed on ground floor households because they are vulnerable to flooding and consequently at high risk for leptospiral transmission. The criterion for determining whether a resident is currently living at a household location is commonly applied in this context to account for resident mobility.
During each survey trained phlebotomists collected blood samples from participants and administered a modified version of the standardised questionnaire used previously (Costa et al., 2014b; Hagan et al., 2016). Information was collected on demographic and socioeconomic indicators, household environmental characteristics and exposures to potential sources of environmental contamination in the previous six months (the average time between the two serosurveys). Study data were collected and managed using REDCap electronic data capture tools (Harris et al., 2009) and all individual data were anonymised. The locations of sampled households are shown in Figure 1 - panel A. If an individual was not found during a sample collection visit their house was revisited at least five times on different days of the week.
The microscopic agglutination test (MAT) was used to determine titers of agglutinating antibodies against pathogenic Leptospira in sera obtained from the blood samples collected in each serosurvey. Serological samples were reacted with a panel of two Leptospira reference strains that are dominant in Pau da Lima: Leptospira interrogans serovars Copenhageni (COPL1) and Cynopteri 3522 C (C3522C). These two strains have been shown to have the same performance in identifying MAT seroconversion in our prospective studies as the WHO recommended battery of 19 reference serovars. When agglutination was observed at a dilution of 1:50, the sample was titrated in serial twofold dilutions to determine the highest agglutination titer. The study outcome of leptospiral infection was defined as seroconversion, an MAT titer increase from negative to ≥1:50, or a fourfold increase in titer for either serovar between paired samples from cohort subjects. All laboratory analyses were performed in the Laboratory Pathology and Molecular Biology at Fiocruz, Salvador. As part of quality control procedures two independent evaluations were conducted by Yale University for all infected subjects and 8% of all samples, with high concordance between results.
Rat ecology study
Request a detailed protocolTo estimate exposure risk due to local rat contamination between the two serosurveys, a cross-sectional rat ecology study was conducted from October to December 2014. As has been described previously (Eyre et al., 2020), the aim of this study was to collect data on the fine-scale spatial variation in rat reservoir population abundance. Data were collected for five indices of rat abundance: live trapping, track plates, number of active burrows present, presence of faecal droppings and presence of trails. Rat trapping was carried out at 189 locations, randomly distributed across the study area (see Panti-May et al., 2016). Two traps were deployed for 4 consecutive 24 hr trapping periods at each location. Trapping success and trap closure without a rat, a common malfunction, were recorded after each 24 hr period. Track plates were placed at 415 locations for two consecutive 24 hr periods following the standardised protocol for placement and survey developed and validated previously (Hacker et al., 2016), with five plates placed at each location in the shape of a ‘five’ on a die. After each 24 hr period, plates were repainted and any lost plates were recorded and replaced. On the first day of trapping or plate placement, a survey for signs of rat infestation, adapted from the Centers for Disease Control and Prevention, 2006 and validated in the study area (Costa et al., 2014b), was conducted within an area of 10 m radius around each trapping or plate location to record the number of active burrows and the presence of faecal droppings and trails. In total, 595 independent locations were sampled for traps, track plates and the three survey indices for signs of rat infestation. The spatial distribution of these locations is shown in Figure 1 - panel A. At 21 locations, theft and local gang violence meant that data for track plates and traps was not collected and only the three survey indices for signs of rat infestation were used.
Environmental data
Request a detailed protocolIn addition to the environmental survey conducted at each household location, we also collected information for three spatially continuous environmental variables: elevation relative to the bottom of each valley, distance to large public refuse piles and the proportion of land cover classified as impervious (man-made structures) within a 30 m radius. The land cover variable was created from Digital Globe’s WorldView-2 satellite imagery (8 bands) taken on February 17, 2013 which was classified using a maximum likelihood supervised algorithm and validated with ground truthed data collected from 20 randomly selected sites of size 5 m by 5 m. The classification map is shown in Figure 1 - panel B.
Ethics
Participants were enrolled according to written informed consent procedures approved by the Institutional Review Boards of the Oswaldo Cruz Foundation and Brazilian National Commission for Ethics in Research, Brazilian Ministry of Health (CAAE: 01877912.8.0000.0040) and Yale University School of Public Health (HIC 1006006956).
For the rat ecology study, the ethics committee for the use of animals from the Oswaldo Cruz Foundation, Salvador, Brazil, approved the protocols used (protocol number 003/2012), which adhered to the guidelines of the American Society of Mammalogists for the use of wild mammals in research (Sikes and Gannon, 2011) and the guidelines of the American Veterinary Medical Association for the euthanasia of animals (Leary et al., 2013). These protocols were also approved by the Yale University’s Institutional Animal Care and Use Committee (IACUC), New Haven, Connecticut (protocol number 2012–11498).
Joint modelling rat abundance and human infection: the rattiness-infection framework
The developed geostatistical modelling framework jointly models multiple rat abundance indices as measurements of a common latent process, called rattiness. Rattiness at each household location contributes to the risk of infection for all inhabitants, in addition to other measured individual or household-level explanatory variables.
We model the rat abundance data following a similar structure to that previously outlined (Eyre et al., 2020). Let denote a spatially continuous stochastic process, representing rattiness. The rat data then consist of a set of outcomes , for , collected at a discrete set of locations . The outcome variables are the set of five rat abundance indices that provide information about : traps (), track plates (), number of burrows (), presence of faecal droppings () and presence of trails ().
Human data are collected from households and consist of an infection outcome for individual at household location , for , collected at a discrete set of locations .
Let ‘[·]’ be a shorthand notation for ‘the probability distribution of .’ We write , and . We assume that the and are conditionally independent given , from which it follows that
where is a shorthand notation for ‘the distribution of’ and denotes the number of individuals at household . This model structure is shown schematically in Figure 2. The conditional independence assumption in Equation 1 is reasonable for a vector-borne disease or one that is transmitted indirectly, in which context the observed rat indices are to be considered as noisy indicators of the unobservable spatial variation in the extent to which the environment is contaminated with rat-derived pathogen. It would be more questionable for applications in which the disease of interest is spread by direct transmission from rat to human.
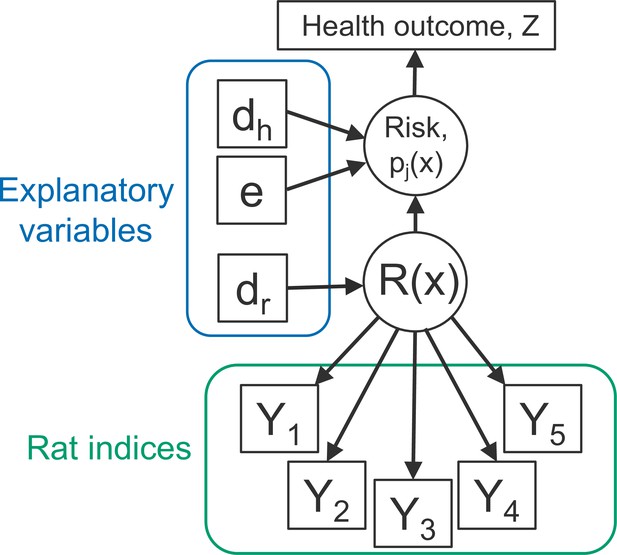
Directed acyclic graph (DAG) of the rattiness-infection model framework.
is the value of a spatially continuous stochastic rattiness process at location . The outcome variables are the set of five rat abundance indices that provide information about : traps (), track plates (), number of burrows (), presence of faecal droppings () and presence of trails (). The outcome variable is the observed health outcome, in this case this represents infection status. The terms dh and dr represent the sets of spatially continuous explanatory variables which contribute to spatial variation in infection risk in humans and , respectively. The terms dh and dr are not mutually exclusive groups of explanatory variables and the same variables may contribute to both infection risk and . The term represents a set of individual- and household-level explanatory variables which contribute to variation in infection risk. Square objects correspond to observable variables, and circles to latent random variables.
Rattiness
Request a detailed protocolWe define rattiness at location as
The terms on the right-hand side of Equation 2 have the following interpretations: is a vector of explanatory variables with associated regression coefficients is a set of independently and identically distributed zero-mean Gaussian variables with unit variance; is a stationary and isotropic spatial Gaussian process; regulates the relative contributions of spatially structured variation, , and unstructured random variation, , to .
For the Gaussian process, , we specify an exponential spatial correlation function:
where is the Euclidean distance between and , and regulates how fast the spatial correlation decays to zero with increasing distance u.
Rat abundance outcomes
Request a detailed protocolThe variable , conditionally on , is a binomial variable representing the number of traps, out of , in which rats were captured. We assume that the times of rat captures from a trap follow a time-varying inhomogeneous Poisson process with intensity , where ti is the time (in days) for which a trap is operative and . It follows that the probability of capturing a rat is
If a trap is found closed without a rat, we assume that the trap was disturbed and set . In all other cases, day. We conducted a sensitivity analysis for this assumption (see ‘Appendix 6’) and found that it did not materially affect rattiness parameter estimates (Appendix 6—table 1).
, is the number of track-plates, out of , that show presence of rats. We model this as a binomial variable with trials and probability where .
, is the number of active rat burrows found at location xi. We model this as a Poisson variable with rate where .
The variables and are binary indicators taking value 1, if at least one faecal dropping or trail, respectively, was found at location xi and 0 otherwise. We model the probability of finding a sign of faecal droppings or trails, and , using logit-linear regressions and .
Human infection outcome
Request a detailed protocolConditionally on , we model the binary human infection outcome as a Bernoulli variable with the probability, , that individual at location is infected. This is modelled with a logit link function and the following linear predictor
where: is a vector of spatially continuous explanatory variables with associated regression coefficients is a vector of household-level and individual-level explanatory variables with associated regression coefficients ; is a set of independently and identically distributed zero-mean Gaussian variables with variance representing unexplained household-level variation; regulates the contribution of rattiness to risk of infection.
