Alasemenia, the earliest ovule with three wings and without cupule
eLife assessment
This useful study describes the second earliest known winged ovule without a capule in the Famennian of Late Devonian. Using solid mathematical analysis, the authors demonstrate that three-winged seeds are more adapted to wind dispersal than one-, two- and four-winged seeds. The manuscript will help the scientific community to understand the origin and early evolutionary history of wind dispersal strategy of early land plants.
https://doi.org/10.7554/eLife.92962.3.sa0Useful: Findings that have focused importance and scope
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Solid: Methods, data and analyses broadly support the claims with only minor weaknesses
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
The ovules or seeds (fertilized ovules) with wings are widespread and especially important for wind dispersal. However, the earliest ovules in the Famennian of the Late Devonian are rarely known about the dispersal syndrome and usually surrounded by a cupule. From Xinhang, Anhui, China, we now report a new taxon of Famennian ovules, Alasemenia tria gen. et sp. nov. Each ovule of this taxon possesses three integumentary wings evidently extending outwards, folding inwards along abaxial side and enclosing most part of nucellus. The ovule is borne terminally on smooth dichotomous branches and lacks a cupule. Alasemenia suggests that the integuments of the earliest ovules without a cupule evolved functions in probable photosynthetic nutrition and wind dispersal. It indicates that the seed wing originated earlier than other wind dispersal mechanisms such as seed plume and pappus, and that three- or four-winged seeds were followed by seeds with less wings. Mathematical analysis shows that three-winged seeds are more adapted to wind dispersal than seeds with one, two or four wings under the same condition.
eLife digest
Many plants need seeds to reproduce. Seeds come in all shapes and sizes and often have extra features that help them disperse in the environment. For example, some seeds develop wings from seed coat as an outer layer, similar to fruits of sycamore trees that have two wings to help them glide in the wind.
The first seeds are thought to have evolved around 372-359 million years ago in a period known as the Famennian (belonging to the Late Devonian). Fossil records indicate that almost all these seeds were surrounded by an additional protective structure known as the cupule and did not have wings. To date, only two groups of Famennian seeds have been reported to bear wings or wing-like structures, and one of these groups did not have cupules. These Famennian seeds all had four wings.
Wang et al. examined fossils of seed plants collected in Anhui province, China, which date to the Famennian period. The team identified a new group of seed plants named the Alasemenia genus. The seeds of these plants each had three wings but no cupules. The seeds formed on branches that did not have any leaves, which indicates the seeds may have performed photosynthesis (the process by which plants generate energy from sunlight). Mathematical modelling suggested that these three-winged seeds were better adapted to being dispersed by the wind than other seeds with one, two or four wings.
These findings suggest that during the Famennian the outer layer of some seeds that lacked cupules evolved wings to help the seeds disperse in the wind. It also indicates that seeds with four or three wings evolved first, followed by other groups of seed plants with fewer seed wings. Future studies may find more winged seeds and further our understanding of their evolutionary roles in the early history of seed plants.
Introduction
Since plants colonized the land, wind dispersal (anemochory) became common with the seed wing representing a key dispersal strategy through geological history (Taylor et al., 2009; Ma, 2009; McLoughlin and Pott, 2019). Winged seeds evolved numerous times in many lineages of extinct and extant seed plants (spermatophytes) (Schenk, 2013; Stevenson et al., 2015). Lacking wings as integumentary outgrowths, the earliest ovules in the Famennian (372–359 million years ago [Ma], Late Devonian) rarely played a role in wind dispersal (Rowe, 1997). Furthermore, nearly all Famennian ovules are cupulate, i.e., borne in a protecting and pollinating cupule (Prestianni et al., 2013; Meyer-Berthaud et al., 2018).
Warsteinia was a Famennian ovule with four integumentary wings, but its attachment and cupule remain unknown (Rowe, 1997). Guazia was a Famennian ovule with four wings and it is terminally borne and acupulate (devoid of cupule) (Wang et al., 2022). This paper documents a new Famennian seed plant with ovule, Alasemenia tria gen. et sp. nov. It occurs in Jianchuan mine of China, where Xinhang fossil forest was discovered to comprise in situ lycopsid trees of Guangdedendron (Wang et al., 2019). The terminally borne ovules are three-winged and clearly acupulate, thus implying additional or novel functions of integument. Based on current fossil evidence and mathematical analysis, we discuss the evolution of winged seeds and compare the wind dispersal of seeds with different number of wings.