Parameterising to test for an interaction with relative elevation
Request a detailed protocolTo explore variation in the role of local rat populations in transmission within sections of the study area with different flooding risk profiles, ξ was parameterised to test for an interaction between rattiness and a categorical parameterisation of household elevation relative to the bottom of the valley (modelled as a piecewise constant function with breaks at 6.7 and 15.6 m, resulting in three categories: low, medium and high elevation levels.) on human infection risk. This was implemented by first dividing the study area into three elevation categories with different flooding risk profiles (as observed during our work in the study area over the last 15 years): low ( from bottom of valley; high flooding risk with maintenance of floodwater for long periods), medium (; moderate flooding with high water runoff), and high (; limited flooding and water runoff). Our study was then designed to evenly sample across this elevation gradient and minimum and maximum values for each elevation category were chosen to include an equal number of households in each level. We then define the set of household locations in each low, medium, and high elevation category as , , and , respectively. Three values of ξ were then estimated such that:
Variable selection
Predictors of rattiness
Request a detailed protocolThe exploratory analysis for the rattiness model followed the steps developed and described previously (Eyre et al., 2020). Firstly, we explored the functional form of the relationship between rattiness and three continuous explanatory variables: relative elevation, distance to large refuse piles and land cover type. To do this, we fitted a simplified rattiness model that did not include covariates or account for spatial correlation. Rattiness is consequently modelled purely as unstructured random variation; hence (Eyre et al., 2020). We then computed the predictive expectation of this simplified rattiness process, , at all locations for which rat index measurements were observed. A generalized additive model (GAM) (Hastie and Tibshirani, 1987) was then fitted to the with the three explanatory variables and the shape of each fitted smooth function was used to assess whether the relationship between each variable and rattiness was linear. Non-linear relationships were modelled using linear splines based on the identified functional form, with knots placed at relative elevations of 8 m and 22 m, and at a distance from large refuse piles of 50 m (see Appendix 1—figure 1). For variable selection, linear models with all combinations of these variables were fitted and ranked by their Akaike Information Criterion (AIC) value (Bozdogan, 1987). The model with the lowest AIC included all of the variables and their linear splines (Appendix 2—table 1).
Following the methodology outlined previously (Eyre et al., 2020), we fitted the full geostatistical rattiness model using the variables selected in ‘Predictors of rattiness’. We then plugged in the maximum likelihood estimates and made predictions for rattiness at all human household locations; here, the predictive target is rather than as defined by Equation 2 because the predicted value of the spatially uncorrelated at any location where rat abundance indices have not been recorded is zero. The expectation of this predictive distribution was then computed to provide an estimate of mean predicted rattiness at all household locations. This was then used as an exploratory covariate in the following section.
Risk factors for human infection
Request a detailed protocolAll explanatory variables were grouped into the following four domains: social status, household environment, occupational exposures and behavioural exposures (see Table 2 for the full list of considered variables by group). A group of a priori confounding variables was then identified, with age, gender and household per capita income selected based on previous findings (Hagan et al., 2016; Reis et al., 2008; Felzemburgh et al., 2014), and valley also included to account for otherwise unmeasured differences between the three valley regions within the study area. In the household environment domain, two variables were used to capture risk due to sewer flooding close to the household: (i) the presence of an open sewer within 10 metres of the household location and (ii) a binary ‘unprotected from open sewer’ variable which identified those households within 10 metres of an open sewer that did not have any physical barriers erected to prevent water overflow. Three high-risk occupations were included in the occupational exposures domain as binary variables. Construction workers and refuse collectors have direct contact with potentially contaminated soil, building materials and refuse in areas that provide harbourage and food for rats. Travelling salespeople have regular and high levels of exposure to the environment (particularly during flooding events) as they move from house to house by foot. Two other binary occupational exposure variables were included that measured whether a participant worked in an occupation that involves contact with floodwater or sewer water.
The relationship between continuous explanatory variables and infection risk (on the log-odds scale) was assessed for linearity by fitting a GAM while controlling for the four confounders. As before, non-linear relationships were modelled using linear splines based on the identified functional form. Age was modelled with a knot at 30 years old, education at 5 years and relative elevation at 20 m (Appendix 1—figure 2). A univariable analysis was conducted to explore the relationship between each explanatory variable and infection risk while controlling for the four a priori confounding variables. Crude and adjusted odds ratios were estimated using a mixed effects logistic regression with a random effect to account for unexplained variation at the household-level.
For the multivariable model, variable selection was conducted within each domain separately. Mixed-effect logistic regression models were fitted for all combinations of the variables in each domain and were ranked by their Akaike Information Criterion (AIC) value (Appendix 2—table 2). Variables in the model with the minimum AIC value were selected for each domain. Age, gender, household per capita income and valley were controlled for in all models throughout this process. Then, the variables selected from each domain were combined and the mean predicted rattiness estimate (obtained in ‘Predictors of rattiness at each household location’) was included with an interaction with relative elevation category. This set of variables was reduced once more following the same process and all selected variables were included in the final multivariable model (‘Appendix 3’).
Model fitting
Request a detailed protocolAll rat and human variables selected in ‘Variable selection’ were then included in the full joint model defined in Equation 2 and Equation 3. We fit this model using the Monte Carlo maximum likelihood (MCML) method (Christensen, 2004) as described in ‘Appendix 4’, and compute 95% confidence intervals by re-fitting the model for 1000 parametric bootstraps. A formal diagnostic investigation of randomized quantile residuals (Dunn and Smyth, 1996; Smyth et al., 2021) is included ‘Appendix 7’. We found no evidence in the diagnostic plots (Appendix 7—figure 1) to suggest that there were issues with our modelling approach.
Prediction maps
Request a detailed protocolThe maximum likelihood parameter estimates were then used to make prediction maps for rattiness and infection risk as follows.
To map a general predictive target, say, we first define , where is a finely spaced grid of locations to cover the region of interest. We then draw samples from the predictive distribution of , that isits conditional distribution given all relevant data. These samples can then be used to compute any desired summary of the predictive distribution. In our analysis, we used as summaries the expectation and 95% prediction interval.
Our first predictive target is rattiness, for which . Our second is human infection risk, for which . In either case, we first sample from using the same sampling algorithm as for maximizing the likelihood in ‘Joint modelling rat abundance and human infection: the rattiness-infection framework’, with the parameters θ and ω fixed at their maximum likelihood estimates. After obtaining samples , , we then sample from , which in both cases follows a multivariate Gaussian distribution with mean and covariance matrix easily obtained from their joint Gaussian distribution, . The resulting values, , constitute samples drawn from as required. Note that each is a sample from the joint predictive distribution of the complete surface of over the whole of the region of interest and can therefore be used to make inferences about spatially aggregated properties of if required.
Data and code accessibility
Request a detailed protocolData and code used in this analysis are publicly available at https://github.com/maxeyre/Rattiness-infection-framework, (copy archived at swh:1:rev:e7953d38269ce97221dbdd83c0be2c65d92dff40, Eyre, 2022) and have been published (Eyre et al., 2021). However, household coordinates and valley ID have been removed from the human data to ensure participant anonymity. The analysis was conducted using R (R Development Core Team, 2016) and the following packages: tidyverse (Wickham, 2017), mgcv (Wood and Wood, 2015), PrevMap (Giorgi and Diggle, 2017), MuMIn (Barton, 2020), lme4 (Bates et al., 2007), and statmod (Smyth et al., 2021). We also include a step-by-step explanation of the model building process to guide future users of the rattiness-infection framework in 'Appendix 8'.
Results
Study overview
In Pau da Lima, we identified 3179 eligible residents using a baseline community census, household visits and through other members of the household. Of these, 2018 (63.4%) individuals consented to join the study and provided a blood sample in the first serosurvey (August-October 2014). As a result of loss to follow-up, only 1401 (69.4%) of these participants (from 669 households) completed the second serosurvey (January-April 2015). Individuals were lost to follow-up because they could not be found after at least five attempts (44.4%), had moved out of the study area (31.1%) or did not wish to provide a second blood sample (19.8%). An overview of participant recruitment is provided in Figure 3. Individuals lost to follow-up were similar in age to those who remained in the study cohort (mean 29.0 and 28.8 years old, respectively, t-value , , ) but were more likely to be male (49.8% male compared to 42.6%, , , ). A full description of the study cohort is included in Appendix 5—table 1.
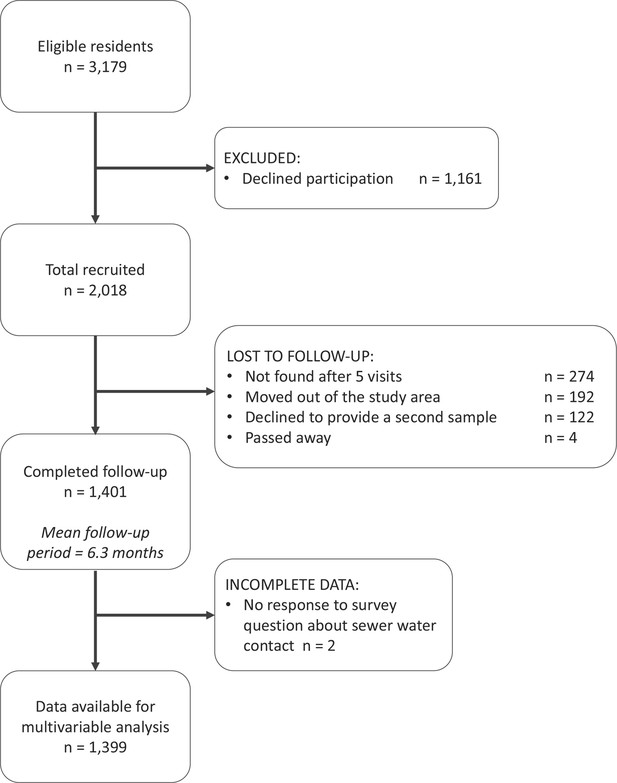
The study participant flow chart in line with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement (http://www.strobestatement.org).