Results
Locality, stratigraphy, and material
All fossils came from Upper Devonian Wutong Formation at Jianchuan mine in Xinhang town, Guangde City, Anhui Province, China. Details on locality are available in previous works (Wang et al., 2019; Xu et al., 2022). At Jianchuan mine, Wutong Formation consists of Guanshan Member with quartzose sandstone and a little mudstone, and the overlying Leigutai Member with inter-beds of quartzose sandstone, siltstone and mudstone. Spore analysis indicates that the Leigutai Member here is late Famennian in age (Gao et al., 2023). Progymnosperm Archaeopteris and lycopsid Leptophloeum occur in Leigutai and/or Guanshan members, and they were distributed worldwide in the Late Devonian (Taylor et al., 2009). Fernlike plant Xinhangia (Yang and Wang, 2022) and lycopsid Sublepidodendron (Xu et al., 2022) were found at the basal part of Leigutai Member. In situ lycopsid trees of Guangdedendron with stigmarian rooting system appear in multiple horizons of Leigutai Member and they formed the Xinhang forest (Wang et al., 2019; Gao et al., 2022). From many horizons of siltstone and mudstone of Wutong Formation (Leigutai Member) at Jianchuan mine, numerous ovules of Alasemenia were collected (Figure 1, Figure 2, Figure 3) and some were transversely sectioned to show the ovular structure (Figure 4, Figure 4—figure supplement 1, Figure 4—figure supplement 2, Figure 4—figure supplement 3, Figure 4—figure supplement 4, Figure 4—figure supplement 5).
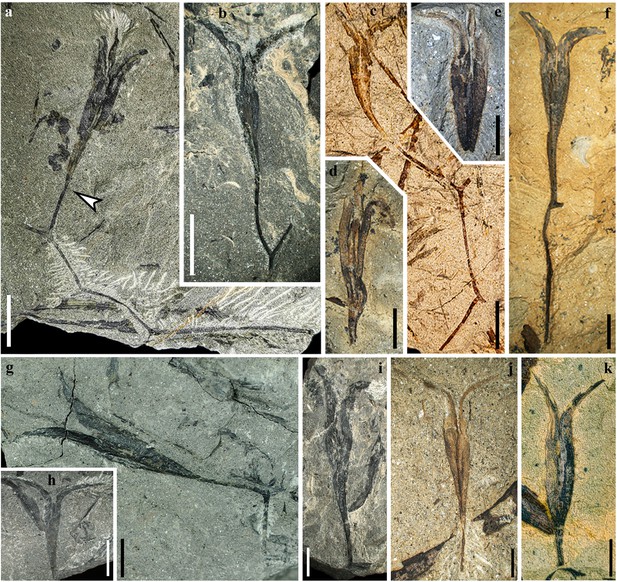
Fertile branches and seeds of Alasemenia tria gen. et sp. nov.
(a) Thrice dichotomous branch with a terminal ovule. Arrow indicating boundary between ovule and ultimate axis (PKUB21721a). (b, f, g, i) Once dichotomous branch with a terminal ovule (PKUB21781, PKUB23132, PKUB19338a, PKUB17899). (c) Twice dichotomous branch with a terminal ovule (PKUB19713a). (d, e) Ovule with three integumentary wings (PKUB19321, PKUB19316). (h) Ovule showing two integumentary wings (PKUB19282). (j, k) Ovule terminating short ultimate axis (PKUB23114, PKUB23129). Scale bars, 1 cm (a–c, h), 5 mm (d–g, i–k).

Fertile branches and seeds of Alasemenia tria gen. et sp. nov.
(a–c) Once dichotomous branch with a terminal ovule (PKUB16876a, b, PKUB17767). a, b, Part and counterpart. (d, e) Part and counterpart, arrow showing the third integumentary wing (PKUB19322a, b). (f) Ovule on ultimate axis (PKUB21752). (g, h, k–m) Ovules lacking ultimate axis (PKUB16788, PKUB21631, PKUB16522, PKUB21647, PKUB21656). (i, j) Part and counterpart, showing limit (arrows) between nucellus and integument (PKUB19339a, b). (n) Four detached ovules (arrows 1–4) (PKUB19331). (o) Enlarged ovule in n (arrow 2), showing three integumentary wings (arrows). Scale bars, 1 cm (n), 5 mm (a–h, k–m, o), 2 mm (i, j).

Seeds of Alasemenia tria gen. et sp. nov.
(a, b) Part and counterpart, enlarged ovule in Figure 1a (PKUB21721a, b). (c) Enlarged ovule in Figure 1c. (d) Counterpart of ovule in c (PKUB19713b). (e) Dégagement of ovule in d, exposing the base of the third integumentary wing (arrow). (f) Enlarged ovule in Figure 1d. (g, h) Enlarged ovule in Figure 2i and j, respectively. Scale bars, 5 mm (a–e), 2 mm (f–h).

Transverse sections of seeds of Alasemenia tria gen. et sp. nov.
(a, b) Part and counterpart. (c–e) Sections of seed in a and b (at three lines, in ascending orders). Arrow in d indicating probable nucellar tip (Slide PKUBC17913-12b, 10a, 9b). (f, g) Part and counterpart. (h–k) Sections of seed in f and g (at four lines, in ascending orders) (Slide PKUBC19798-8b, 6b, 4a, 4b). (l, m) Part and counterpart. (n–r) Sections of seed in l and m (at five lines, in ascending orders), showing three wings departing centrifugally (Slide PKUBC17835-5a, 7b, 8b, 9a, 10a). (s, v, A), One seed sectioned. (t, u) Sections of seed in s (at two lines, in ascending orders) (Slide PKUBC18716-8b, 7a). (w–z) Sections of seed in v (at four lines, in ascending orders) (Slide PKUBC20774-7a, 6b, 3a, 3b). (B–E) Sections of seed in A (at four lines, in ascending orders), showing three wings departing centrifugally (Slide PKUB17904-5b, 4a, 4b, 3b). Scale bars, 2 mm (a, b, f, g, l, m, s, v, A), 1 mm (c–e, h–k, n–r, t, u, w–z, B–E).
Systematic palaeontology
Division Spermatophyta
Order and family incertae sedis
Alasemenia tria gen. et sp. nov.
Etymology
The generic name from the Latin ‘ala’ and ‘semen’, meaning wing and seed, respectively; the specific epithet from the Latin ‘tri’ (three), referring to wing number of a seed.
Holotype designated here
PKUB21721a, b (part and counterpart housed in Department of Geology, Peking University, Beijing) (Figure 1a, m and n).
Locality and horizon
Xinhang, Guangde, Anhui, China; Leigutai Member of Wutong Formation, Upper Devonian.
Diagnosis
Dichotomous branches bearing terminal and acupulate ovules. Three broad wing-like integumentary lobes radially and symmetrically attached to each nucellus, distally tapered and proximally reduced. Integumentary lobes evidently extending outwards, with their free parts ca. 40% of ovule length. Individual integumentary lobes folding inwards along abaxial side. Nucellus largely adnate to integument.
Description
Some ovules are borne terminally on smooth branches that are thrice (Figure 1a), twice (Figure 1c) or once (Figure 1b, f, g and i, Figure 2a–c) dichotomous at 40–135°. The boundary between ovule and ultimate axis below refers to position where the ovule width just begins to increase (e.g. Figure 1a, arrow). The branches excluding ovules are up to 76 mm long and 0.4–0.9 mm wide. Most ovules terminate ultimate axis (Figure 1j, Figure 2d–f) or are detached (Figure 1d, e, h and k, Figure 2g–o). The ovules are 25.0–33.0 mm long and 3.5–5.6 mm at the maximum width (excluding the width of outward extension of integumentary wings). Compressions of ovules (Figure 1, Figure 2, Figure 3) and their serial transverse sections (Figure 4, Figure 4—figure supplement 1, Figure 4—figure supplement 2, Figure 4—figure supplement 3, Figure 4—figure supplement 4, Figure 4—figure supplement 5) do not show any cupules.