Between the two serosurveys there was serological evidence of 72 leptospiral infections in the cohort, with an overall infection rate of 51.4 (95%CI 40.4, 64.2) infections per 1,000 follow-up events. Valleys 2 and 3 had high estimated infection rates with 66.4 (95%CI 47.3, 90.2) infections per 1000 follow-up events and 49.6 (95%CI 33.6, 69.9) infections per 1000 follow-up events, respectively, compared to 23.2 (95%CI 9.2, 46.9) infections per 1000 follow-up events in Valley 1. The number of infected participants in each household are mapped in Figure 4 - panel A, with relative elevation shown for reference in Figure 4 - panel B.
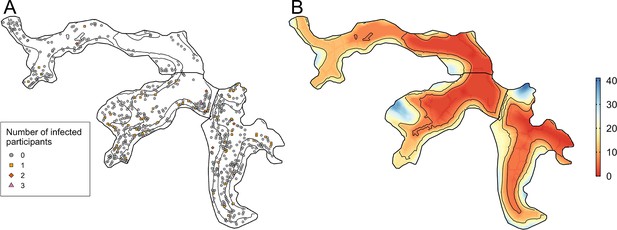
Household infection and elevation maps.
(A) Map of participant household locations with the number of leptospiral infections in each household marked (grey circle - no infections; orange square - 1 infection; red diamond - 2 infections; pink triangle - 3 infections) and contours marking low, medium, and high relative elevation category; (B) Elevation (metres) relative to the bottom of the valley with contours marking low, medium, and high relative elevation levels.
In the rat ecology study a rat was captured in 129 (9.0%) out of 1,512 trapping-days, 263 (37.4%) out of 703 track plate days had at least one positive plate and 28.5%, 19.7%, and 25.9% of the 580 sampled locations had at least one sign of active burrows, faecal droppings and trails, respectively.
Exploratory analysis and model selection
The results from the exploratory multivariable analysis of rattiness are shown in Table 1. The linear splines used were informed by the functional forms shown in Appendix 1—figure 1. The relationship between rattiness and relative elevation demonstrates a trade-off between the high availability of food sources at the bottom of the valley and high risk of flooding which prevents the establishment of burrows. In the lowest elevation areas (0–8 m above the bottom of the valley), relative elevation and rattiness were positively associated with an increase of 0.04 (95%CI 0.00, 0.07) rattiness units per 1 m; when interpreting the magnitude of effect estimates note that, by definition, rattiness is defined so as to have variance one. Rattiness then peaked at an elevation of 8 m before declining with increasing elevation by 0.04 (95%CI –0.09, 0.01) units until an elevation of 22 m. Rattiness started to increase again above this elevation by 0.06 (95%CI 0.00, 0.10) units per metre. Rattiness decreased with increasing distance from large refuse piles, a source of food and harbourage, by 0.07 (95%CI −0.13,–0.01) units per 10 m distance until a distance of 50 m, beyond which there was a smaller increase in rattiness of 0.02 (95%CI –0.05, 0.09) per 10 m. Impervious land cover (defined as the proportion of the area within a 30 m radius around each sampling location classified as pavement or building) was negatively associated with rattiness, decreasing by –0.05 (95%CI −0.08,–0.01) units for every 10% increase in impervious cover.
Multivariable linear regression analysis of predictors for rattiness (note that rattiness is a unit-variance random variable when interpreting the magnitude of effect estimates).
| Variable | Estimate (95% CI) * |
|---|---|
| Relative elevation (per 1 m increase)† | |
| 0–8 m | 0.04 (0.00, 0.07) |
| 8–22 m | –0.04 (-0.09, 0.01) |
| >22 m | 0.06 (0.00, 0.10) |
| Distance to large refuse piles (per 10 m increase)† | |
| 0–50 m | –0.07 (-0.13,–0.01) |
| >50 m | 0.02 (-0.05, 0.09) |
| Impervious land cover (per 10% increase) | –0.05 (-0.08,–0.01) |
-
*
CI, Confidence interval.
-
†
The effects of relative elevation and distance to refuse are modelled as broken linear models with transitions at 8m and 22m, and 50m, respectively. This was informed by the relationship described by Generalized Additive Modelling in Appendix 1—figure 1.
In the community cohort data, the univariable analysis identified several risk factors that increased a resident’s risk of leptospiral infection (Table 2). Variables in two of the four domains (demographic and social status and behavioural exposures) had estimated effect sizes with 95% confidence intervals that did not include an odds ratio of one (statistically significant at the conventional 5% level). Within the demographic and social status domain, risk of infection increased with age and was found to be higher for male participants and those living in Valleys 2 and 3. In the behavioural exposures domain, participants who had had frequent contact with floodwater in the last six months were more likely to be infected. Two individuals were excluded from the multivariable analysis (n=1399) because of missing data for the floodwater exposure survey question.
Univariable mixed effects logistic regression analysis of human risk factors for leptospiral infection.
| Variable | OR (95% CI)* | aOR (95% CI)* |
|---|---|---|
| Demographic and social status | ||
| Age (per year)† | ||
| 0–30 years old | 1.08 (1.03, 1.13) | 1.09 (1.04, 1.15) |
| >30 years old | 1.02 (0.96, 1.09) | 1.02 (0.95, 1.08) |
| Male gender | 2.22 (1.31, 3.85) | 2.78 (1.56, 4.96) |
| Daily per capita household income (US$/day) | 1.01 (0.89, 1.11) | 0.92 (0.80, 1.05) |
| Valley | ||
| 1 | REF | REF |
| 2 | 3.35 (1.33, 10.37) | 3.52 (1.23, 10.05) |
| 3 | 2.39 (0.93, 7.38) | 2.53 (0.88, 7.27) |
| Adult illiteracy | 1.34 (0.61, 2.79) | 0.66 (0.29, 1.49) |
| Education (per year of education)† | ||
| 0–5 years | 1.05 (0.85, 1.32) | 1.14 (0.91, 1.44) |
| >5 years | 0.96 (0.73, 1.27) | 0.96 (0.75, 1.26) |
| Household environment | ||
| Impervious land cover (per 10% increase) | 0.87 (0.76, 0.99) | 0.82 (0.71, 0.95) |
| Relative elevation (per 1 m increase)† | ||
| 0–20 m | 0.94 (0.89, 0.99) | 0.93 (0.88, 0.99) |
| >20 m | 1.12 (0.98, 1.29) | 1.12 (0.97, 1.29) |
| Relative elevation category ‡ | ||
| Low (0–6.7 m) | REF | REF |
| Medium (6.7–15.6 m) | 0.72 (0.37, 1.39) | 0.72 (0.36, 1.44) |
| High (>15.6 m) | 0.58 (0.27, 1.20) | 0.51 (0.23, 1.11) |
| Open sewer within 10 m | 1.60 (0.85, 3.17) | 1.69 (0.85, 3.37) |
| Unprotected from open sewer | 1.00 (0.55, 1.79) | 1.11 (0.61, 2.03) |
| Live on hillside | 0.99 (0.52, 1.86) | 0.89 (0.46, 1.71) |
| Occupational exposures | ||
| Work in construction § | 1.36 (0.51, 3.21) | 0.62 (0.23, 1.67) |
| Work as travelling salesperson § | 4.81 (1.12, 18.78) | 2.97 (0.71, 12.40) |
| Work in refuse collection § | 2.95 (1.04, 7.89) | 1.57 (0.56, 4.42) |
| Work involves contact with floodwater § | 0.89 (0.04, 5.61) | 0.52 (0.05, 4.96) |
| Work involves contact with sewer water § | 3.61 (0.45, 20.38) | 1.92 (0.29, 12.80) |
| Behavioural exposures | ||
| Contact with floodwater in last 6 months | ||
| Never/rarely | REF | REF |
| Sometimes | 0.61 (0.27, 1.25) | 0.66 (0.30, 1.47) |
| Frequently | 2.14 (0.91, 4.94) | 2.84 (1.18, 6.86) |
| Contact with sewer water in last 6 months | ||
| Never/rarely | REF | REF |
| Sometimes | 0.55 (0.19, 1.31) | 0.67 (0.25, 1.78) |
| Frequently | 1.42 (0.51, 3.50) | 1.63 (0.61, 4.41) |
-
*
OR, Odds ratio; aOR, Adjusted odds ratio; CI, Confidence interval; REF, Reference level.
-
†
The effect of age, education and relative elevation are modelled as broken linear models with transitions at 30 years old, 5 years of education and an elevation of 20m. This was informed by the relationship described by Generalized Additive Modelling (Appendix 1—figure 2).
-
‡
Relative elevation category consists of three discrete groups representing three regions with different floodingrisk profiles.
-
§
Binary variable with reference category of ‘no occupational exposure’.
In the exploratory results from the multivariable model there was strong evidence of an interaction between rattiness and household relative elevation category on human infection risk (see Appendix 3—table 1 for all parameter estimates for this model). In the high elevation category area, a unit increase in mean predicted rattiness at the household location was estimated to increase the odds of infection by 6.92 (95%CI 1.88, 25.47). In contrast, in the low and medium elevation category areas there was no evidence of a relationship between rattiness and infection risk, as shown in Figure 5. Consequently, this interaction effect was also included in the rattiness-infection joint model.
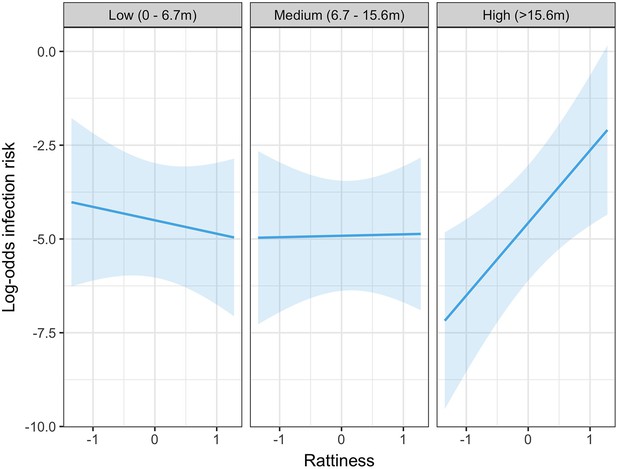
Predicted relationship between rattiness and infection risk from the multivariable mixed effects logistic regression demonstrating evidence of an interaction with relative elevation category (low, medium and high).
Shown on the log-odds scale with shaded areas corresponding to 95% confidence intervals.
The explanatory variables selected in the rat and human multivariable analyses were then entered into the full rattiness-infection joint model with the functional forms included in Table 1. To test for residual spatial correlation in the human infection data after controlling for explanatory variables and rattiness, we fitted the joint model with an additional spatial Gaussian process in the human infection linear predictor. The estimated value for the scale of spatial correlation for this Gaussian process was less than 1 m and indistinguishable from household-level variation. We consequently fitted the joint model specified in Equation 3 which assumes that there is no residual spatial correlation in the human infection data.
Joint rattiness-infection model
Human infection risk factors, rattiness predictors and other model parameters estimated using the joint rattiness-infection model are shown in Table 3. Infection risk was strongly associated with age, with an individual experiencing an increased odds of infection of 1.09 (95%CI 1.04, 1.19) for every year of life up until 30 years of age, and 1.02 (95%CI 0.92, 1.09) for each additional year thereafter. Male participants were more likely to be infected than female participants (OR 2.69 95% CI 1.58, 5.89). Compared with individuals living in Valley 1, those living in Valley 2 had a higher estimated odds of infection (OR 2.91 95% CI 1.03, 20.82). Individuals living in the medium (OR 0.77 95% CI 0.31, 1.66) and high (OR 0.67 95% CI 0.11, 1.64) elevation areas had a lower estimated odds of infection relative to those living in the low relative elevation category area where there are open sewers and flooding risk is higher, however these confidence intervals included an odds ratio of one (not statistically significant at the conventional 5% level).
Parameter estimates for the full joint rattiness-infection model.
| Parameter | Estimate (95% CI) |
|---|---|
| Human infection risk factors | OR |
| Age (per year) | |
| 0–30 years old | 1.09 (1.04, 1.19) |
| >30 years old | 1.02 (0.92, 1.09) |
| Male gender | 2.69 (1.58, 5.89) |
| Daily per capita household income (US$/day) | 0.93 (0.74, 1.05) |
| Valley | |
| 1 | REF |
| 2 | 2.91 (1.03, 20.82) |
| 3 | 2.28 (0.86, 14.00) |
| Relative elevation category | |
| Low (0–6.7 m) | REF |
| Medium (6.7–15.6 m) | 0.77 (0.31, 1.66) |
| High (>15.6 m) | 0.67 (0.11, 1.64) |
| Work as travelling salesperson | 3.16 (0.38, 20.57) |
| Contact with floodwater in last 6 months | |
| Never/rarely | REF |
| Sometimes | 0.62 (0.18, 1.39) |
| Frequently | 2.47 (0.67, 7.41) |
| Rattiness (per unit rattiness) | |
| 1.14 (1.05, 1.53) | |
| 1.25 (1.08, 1.74) | |
| 3.27 (1.68, 19.07) | |
| (variance of household-level random effect) | 1.36 (0.23, 5.35) |
| Rattiness variables | |
| Relative elevation (per 1 m increase)2 | |
| 0–8 m | 0.05 (-0.01, 0.13) |
| 8–22 m | –0.06 (-0.16, 0.02) |
| >22 m | 0.05 (-0.03, 0.14) |
| Distance to large refuse piles (per 10 m increase)3 | |
| 0–50 m | –0.10 (-0.21, 0.02) |
| >50 m | 0.03 (-0.11, 0.17) |
| Impervious land cover (per 10% increase) | –0.07 (-0.14,–0.01) |
| Rattiness parameters | |
| –2.94 (-3.27,–2.65) | |
| –2.06 (-2.50,–1.74) | |
| –1.41 (-1.67,–1.16) | |
| –2.82 (-3.83,–2.32) | |
| –2.22 (-2.96,–1.76) | |
| 0.72 (0.45, 0.97) | |
| 2.37 (2.05, 2.68) | |
| 1.28 (1.08, 1.45) | |
| 2.36 (1.80, 3.34) | |
| 2.43 (1.85, 3.12) | |
| 0.67 (0.29, 1.00) | |
| 9.23 (3.21, 18.24) |
Infection risk was positively associated with rattiness for households situated in all three levels of the relative elevation category variable. However, while the effect size (per unit increase in rattiness) was similar in the low (OR 1.14 95% CI 1.05, 1.53) and medium (OR 1.25 95% CI 1.08, 1.74) elevation areas, in the high elevation area the effect of increasing rattiness on infection risk was significantly stronger (OR 3.27 95% CI 1.68, 19.07). This interaction effect between rattiness and household relative elevation category on human infection risk was confirmed with a test for evidence against the null hypothesis that (, , ).
Parameter estimates for the rattiness variables were very similar to the estimates from the exploratory linear regression (Table 1), with a slightly higher effect size for the distance to refuse piles and land cover variables. There was evidence of small-scale spatial correlation in rattiness ( = 9.23m 95% CI 3.21, 18.24 m) corresponding to a spatial correlation range (the distance at which the correlation reduces to 5%) of approximately 28 m. The estimate for of about 0.67 (95%CI 0.29, 1.00) indicates that the majority of the unexplained variation in rattiness is spatially structured, with the remainder modelled as a nugget effect.
Spatial prediction
There was heterogeneous spatial variation in predicted rattiness. The numerous small regions of high rattiness in Figure 6 - panel A are indicative of the small-scale spatial correlation in the data. The low elevation areas in the central length of each valley (relative elevation is shown in Figure 6 - panel E with the contours marking the low, medium and high elevation areas) had high mean predicted rattiness. High rattiness was also predicted in several high elevation areas, for example the northern tip of Valley 3 and several small hotspots along the three valley’s high elevation sides.
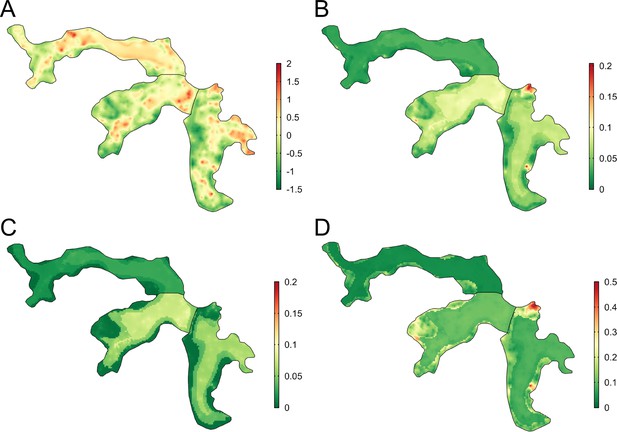
Joint rattiness-infection model predictions.
(A) Mean predicted rattiness; (B) Mean predicted leptospiral infection risk for 30-year-old male participants with a household per capita income of USD$1 /day who never/rarely have contact with floodwater and do not work as a travelling salesperson; (C) lower 95% prediction interval for predicted infection risk; (D) upper 95% prediction interval for predicted infection risk.
To illustrate the spatial variation in infection risk within the study area, prediction maps are shown in Figure 6 - panel B for a 30-year-old male participant with a household per capita income of USD$1 /day who never or rarely had contact with floodwater in the previous six months and did not work as a travelling salesperson. Infection risk was low across most of Valley 1 (<2.5%), with marginally higher average values found in the central low elevation area (2.5–5%). Risk was consistently higher across most of Valleys 2 and 3 (7.5–15%), with the effect of elevation on risk clearly visible. In areas with higher and more spatially heterogeneous predicted infection risk, for example in the central region of Valley 2, this was driven by high levels of predicted rattiness. The stronger estimated effect of rattiness on infection risk in higher elevation areas was particularly visible in Valleys 2 and 3, as seen in the three hotspots with risk reaching 20% and the moderate risk hotspots along the sides of both valleys. Prediction intervals were relatively narrow across most of the study area (Figure 6 - panel C and Figure 6 - panel D) with greater uncertainty in the high risk areas.
Discussion
We developed and applied a novel framework for joint spatial modelling of disease reservoir abundance and human infection risk to a community-based cohort study and fine-scale rat ecology study. We found that higher levels of rattiness, our proxy for rat abundance, at the household location were associated with a higher risk of leptospiral infection for residents across the entire study area. Importantly, we found that a unit increase in rattiness in high elevation areas was associated with an almost three times higher odds ratio for infection than in low and medium elevation areas. To our knowledge, this is the first study to jointly model rodent abundance and human infection data for a rodent-borne zoonosis. The findings provide new insights into how the dominant mechanisms of Leptospira transmission within complex urban settings may vary over small distances, as a result of interactions between rats, the environment, geography, and local epidemiology.