Each ovule possesses a layer of integument with three radially arranged and wing-like integumentary lobes (Figure 1a, d and e, Figure 2d, e and o, arrows, Figure 3a–f, Figure 4, Figure 4—figure supplement 1, Figure 4—figure supplement 2, Figure 4—figure supplement 3, Figure 4—figure supplement 4, Figure 4—figure supplement 5). They are broad, acropetally tapered and proximally reduced to merge with the ultimate axis (Figure 1a–c and f, Figure 2a, d–i and n). The integumentary lobes are 1.2–2.3 mm at the maximum width and free for 8.3–14.8 mm distance (32–45% of the ovule length), and the free lobe parts extend well above the nucellar tip and greatly curve outward. Usually, two lobes of a single ovule are evident and the third one is sometimes exposed through dégagement (Figure 1a, Figure 2n, arrow 2, o, middle arrow, Figure 3a and e, arrow). Such situation indicates that the lobes of an ovule are present on different bedding planes.
In transverse sections of an ovule, two integumentary lobes extend along the bedding plane, and the third lobe is either originally perpendicular to or compressed to lie somewhat along the bedding plane (Figure 4c–e, h–k, n–r, t and w–y, Figure 4—figure supplement 1, Figure 4—figure supplement 2, Figure 4—figure supplement 3, Figure 4—figure supplement 4, Figure 4—figure supplement 5). The integumentary lobes are narrow, flattened and fused in the lower part of an ovule, and acropetally become wide, thick, separated and far away (Figure 4c–e, Figure 4—figure supplement 1a–k, Figure 4—figure supplement 2). Because of great outward curving of lobes, it is difficult to observe their distal parts in the sections. When thick, the lobes present a V or U shape. Therefore, they are symmetrically folded along the abaxial side and toward the ovule center.
A few ovules show the outline of a nucellus, which is ca. 10–11.7 mm long and 1.2–1.7 mm at the maximum width (Figures 1d and 2i, arrows, j, Figure 3f–h). Transverse sections occasionally meet the nucellar tip (Figure 4d, arrow). With the exception of tip, the nucellus is adnate to the integument and is distally surrounded by the free parts of integumentary lobes. The ovule is reconstructed to show three integumentary wings (Figure 5a) and nucellar tip (Figure 5b).
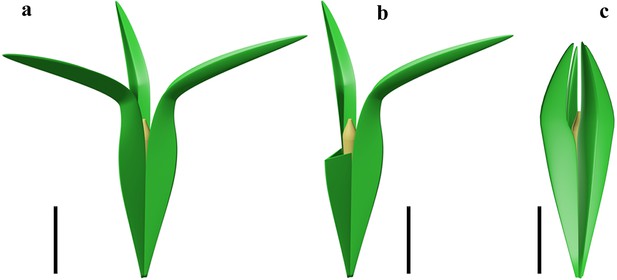
Reconstruction of two acupulate ovules with integumentary wings.
(a) Alasemenia tria with three wings distally extending outwards. (b), A. tria with one of three wings partly removed to show nucellar tip. (c) Guazia dongzhiensis with four wings distally extending inwards (Wang et al., 2022). Scale bars, 5 mm.
Discussion
Late Devonian acupulate ovules and their functions
Except few taxa including Guazia (Wang et al., 2022), Dorinnotheca (Fairon-Demaret, 1996), Cosmosperma (Liu et al., 2017), Elkinsia (Serbet and Rothwell, 1992) and Moresnetia (Fairon-Demaret and Scheckler, 1987), the earliest ovules in the Late Devonian (Famennian) have not been preserved to be connected to the branches. These ovules are usually surrounded by cupules. Now, as in Guazia, the ovules of Alasemenia terminate the dichotomous branches and are transversely sectioned to show the ovular structures. The ovules of these two genera provide rare evidence for acupulate ovules in the earliest seed plants.