The finding that rattiness was associated with infection risk indicates that the spatial distribution of rat populations was an important driver of transmission close to the household across the entire study area. This is consistent with a recent study investigating the predictive power of household rat infestation scores for human infection (Costa et al., 2021). There was no residual spatial correlation in the infection data after accounting for rattiness in our analysis, possibly suggesting that previously unexplained spatial heterogeneity in risk could be driven by variation in rattiness (Hagan et al., 2016). Our model also predicted high average rattiness across the low elevation areas where leptospiral transmission is high (Hagan et al., 2016; Reis et al., 2008). This supports the hypothesis that abundant rat populations are responsible for high levels of observed environmental contamination across these lower areas (Casanovas-Massana et al., 2018; Casanovas-Massana et al., 2022), and consequently increased infection risk.
The identified interaction between elevation and rattiness on infection risk suggests that relatively small changes in environment and topography can modify transmission pathways within an urban community. The weaker effect of rattiness on infection risk at low and medium elevation areas relative to high elevation areas may be explained by differences in their hydrological profiles. While high rat abundance in low and medium areas results in high leptospiral contamination, these areas are prone to high levels of water runoff and flooding. This disperses the pathogen across low elevation areas. The ability of leptospires to persist in the environment for weeks or months means that this process can significantly increase environmental risk in low elevation areas for long periods. This process disconnects shedding and infection events in space and time (Plowright et al., 2017) and obscures the relationship between infection risk and rattiness in low and medium elevation households.
In contrast, high elevation areas have lower levels of water runoff and flooding due to improved drainage and sewage systems, and a smaller upstream catchment area for rainfall. Leptospires are consequently less likely to be washed away from the location at which they were shed, and environmental risk remains more localised and strongly associated with the spatial distribution of rats. This hypothesised role of hydrology in the aggregation and dispersal of leptospires (Plowright et al., 2017) is supported by a recent study in low elevation areas of Pau da Lima which found that soil contamination was not associated with local rat activity (Schneider et al., 2018). However, our finding that rattiness was associated with infection in low and medium elevation areas suggests that the spatial distribution of environmental risk in these areas is not entirely determined by water dispersal.
Interestingly, a previous study of surface waters in Pau da Lima found that the probability of a sample being positive for Leptospira was highest in low elevation areas and lowest in medium elevation areas, with no significant difference between low and high elevation areas (Casanovas-Massana et al., 2018). This is consistent with our findings and suggests that there may be a ‘washing out’ of locally deposited leptospires in medium elevation areas but not in high elevation areas.
This has several implications for disease control strategies which aim to reduce environmental risk. Improving drainage systems at all elevation levels can reduce the dispersal of leptospires from high to low elevation areas. Closure of sewer systems, which generally run through low elevation areas, can protect local residents from exposure and reduce the introduction of additional contamination from upstream sewer water. Paving over soil surfaces can reduce the surface area over which leptospires can persist (Bierque et al., 2020), reducing environmental risk further.
A reduction in the dispersal and accumulation of bacteria will result in more localised environmental risk, as was observed in the high elevation areas in this study. Higher risk will then be found in areas with a high abundance of infected rats. This may also reduce environmental exposure for rats, thereby lowering shedding rates and acting as a feedback loop into the Leptospira transmission cycle (Minter et al., 2018). Given the limited and short-term impact of chemical rodenticide campaigns on Norway rat abundance in these settings (de Masi et al., 2009), longer-term environment management strategies targeted at rattiness hotspots may also be needed to reduce the availability of key predictors of rattiness, such as large refuse piles and vegetation and soil land cover. Funding and political will for large-scale infrastructural interventions is often limited in marginalised urban settings and small-scale community-based interventions which target these mechanisms should be evaluated.
Transmission is dynamic in space and time and the alignment of conditions which enable spillover infection can vary over time (Plowright et al., 2017). Our study was designed to explore the spatial variation in rattiness and infection risk in Pau da Lima during the driest period of the year, and it may not be representative of transmission mechanisms during the rainy season. There is some evidence, however, that this may not necessarily be the case, with two recent studies in Pau da Lima reporting low seasonal variation in both rat abundance (Panti-May et al., 2016) and spatial infection risk patterns (Hagan et al., 2016). Nonetheless, future studies across different time periods are needed to establish the role of rat abundance in Leptospira transmission.
In this study we used household location to link rattiness to an individual, under the assumption that the majority of their exposure occurs close to home. Given the spatially heterogeneous distribution of rattiness and environmental contamination (Casanovas-Massana et al., 2018) within the community, future epidemiological studies of leptospirosis and zoonotic spillover could benefit from trying to pinpoint key sources of infection away from the household using GPS mobility data, as has been attempted in a small study previously (Owers et al., 2018). The rattiness-infection framework could then be extended to model cumulative environmental exposure to the rattiness surface by integrating along a person’s trajectory as they move around the community.
Our framework did not account for disease dynamics within rat populations. Given that 80% of rats are estimated to be actively shedding Leptospira in Pau da Lima (Costa et al., 2015b; de Faria et al., 2008) and prevalence in rats is generally high in urban areas globally (Pellizzaro et al., 2019; Costa et al., 2014a; Boey et al., 2019; Yusof et al., 2019; Krøjgaard et al., 2009), the use of rattiness as a proxy for rat shedding appears reasonable and it may be a useful proxy in other epidemiological studies. Despite this, non-shedding rats may be spatially clustered and future work would benefit from the collection of georeferenced rat infection data. For other zoonotic spillover systems where pathogen release does not occur at a high and homogeneous rate across the reservoir host population, accounting for spatially heterogeneous or time-varying (Davis et al., 2005) disease dynamics will be important.
A possible limitation of this study is the titre rise cut-off values used for classifying seroconversion and reinfection in the cohort that determine the sensitivity and specificity of the infection criteria. However, these criteria were used because they are the standard definitions for serological determination of infection that are commonly applied for leptospirosis and a wide range of other infections, and they enable the comparison of results with other previous leptospirosis studies.
The rattiness-infection modelling framework is a flexible tool for exploring the spatial association between reservoir abundance, the environment and human health outcomes. It provides a statistically principled method for joint spatial modelling of infection risk and multiple indices of reservoir abundance, pooling data between indices and directly accounting for uncertainty in their measurement in all parameter estimates and predictions. The framework’s geostatistical structure includes spatially continuous predictors for abundance and accounts for spatial correlation, enabling mapping of both infection risk and rattiness. This can be useful for identifying high-risk areas and targeting control. One inherent limitation is its dependence on the availability of spatially continuous environmental variables and abundance data, both of which are prone to high measurement error. This can result in high uncertainty in the model parameter estimates and predictions, as demonstrated by the wider confidence intervals for risk factors in the joint model compared to the standard mixed-effects logistic regression analysis. An additional benefit of the geostatistical structure is that abundance measurements do not have to be taken at the household location, providing some flexibility in the design of eco-epidemiological studies and indices used. The framework may have important applications beyond the study of zoonotic spillover, with the rattiness component replaced by other exposure measures for example mosquito density or ecological indices (such as pollution, where there are multiple, related measures of air or groundwater quality) to model associations with human or animal health outcomes.
In conclusion, we have developed a framework that may have broad applications in delineating complex animal-environment-human interactions during zoonotic spillover and identifying opportunities for public health intervention. We demonstrate its potential by applying it to Leptospira in an urban setting, finding evidence that the extent to which local rat shedding drives spillover transmission is moderated by elevation, most likely a proxy for water runoff. Future work examining these transmission mechanisms in similar settings and across different time points will be key to establishing how generalisable these results are.
Appendix 1
Functional form of continuous explanatory variables
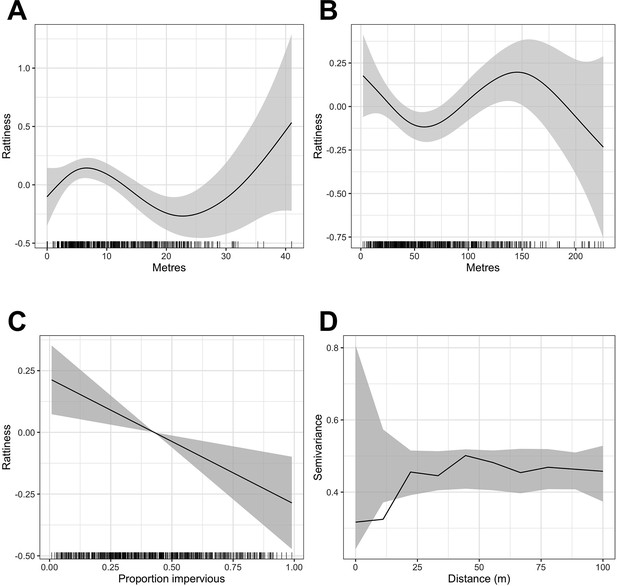
Generalized Additive Model (GAM) partial dependence plots for the unstructured random variation in rattiness,, plotted against the continuous explanatory variables considered in the analysis (shaded areas correspond to 95% confidence intervals).
(A) elevation relative to the bottom of valley, (B) distance to large refuse piles, (C) impervious land cover in 20 m radius buffer around sampling point. are estimated using a non-spatial model which excludes all covariates. (D) is a variogram computed from using a non-spatial model that includes all of the covariates; the dashed lines correspond to 95% confidence intervals under the assumption of spatial independence.
A single knot point for the distance to refuse piles variable was chosen at 50 m to account for the expected decay in the effect of food resources up to a rat home range distance, beyond which little effect would be expected. We did not include an additional knot point at 145 m despite there being a visible change in gradient in Appendix 1—figure 1 - panel B for two reasons. Firstly, the home range of Norway rats is estimated to be less than 100 m in these urban settings (Feng and Himsworth, 2014; Davis et al., 1948; Byers et al., 2019), meaning that rat abundance is very unlikely to be affected by the availability of anthropogenic food sources beyond this distance, particularly given the high availability of food across the study area. Secondly, we could offer no scientific rationale for why rattiness would start to increase again beyond 50 m before peaking at a very large distance of 145 m from a refuse pile and decreasing thereafter.
In contrast, the mechanisms by which rattiness varies with elevation are more complex, with significant changes in the environment occurring at different elevations. For example, the relationship identified in Appendix 1—figure 1 - panel A can be explained by the high risk of flooding at the bottom of the valley, which carries resources down to lower elevations (resulting in a peak of rattiness at about 7 m) but makes the very lowest elevations unsuitable for rat burrows. The highest elevations in our study area are close to a main road with food markets where large quantities of food waste are left out in the street for collection. Although there is large uncertainty about this relationship, it is highly possible that this may be driving the positive relationship between 23 m and 40 m.
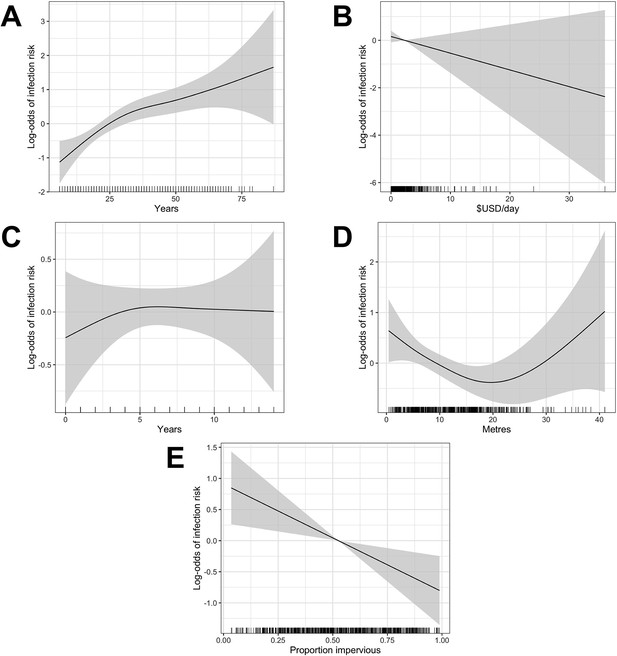
Generalized Additive Model (GAM) partial dependence plots for human infection risk plotted against the continuous explanatory variables considered in this analysis (shaded areas correspond to 95% confidence intervals).
(A) age, (B) household per capita income (in USD), (C) years of education, (D) household elevation relative to the bottom of valley, (E) impervious land cover in 20 m radius buffer around household.
Single knot points were considered for age at 30 years (Appendix 1—figure 2 - panel A), education at 5 years (Appendix 1—figure 2 - panel C) and relative elevation at 20 metres (Appendix 1—figure 2 - panel D) based on the value of the explanatory variable at which the gradient of the relationship changed in these plots.
Appendix 2
Model selection tables
AIC fit of the five highest ranked multivariable rattiness models (’+’ indicates that a variable was selected in the model).
AIC fit of the five highest ranked multivariable human infection models (’+’ indicates that a variable was selected in the model).
| Model | Age (0–30) | Age (>30) | Sex | Valley | Floodwater | Income | Land cover | Salesperson | Elevation level | Rattiness | Ratt:Elev | df* | AICc * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | + | + | + | + | + | + | + | + | + | + | 16 | 523.14 | |
| M2 | + | + | + | + | + | + | + | + | + | 15 | 523.52 | ||
| M3 | + | + | + | + | + | + | + | + | + | + | + | 17 | 523.72 |
| M4 | + | + | + | + | + | + | + | + | + | + | 16 | 524.11 | |
| M5 | + | + | + | + | + | + | + | + | + | 14 | 525.04 | ||
| M*† | + | + | + | + | + | + | + | + | 13 | 532.13 |
-
*
df, degrees of freedom; AICc, corrected Akaike Information Criterion
-
†
Model M* was ranked outside of the top 5 models but is included here for reference to demonstrate the improvement in model fit when rattiness is included.
Appendix 3
Exploratory multivariable analysis of human risk factors
Multivariable mixed effects logistic regression analysis of risk factors for leptospiral infection in community members.
Note: there was missing information for the contact with floodwater question for two individuals and consequently only 1399 participants from 668 households were included in this analysis.
| Variable | OR (95% CI) |
|---|---|
| Demographic and social status | |
| Age (per year)* | |
| 0–30 years old | 1.10 (1.04, 1.16) |
| >30 years old | 1.02 (0.96, 1.09) |
| Male gender | 2.90 (1.59, 5.28) |
| Daily per capita household income (US$/day) | 0.93 (0.81, 1.06) |
| Valley | |
| 1 | REF |
| 2 | 3.91 (1.33, 11.68) |
| 3 | 2.26 (0.74, 6.93) |
| Household environment | |
| Relative elevation level | |
| High (>15.6 m) | REF |
| Medium (6.7–15.6 m) | 0.71 (0.30, 1.70) |
| Low (0–6.7 m) | 1.08 (0.44, 2.62) |
| Occupational exposures | |
| Work as travelling salesperson † | 3.38 (0.77, 14.87) |
| Behavioural exposures | |
| Contact with floodwater in last 6 months | |
| Never/rarely | REF |
| Sometimes | 0.64 (0.28, 1.43) |
| Frequently | 2.48 (1.02, 6.02) |
| Rattiness | |
| Rattiness at high elevation level (per unit rattiness) | 6.92 (1.88, 25.47) |
| Elevation level: Low × rattiness | 0.10 (0.02, 0.62) |
| Elevation level: Medium × rattiness | 0.15 (0.02, 0.91) |
| (variance of household random effect) | 1.78 |
-
*
The effect of age is modelled as a broken linear model with a transition at 30 years old, as informed by the relationship described by Generalized Additive Modelling (Appendix 1—figure 2).
-
†
Binary variable with reference category of ‘no occupational exposure’.
Appendix 4
Model fitting
To fit the joint model, we proceed as follows. Let and and be the vector of unknown parameters associated with and . The likelihood function is then given by
The integral in Equation 5 cannot be solved analytically so we approximate it using Monte Carlo methods. Specifically, let and be our initial best guesses for θ and ω, respectively. Since we re-write the integral in Equation 5 using an importance sampling distribution to give
where the expectation is taken with respect to the distribution of .
Based on Equation 6, we then approximate Equation 5 with
where is the -th sample from . To obtain the maximum likelihood estimates for θ and ω, we maximize Equation 7 using numerical optimization. To simulate from , we use the Laplace sampling algorithm described in detail by Christensen, 2004 and Giorgi and Diggle, 2017. We draw 110,000 samples from , with a burn in of 10,000 samples and thin by 10%, leaving 10,000 MCMC samples.
To improve the approximation of the likelihood function, we also update our guesses and by plugging their estimated values into the denominator of Equation 7 and iterate its maximization until convergence.
Appendix 5
Baseline cohort characteristics
Summary of demographic, socioeconomic and environmental risk factors.
| Variable | No. or Median (% or IQR) * |
|---|---|
| Demographic and social status | |
| Age (years) | 27 (15–41) |
| Male gender | 597 (42.6%) |
| Daily per capita household income (US$/day) | 1.6 (0.8–2.8) |
| Valley 1 | 259 (18.5%) |
| Valley 2 | 557 (39.8%) |
| Valley 3 | 585 (41.8%) |
| Literacy | 1125 (80.3%) |
| Education (years) | 6 (4-9) |
| Household environment | |
| Impervious land cover (%) | 49.6 (35.1–70.6) |
| Relative elevation (metres) | 11.0 (5.9–16.3) |
| Elevation level | |
| Low (0–6.7 m) | 474 (33.8%) |
| Medium (6.7–15.6 m) | 524 (37.4%) |
| High (>15.6 m) | 403 (28.8%) |
| Open sewer within 10 m | 926 (66.1%) |
| Unprotected from open sewer | 666 (47.6%) |
| Live on hillside | 453 (32.4%) |
| Occupational exposures | |
| Work in construction | 105 (7.5%) |
| Work as travelling salesperson | 24 (1.7%) |
| Work in refuse collection | 61 (4.4%) |
| Work involves contact with mud | 27 (1.9%) |
| Work involves contact with floodwater | 23 (1.6%) |
| Work involves contact with sewer water | 16 (1.1%) |
| Behavioural exposures | |
| Contact with floodwater in last 6 months | |
| Never/rarely | 986 (70.5%) |
| Sometimes | 299 (21.4%) |
| Frequently | 114 (8.1%) |
| Contact with sewer water in last 6 months | |
| Never/rarely | 1120 (80.2%) |
| Sometimes | 180 (12.9%) |
| Frequently | 97 (6.9%) |
-
*
No., number; IQR, interquartile range; Percentages are calculated without missing values. All variables had ≤ 5 missing values.
Appendix 6
Sensitivity analysis for disturbed trap modelling assumption
In the rattiness-infection framework we assumed that a trap was disturbed when it was found closed without a rat and set (see ‘Rat abundance outcomes’) in the equation for the probability of capturing a rat
This occurred in 554 (36.6%) out of 1,512 trapping-days. To ascertain the potential impact of this on model parameter estimates we conducted a sensitivity analysis as follows:
Draw values for t from for all trap observations that were found closed.
Fit a simplified rattiness model with covariates that did not account for spatial correlation by setting in Equation 2 in ‘Rattiness’.
Repeat steps 1–2 a total of 1,000 times.
Estimate the between-imputation standard error for each parameter, defined as:
for imputation of a total imputed datasets.
The results for each parameter can be seen in Appendix 6—table 1 below. Estimated between-imputation standard errors were small relative to parameter estimates, indicating that uncertainty due to the missing trap disturbance information is unlikely to have significantly affected parameter estimates in the full rattiness-infection model.