Both cupule and integument of an early ovule perform the protective and pollinating functions (Meyer-Berthaud, 2022). The integument of Alasemenia is adnate to most part of the nucellus and its three lobes extend long distance above the nucellar tip and then evidently outwards. Such structure leads to efficient protection of nucellus and adaptation for pollination. In early seed plants, the fertile branches terminated by cupulate ovules consistently lack leaves and thus the cupules probably serve a nutritive function as in photosynthetic organs; this function may be transferred to the integuments of acupulate ovules such as Guazia (Meyer-Berthaud, 2022). Regarding Alasemenia, the nutritive function would also apply to the integuments since the acupulate ovules are terminal on various orders of naked branches and ultimate axes. The surface of integuments is enlarged through the outgrowths of wings and thus promotes the photosynthesis. Little is known about the dispersal function of Famennian ovules, because the integumentary wings as a derived character have been rarely documented. Warsteinia (Rowe, 1997), Guazia (Wang et al., 2022) and now Alasemenia indicate that the anemochory originated in the Famennian. However, the ovule of Warsteinia remains unclear in attachment to branches and possession of a cupule. Alasemenia confirms that, like Guazia, the integuments of acupulate ovules developed a new function in wind dispersal.
Late Devonian winged ovules and evolution of ovular wings
In the Late Devonian ovules, besides Alasemenia, both Warsteinia (Rowe, 1997) and Guazia (Wang et al., 2022) possess integumentary lobes forming radially arranged wings. Their integumentary wings illustrate diversity in number (three or four per ovule), length, folding or flattening, and being straight or curving distally. As in Alasemenia (Figure 5a), the integumentary wings of acupulate ovule of Guazia are broad, thin and fold inwards along the abaxial side, but their numbers are four in each ovule and their free portions usually arch centripetally (Figure 5c; Wang et al., 2022, Figure 5). In contrast to Alasemenia, Warsteinia has four integumentary wings without folding and their free portions are short and straight (Rowe, 1997, TEXT-FIGURE 4). Ovules of Alasemenia and Guazia terminating long and narrow branches suggest easy abscission of diaspores (ovules with or without an ultimate axis) and better preparation for dispersal. Compared to Warsteinia with short and straight wings and Guazia with long but distally inwards curving wings, Alasemenia with longer and outwards extending wings would efficiently reduce the rate of descent and be more capably moved by wind. Furthermore, the quantitative analysis in mathematics indicates that three-winged ovules such as Alasemenia are more adapted to wind dispersal than four-winged ovules including Warsteinia and Guazia (see following).
Alasemenia, Guazia, and Warsteinia suggest that the evolutionarily novel wings, as integument outgrowths and the most important mechanism for seed dispersal by wind, appeared early in the spermatophytes and had been manifested in younger lineages. Other wind dispersal mechanisms including plumes, pappi and parachutes of seeds appeared later in the Permo-Carboniferous and Mesozoic, respectively (Axsmith et al., 2013). Current evidence indicates that seeds with three or four wings occurred first in the Late Devonian. They were followed by two- or three-winged seeds in the Carboniferous (Long, 1960; Long, 1969), and then by single-winged seeds in the Permian (Stevenson et al., 2015; Prevec et al., 2008). Relating to wind dispersal, the diaspores (seeds/fruits) of living spermatophytes possess multiple mechanisms and variable number of wings (Ma, 2009).
Mathematical analysis of wind dispersal of ovules with 1-4 wings
The rate of diaspore descent in still air is an important indicator of the potential ability of modern samaras dispersal (Augspurger et al., 2016; Augspurger et al., 2017). In examining samaras, it has been demonstrated that the value of angular velocity is smaller than that of terminal velocity (Green, 1980), and the relationship between the samaras’ wing loading and terminal velocity is:
where is the weight of samaras, AW is the surface area of the wing, is defined as the samaras’ wing loading.
As for ovules, we also use terminal velocity as an indicator of dispersal ability. Since the broad integumentary wings well extend outwards, the wing loading of Alasemenia is obviously less than that of Guazia. When the winged seeds fall in the air, the predictable spinning can lead to the reduction of fall rate, and result in the increase of the horizontal dispersal distance. This has been observed in the field experiments or proved in the modelling reconstruction experiments (Green, 1980; Habgood et al., 1998).
However, the tiny asymmetry of ovules will be amplified in the running geometry by centrifugal and aerodynamic loads, result in vibrations and thus significantly reduce the efficiency (Lu et al., 2019). The transverse wave caused by vibrations will arrive the tip of wing and form stationary waves. In the ovules with even number of wings like Guazia (Wang et al., 2022) and Warsteinia (Rowe, 1992; Rowe, 1997), the center symmetry structure will lead to stronger resonance than the ovules with odd number of wings (like Alasemenia). It means the ovules with odd number of wings are more stable in high rate spinning and spend more falling time in the dispersal process.
Another consideration is the capacity of airflow in horizontal direction. The Reynolds number (Re) is the indicator of patterns in fluid flow situations, and for ovules, the Reynolds numbers are mainly falling in 103–104, suggesting that the inertia forces are much stronger than viscous forces (Burrows, 1975; Seter and Rosen, 1992). In this situation, the thrust of airflow can be represented as:
where c is the coefficient, is the density of air, is the windward face, is the velocity of relative movement.
It means that we can transform the comparison of capacity of airflow into the area of windward. Supposing the maximum windward area of each wing is and represents the number of wings revolving on its own axis, we define a function to represent the area of windward when the angle between airflow and wings is . We introduce relative efficiency for comparison. Here, we list the following 5 ideal basic situations and the summary results are shown in Figure 6.
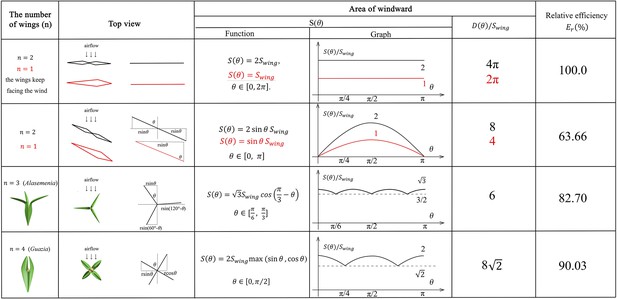
The mathematical analysis of wind dispersal ability of ovules with 1–4 wings.
The maximum windward area of each wing is and represents the number of wings per ovule. is the distance from the tip of wing to the axis of ovules. represents the area of windward when the angle between airflow and wings is , and represents the accumulated area of windward in a cycle. means relative efficiency for wind dispersal. Red lines and expressions show the situation of .
and the wings keep facing the wind (wings without rotation, as a control group). In this situation, the area of windward is identical to , which can be written as:
We define a function to represent the accumulated area of windward in a cycle. By definition,
. In this situation, we discuss the condition when .
Based on symmetry,
. In this situation,
As can be seen in Figure 6, the relative efficiency is equal to the situation of ,
(Alasemenia).
Based on symmetry,
(Guazia, Warsteinia).
When
Based on symmetry,
Generally, the above one- to four-winged seeds are quantitatively analysed for their wind dispersal capability and the results are shown in Figure 6. The relative efficiency for wind dispersal (Er) of these seeds in five ideal basic situations is calculated for comparison. The one- or two-winged seeds are treated as a control group when the wings keep facing the wind and do not rotate. In this case, the value of Er is 100%. When descending through autorotation, one- to four-winged seeds present different values of Er. The relative wind dispersal efficiency of three-winged seeds is obviously better than that of single- and two-winged seeds, and is close to that of four-winged seeds (Figure 6). In addition, three-winged seeds have the most stable area of windward, which also ensures the motion stability in wind dispersal. Significantly, the maximum windward area of each wing of Alasemenia is greater than that of Guazia and Warsteinia with four wings. All these factors suggest that Alasemenia is well adapted for anemochory.