Trap disturbance sensitivity analysis: non-spatial rattiness model parameter estimates and between-imputation standard errors.
| Parameter | Estimate | |
|---|---|---|
| –2.8274 | 0.0128 | |
| –1.9058 | 0.0004 | |
| –1.3794 | 0.0008 | |
| –2.8617 | 0.0027 | |
| –2.1538 | 0.0023 | |
| 0.7010 | 0.0120 | |
| 2.4016 | 0.0004 | |
| 1.3820 | 0.0008 | |
| 2.6704 | 0.0031 | |
| 2.6431 | 0.0036 | |
| Relative elevation (per 1 m increase)2 | ||
| 0–8 m | 0.0525 | 0.0001 |
| 8–22 m | –0.0583 | 0.0001 |
| >22 m | 0.1112 | 0.0002 |
| Distance to large refuse piles (per 10 m increase)3 | ||
| 0–50 m | –0.1090 | 0.0002 |
| >50 m | 0.0405 | 0.0001 |
| Impervious land cover (per 10% increase) | –0.0592 | 0.0001 |
Appendix 7
Residual diagnostics
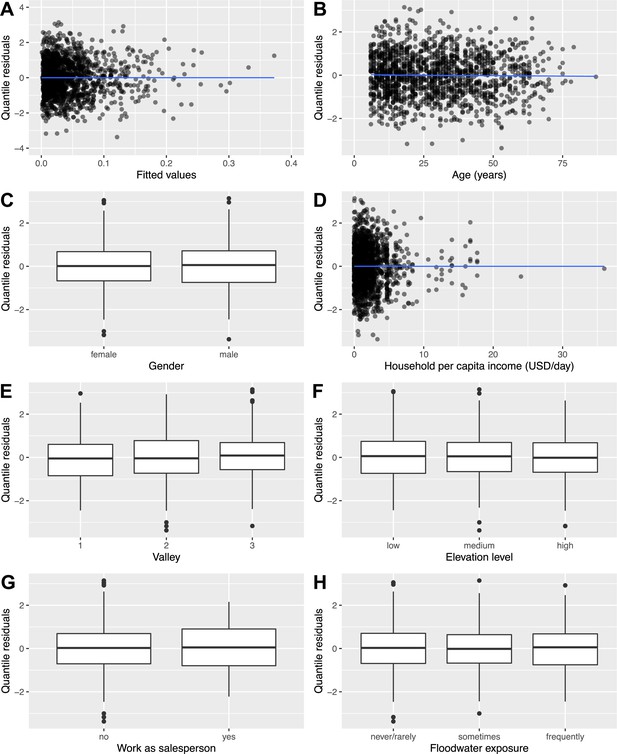
To examine the fit of the full rattiness-infection model to the human infection data, a formal diagnostic investigation was conducted using randomized quantile residuals (Dunn and Smyth, 1996; Smyth et al., 2021). The residual plots in Appendix 7—figure 1 exhibit no trends between quantile residuals and fitted values (Panel A) or variables in the model (Panels B-H).
Appendix 8
Model building guidance
To help guide the model building process for future users of the rattiness-infection framework we outline the following key steps (to be viewed with the available R code at https://github.com/maxeyre/Rattiness-infection-framework):
Set up the rat (or any other animal reservoir) component of the model [script: 1-rat-explore.R]:
Fit the non-spatial rattiness model with no explanatory variables and predict rattiness at all sampled rat locations.
Explore the relationship between predicted mean rattiness and explanatory variables using Generalized Additive Models (GAMs) to decide on their functional form. Note: all variables considered must also be measured at household locations
Conduct model selection using a linear model with mean predicted rattiness as the dependent variable.
Fit the non-spatial rattiness model with selected explanatory variables and compute the empirical variogram to check for evidence of residual spatial autocorrelation. If the variogram shows no signs of residual correlation, consider confirming this result by fitting the model in the following step - the estimated scale of spatial correlation should be close to zero (it may also not be able to estimate its value, with the value changing considerably between iterations).
Fit the spatial rattiness model with selected explanatory variables and predict mean rattiness at household locations to create an exploratory rattiness variable. To improve model convergence use parameter estimates from the non-spatial model as the first guess for the parameters and repeat model fitting by plugging in previous estimates.
Set up the human infection component of the model [script: 2-human-explore.R]:
Explore the relationship between infection risk and explanatory variables using Generalized Additive Models (GAMs) to decide on their functional form.
Conduct model selection
Explore the relationship between infection risk and mean predicted rattiness using Generalized Additive Models (GAMs) and consider interactions where relevant.
Test for residual spatial correlation after controlling for selected variables and mean predicted rattiness (we recommend using the PrevMap package).
Using the joint rattiness-infection framework:
Fit the joint model [script: 3-fit-joint-model.R]. In the ‘control’ file, the inclusion of a household-level random effect (or ‘nugget’) and an additional spatial Gaussian process (if there was evidence of residual spatial correlation after controlling for explanatory variables and rattiness in the previous step) in the human linear predictor can be controlled. We recommend monitoring parameter estimates from each iteration to assess how well the model is converging. If parameters for the spatial Gaussian processes are not converging then this may indicate that the data do not support the inclusion of an additional spatial Gaussian process in the human component and a simpler model should be considered. This model fitting process can be time consuming and ideally should be run on a high-end computing network.
Conduct a residual diagnostic analysis for the full rattiness-infection model [script: 5-revision subanalyses.R].
Bootstrap to estimate uncertainty in parameter estimates [script: 3-fit-joint-model.R]
Create prediction maps for rattiness, infection risk and spatial Gaussian processes (if required) [script: 4-spatial-prediction.R]. The prediction grid for your study area must include values for all variables included in the model.
Data availability
Rat and human data analysed in this study have been deposited in OSF (https://doi.org/10.17605/OSF.IO/AQZ2Y). However, household coordinates and valley ID have been removed from the human data to ensure participant anonymity. Modelling functions, R scripts and metadata for analyses in this manuscript are publicly available at https://github.com/maxeyre/Rattiness-infection-framework, (copy archived at swh:1:rev:e7953d38269ce97221dbdd83c0be2c65d92dff40).
-
Open Science FrameworkLinking rattiness, geography and environmental degradation to spillover Leptospira infections in marginalised urban settings: Data sources.https://doi.org/10.17605/OSF.IO/AQZ2Y
References
-
Socio-environmental determinants of the leptospirosis outbreak of 1996 in Western Rio de Janeiro: a geographical approachInternational Journal of Environmental Health Research 10:301–313.https://doi.org/10.1080/0960312002001500
-
SoftwareMu-min: multi-model inference, version 0.2R Package Version.
-
SoftwareThe lme4 package, version 2.0R Package Version.
-
Dynamic and integrative approaches to understanding pathogen spilloverPhilosophical Transactions of the Royal Society of London. Series B, Biological Sciences 374:1–7.https://doi.org/10.1098/rstb.2019.0014
-
A case control study to explore the risk factors for acquisition of leptospirosis in Surat City, after floodIndian Journal of Medical Sciences 62:431–438.https://doi.org/10.4103/0019-5359.48454
-
Leptospira infection in rats: a literature review of global prevalence and distributionPLOS Neglected Tropical Diseases 13:e0007499.https://doi.org/10.1371/journal.pntd.0007499
-
Transmission ecology of rodent-borne diseases: new frontiersIntegrative Zoology 10:424–435.https://doi.org/10.1111/1749-4877.12149
-
Seroprevalence, risk factors, and rodent reservoirs of leptospirosis in an urban community of Puerto Rico, 2015The Journal of Infectious Diseases 220:1489–1497.https://doi.org/10.1093/infdis/jiz339
-
Rats about town: a systematic review of rat movement in urban ecosystemsFrontiers in Ecology and Evolution 7:13.https://doi.org/10.3389/fevo.2019.00013
-
Genetic evidence for a potential environmental pathway to spillover infection of rat-borne leptospirosisThe Journal of Infectious Diseases 225:130–134.https://doi.org/10.1093/infdis/jiab323
-
ReportIntegrated Pest Management: Conducting Urban Rodent SurveysUS Department of Health and Human Services.