Conclusion
The earliest ovules in the Famennian of Late Devonian, usually preserved to be unconnected with branches, are mostly devoid of integumentary wings and enclosed in cupules; they are thus insufficiently known for integument function and wind dispersal. New ovule Alasemenia in this paper terminates leafless branches, bears three wings and lacks cupules. It represents the second acupulate ovule in the Famennian. Besides protective and pollinating functions, Alasemenia suggests photosynthetically nutritive and anemochorous functions of early ovules. It indicates the diversity of Famennian winged ovules and the evolutionary sequence of ovule wings. Compared to Famennian four-winged ovules of Warsteinia and Guazia, Alasemenia with three distally outwards extending wings shows advantage in anemochory. Mathematical analysis implies that three-winged ovules could be efficiently dispersed by wind.
Methods
All specimens are housed in Department of Geology, Peking University, Beijing, China. Steel needles were used to expose some seeds and fertile axes. Serial dégagement was employed to reveal the morphology and structure of seeds. Seeds were embedded in resin, sectioned and ground to show the integuments and nucelli. All photographs were made with a digital camera and microscope.
Data availability
No data are newly generated or analysed during this study.
References
-
A Triassic seed with an angiosperm-like wind dispersal mechanismPalaeontology 56:1173–1177.https://doi.org/10.1111/pala.12049
-
Wind‐borne seed and fruit movementNew Phytologist 75:405–418.https://doi.org/10.1111/j.1469-8137.1975.tb01404.x
-
Typification and redescription of Moresnetia zalesskyi Stockmans, 1948, an early seed plant from the Upper Famennian of BelgiumBulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre 57:183–199.
-
Dorinnotheca streelii Fairon-Demaret, gen. et sp. nov., a new early seed plant from the upper Famennian of BelgiumReview of Palaeobotany and Palynology 93:217–233.https://doi.org/10.1016/0034-6667(95)00127-1
-
Re-study of Guangdedendron micrum from the Late Devonian Xinhang forestBMC Ecology and Evolution 22:69.https://doi.org/10.1186/s12862-022-02021-w
-
Spore assemblage from the upper devonian wutong formation in Xinhang, AnhuiActa Scientiarum Naturalium Universitatis Pekinensis 59:221–230.
-
The terminal velocity and dispersal of spinning samarasAmerican Journal of Botany 67:1218–1224.https://doi.org/10.1002/j.1537-2197.1980.tb07754.x
-
Modelling of the dispersal of Lepidocarpon based on experiments using reconstructionsReview of Palaeobotany and Palynology 102:101–114.https://doi.org/10.1016/S0034-6667(98)00012-8
-
XII.—On the structure of Samaropsis scotica Calder (emended) and Eurystoma angulare gen. et sp. nov., Petrified Seeds from the Calciferous Sandstone Series of BerwickshireTransactions of the Royal Society of Edinburgh 64:261–280.https://doi.org/10.1017/S0080456800100286
-
VII.—Eurystoma trigona sp. nov., a Pteridosperm ovule borne on a frond of Alcicornopteris KidstonTransactions of the Royal Society of Edinburgh 68:171–182.https://doi.org/10.1017/S0080456800014654
-
ConferenceNonsynchronous vibration associated with transonic fan blade untwistTurbo Expo: Power for Land, Sea, and Air.https://doi.org/10.1115/GT2019-91286
-
Plant mobility in the Mesozoic: Disseminule dispersal strategies of Chinese and Australian Middle Jurassic to Early Cretaceous plantsPalaeogeography, Palaeoclimatology, Palaeoecology 515:47–69.https://doi.org/10.1016/j.palaeo.2017.12.036
-
Were all devonian seeds cupulate? A reinvestigation of Pseudosporogonites hallei, Xenotheca bertrandii, and Aglosperma sppInternational Journal of Plant Sciences 174:832–851.https://doi.org/10.1086/670235
-
Late Devonian winged preovules and their implications for the adaptive radiation of early seed plantsPalaeontology 40:575–595.
-
Evolution of limited seed dispersal ability on gypsum islandsAmerican Journal of Botany 100:1811–1822.https://doi.org/10.3732/ajb.1300075
-
Characterizing the most primitive seed Ferns. I. a reconstruction of Elkinsia polymorphaInternational Journal of Plant Sciences 153:602–621.https://doi.org/10.1086/297083
-
Study of the vertical autorotation of a single winged samaraBiological Reviews 67:175–197.https://doi.org/10.1111/j.1469-185X.1992.tb01018.x
-
BookPaleobotany: The Biology and Evolution of Fossil PlantsBurlington: Academic Press.
-
Guazia, the earliest ovule without cupule but with unique integumentary lobesNational Science Review 9:nwab196.https://doi.org/10.1093/nsr/nwab196
Article and author information
Author details
Funding
National Natural Science Foundation of China (42130201)
- Deming Wang
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank X Gao, Z Z Deng and L Liu for help in fieldwork. Q Y Jia and D B Ni provide assistance in the experiment for seed sections. This work was supported by the National Natural Science Foundation of China (grant no. 42130201).
Version history
- Sent for peer review:
- Preprint posted:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.92962. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Wang, Yang, Liu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 834
- views
-
- 105
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
- Genetics and Genomics
Evolutionary arms races can arise at the contact surfaces between host and viral proteins, producing dynamic spaces in which genetic variants are continually pursued. However, the sampling of genetic variation must be balanced with the need to maintain protein function. A striking case is given by protein kinase R (PKR), a member of the mammalian innate immune system. PKR detects viral replication within the host cell and halts protein synthesis to prevent viral replication by phosphorylating eIF2α, a component of the translation initiation machinery. PKR is targeted by many viral antagonists, including poxvirus pseudosubstrate antagonists that mimic the natural substrate, eIF2α, and inhibit PKR activity. Remarkably, PKR has several rapidly evolving residues at this interface, suggesting it is engaging in an evolutionary arms race, despite the surface’s critical role in phosphorylating eIF2α. To systematically explore the evolutionary opportunities available at this dynamic interface, we generated and characterized a library of 426 SNP-accessible nonsynonymous variants of human PKR for their ability to escape inhibition by the model pseudosubstrate inhibitor K3, encoded by the vaccinia virus gene K3L. We identified key sites in the PKR kinase domain that harbor K3-resistant variants, as well as critical sites where variation leads to loss of function. We find K3-resistant variants are readily available throughout the interface and are enriched at sites under positive selection. Moreover, variants beneficial against K3 were also beneficial against an enhanced variant of K3, indicating resilience to viral adaptation. Overall, we find that the eIF2α-binding surface of PKR is highly malleable, potentiating its evolutionary ability to combat viral inhibition.
-
- Ecology
- Evolutionary Biology
Seasonal polyphenism enables organisms to adapt to environmental challenges by increasing phenotypic diversity. Cacopsylla chinensis exhibits remarkable seasonal polyphenism, specifically in the form of summer-form and winter-form, which have distinct morphological phenotypes. Previous research has shown that low temperature and the temperature receptor CcTRPM regulate the transition from summer-form to winter-form in C. chinensis by impacting cuticle content and thickness. However, the underling neuroendocrine regulatory mechanism remains largely unknown. Bursicon, also known as the tanning hormone, is responsible for the hardening and darkening of the insect cuticle. In this study, we report for the first time on the novel function of Bursicon and its receptor in the transition from summer-form to winter-form in C. chinensis. Firstly, we identified CcBurs-α and CcBurs-β as two typical subunits of Bursicon in C. chinensis, which were regulated by low temperature (10 °C) and CcTRPM. Subsequently, CcBurs-α and CcBurs-β formed a heterodimer that mediated the transition from summer-form to winter-form by influencing the cuticle chitin contents and cuticle thickness. Furthermore, we demonstrated that CcBurs-R acts as the Bursicon receptor and plays a critical role in the up-stream signaling of the chitin biosynthesis pathway, regulating the transition from summer-form to winter-form. Finally, we discovered that miR-6012 directly targets CcBurs-R, contributing to the regulation of Bursicon signaling in the seasonal polyphenism of C. chinensis. In summary, these findings reveal the novel function of the neuroendocrine regulatory mechanism underlying seasonal polyphenism and provide critical insights into the insect Bursicon and its receptor.