-
Monte Carlo maximum likelihood in model-based geostatisticsJournal of Computational and Graphical Statistics 13:702–718.https://doi.org/10.1198/106186004X2525
-
Influence of household rat infestation on Leptospira transmission in the urban slum environmentPLOS Neglected Tropical Diseases 8:e3338.https://doi.org/10.1371/journal.pntd.0003338
-
Global morbidity and mortality of leptospirosis: a systematic reviewPLOS Neglected Tropical Diseases 9:e0003898.https://doi.org/10.1371/journal.pntd.0003898
-
Household rat infestation in urban slum populations: development and validation of a predictive score for leptospirosisPLOS Neglected Tropical Diseases 15:e0009154.https://doi.org/10.1371/journal.pntd.0009154
-
The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infectionPLOS Neglected Tropical Diseases 11:e0005429.https://doi.org/10.1371/journal.pntd.0005429
-
Fluctuating rodent populations and risk to humans from rodent-borne zoonosesVector Borne and Zoonotic Diseases 5:305–314.https://doi.org/10.1089/vbz.2005.5.305
-
Evaluation on the effectiveness of actions for controlling infestation by rodents in campo limpo region, Sao Paulo municipality, BrazilInternational Journal of Environmental Health Research 19:291–304.https://doi.org/10.1080/09603120802592723
-
Randomized quantile residualsJournal of Computational and Graphical Statistics 5:236–244.https://doi.org/10.1080/10618600.1996.10474708
-
Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in guinea, West AfricaVector Borne and Zoonotic Diseases 7:119–128.https://doi.org/10.1089/vbz.2006.0520
-
PrevMap: an R package for prevalence mappingJournal of Statistical Software 78:1–29.https://doi.org/10.18637/jss.v078.i08
-
Leptospirosis: risk factors and management challenges in developing countriesResearch and Reports in Tropical Medicine 7:49–62.https://doi.org/10.2147/RRTM.S102543
-
Influence of rainfall on Leptospira infection and disease in a tropical urban setting, BrazilEmerging Infectious Diseases 26:311–314.https://doi.org/10.3201/eid2602.190102
-
Spatiotemporal determinants of urban leptospirosis transmission: four-year prospective cohort study of slum residents in BrazilPLOS Neglected Tropical Diseases 10:e0004275.https://doi.org/10.1371/journal.pntd.0004275
-
Generalized additive models: some applicationsJournal of the American Statistical Association 82:371–386.https://doi.org/10.1080/01621459.1987.10478440
-
Spatial analysis of Leptospira infection in muskrats in lower saxony, Germany, and the association with human leptospirosisResearch in Veterinary Science 114:351–354.https://doi.org/10.1016/j.rvsc.2017.06.015
-
Risk factors for clinical leptospirosis from Western JamaicaThe American Journal of Tropical Medicine and Hygiene 83:633–636.https://doi.org/10.4269/ajtmh.2010.09-0609
-
Poverty, sanitation, and Leptospira transmission pathways in residents from four Brazilian slumsPLOS Neglected Tropical Diseases 15:e0009256.https://doi.org/10.1371/journal.pntd.0009256
-
High prevalence of Leptospira spp in sewer rats (Rattus norvegicus)Epidemiology and Infection 137:1586–1592.https://doi.org/10.1017/S0950268809002647
-
Climate change, flooding, urbanisation and leptospirosis: fuelling the fire?Transactions of the Royal Society of Tropical Medicine and Hygiene 104:631–638.https://doi.org/10.1016/j.trstmh.2010.07.002
-
ReportAVMA guidelines for the euthanasia of animalsAmerican Veterinary Medical Association.
-
Environmental and behavioural determinants of leptospirosis transmission: a systematic reviewPLOS Neglected Tropical Diseases 9:e0003843.https://doi.org/10.1371/journal.pntd.0003843
-
Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysisJournal of Medical Entomology 33:711–720.https://doi.org/10.1093/jmedent/33.5.711
-
Fine-Scale GPs tracking to quantify human movement patterns and exposure to leptospires in the urban slum environmentPLOS Neglected Tropical Diseases 12:e0006752.https://doi.org/10.1371/journal.pntd.0006752
-
Pathways to zoonotic spilloverNature Reviews. Microbiology 15:502–510.https://doi.org/10.1038/nrmicro.2017.45
-
SoftwareR: a language and environment for statistical computing, version 1.0.1R Foundation for Statistical Computing, Vienna, Austria.
-
Impact of environment and social gradient on Leptospira infection in urban slumsPLOS Neglected Tropical Diseases 2:e0000228.https://doi.org/10.1371/journal.pntd.0000228
-
Quantification of pathogenic Leptospira in the soils of a Brazilian urban slumPLOS Neglected Tropical Diseases 12:e0006415.https://doi.org/10.1371/journal.pntd.0006415
-
Global burden of leptospirosis: estimated in terms of disability adjusted life yearsPLOS Neglected Tropical Diseases 9:e0004122.https://doi.org/10.1371/journal.pntd.0004122
-
ReportLeptospirosis burden epidemiology reference group (LERG)Geneva: World Health Organization.
-
SoftwareThe tidyverse, version 1.1R Package.
-
Combining mosquito vector and human disease data for improved assessment of spatial West Nile virus disease riskThe American Journal of Tropical Medicine and Hygiene 78:654–665.https://doi.org/10.4269/ajtmh.2008.78.654
-
SoftwarePackage ‘ mgcv, version 1.8-40R Package.
Article and author information
Author details
Funding
National Institutes of Health (F31 AI114245)
- Albert I Ko
National Institutes of Health (R01 AI052473)
- Albert I Ko
National Institutes of Health (U01 AI088752)
- Albert I Ko
National Institutes of Health (R01 TW009504)
- Albert I Ko
National Institutes of Health (R25 TW009338)
- Albert I Ko
Medical Research Council (964635)
- Max T Eyre
Wellcome Trust (102330/Z/13/Z)
- Nivison Nery
- Federico Costa
Fundação Oswaldo Cruz
- Federico Costa
Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB/JCB0020/2016)
- Fábio N Souza
- Federico Costa
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. For the purpose of Open Access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Acknowledgements
We thank the residents and community leaders of Pau da Lima community for their support and participation in this study. This work was supported by the Oswaldo Cruz Foundation and Secretariat of Health Surveillance, Brazilian Ministry of Health, the National Institutes of Health of the United States, the Wellcome Trust and by the Fundação de Amparo à Pesquisa do Estado da Bahia. MTE was supported by a UK Research and Innovation (UKRI) doctorate studentship. FNS was supported by a FAPESB doctorate scholarship.
Ethics
Human subjects: Participants were enrolled according to written informed consent procedures approved by the Institutional Review Boards of the Oswaldo Cruz Foundation and Brazilian National Commission for Ethics in Research, Brazilian Ministry of Health (CAAE: 01877912.8.0000.0040) and Yale University School of Public Health (HIC 1006006956).
For the rats captured in the rat ecology study, the ethics committee for the use of animals from the Oswaldo Cruz Foundation, Salvador, Brazil, approved the protocols used (protocol number 003/2012), which adhered to the guidelines of the American Society of Mammalogists for the use of wild mammals in research (Sikes and Gannon, 2011) and the guidelines of the American Veterinary Medical Association for the euthanasia of animals (Leary et al., 2013). These protocols were also approved by Yale University's Institutional Animal Care and Use Committee (IACUC), New Haven, Connecticut (protocol number 2012-11498).
Copyright
© 2022, Eyre et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 981
- views
-
- 182
- downloads
-
- 5
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
- Microbiology and Infectious Disease
Background:
In many settings, a large fraction of the population has both been vaccinated against and infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hence, quantifying the protection provided by post-infection vaccination has become critical for policy. We aimed to estimate the protective effect against SARS-CoV-2 reinfection of an additional vaccine dose after an initial Omicron variant infection.
Methods:
We report a retrospective, population-based cohort study performed in Shanghai, China, using electronic databases with information on SARS-CoV-2 infections and vaccination history. We compared reinfection incidence by post-infection vaccination status in individuals initially infected during the April–May 2022 Omicron variant surge in Shanghai and who had been vaccinated before that period. Cox models were fit to estimate adjusted hazard ratios (aHRs).
Results:
275,896 individuals were diagnosed with real-time polymerase chain reaction-confirmed SARS-CoV-2 infection in April–May 2022; 199,312/275,896 were included in analyses on the effect of a post-infection vaccine dose. Post-infection vaccination provided protection against reinfection (aHR 0.82; 95% confidence interval 0.79–0.85). For patients who had received one, two, or three vaccine doses before their first infection, hazard ratios for the post-infection vaccination effect were 0.84 (0.76–0.93), 0.87 (0.83–0.90), and 0.96 (0.74–1.23), respectively. Post-infection vaccination within 30 and 90 days before the second Omicron wave provided different degrees of protection (in aHR): 0.51 (0.44–0.58) and 0.67 (0.61–0.74), respectively. Moreover, for all vaccine types, but to different extents, a post-infection dose given to individuals who were fully vaccinated before first infection was protective.
Conclusions:
In previously vaccinated and infected individuals, an additional vaccine dose provided protection against Omicron variant reinfection. These observations will inform future policy decisions on COVID-19 vaccination in China and other countries.
Funding:
This study was funded the Key Discipline Program of Pudong New Area Health System (PWZxk2022-25), the Development and Application of Intelligent Epidemic Surveillance and AI Analysis System (21002411400), the Shanghai Public Health System Construction (GWVI-11.2-XD08), the Shanghai Health Commission Key Disciplines (GWVI-11.1-02), the Shanghai Health Commission Clinical Research Program (20214Y0020), the Shanghai Natural Science Foundation (22ZR1414600), and the Shanghai Young Health Talents Program (2022YQ076).
-
- Epidemiology and Global Health
Background:
The role of circulating metabolites on child development is understudied. We investigated associations between children’s serum metabolome and early childhood development (ECD).
Methods:
Untargeted metabolomics was performed on serum samples of 5004 children aged 6–59 months, a subset of participants from the Brazilian National Survey on Child Nutrition (ENANI-2019). ECD was assessed using the Survey of Well-being of Young Children’s milestones questionnaire. The graded response model was used to estimate developmental age. Developmental quotient (DQ) was calculated as the developmental age divided by chronological age. Partial least square regression selected metabolites with a variable importance projection ≥1. The interaction between significant metabolites and the child’s age was tested.
Results:
Twenty-eight top-ranked metabolites were included in linear regression models adjusted for the child’s nutritional status, diet quality, and infant age. Cresol sulfate (β=–0.07; adjusted-p <0.001), hippuric acid (β=–0.06; adjusted-p <0.001), phenylacetylglutamine (β=–0.06; adjusted-p <0.001), and trimethylamine-N-oxide (β=–0.05; adjusted-p=0.002) showed inverse associations with DQ. We observed opposite directions in the association of DQ for creatinine (for children aged –1 SD: β=–0.05; pP=0.01;+1 SD: β=0.05; p=0.02) and methylhistidine (–1 SD: β = - 0.04; p=0.04;+1 SD: β=0.04; p=0.03).
Conclusions:
Serum biomarkers, including dietary and microbial-derived metabolites involved in the gut-brain axis, may potentially be used to track children at risk for developmental delays.
Funding:
Supported by the Brazilian Ministry of Health and the Brazilian National Research Council.