Prolactin-mediates a lactation-induced suppression of arcuate kisspeptin neuronal activity necessary for lactational infertility in mice
Figures
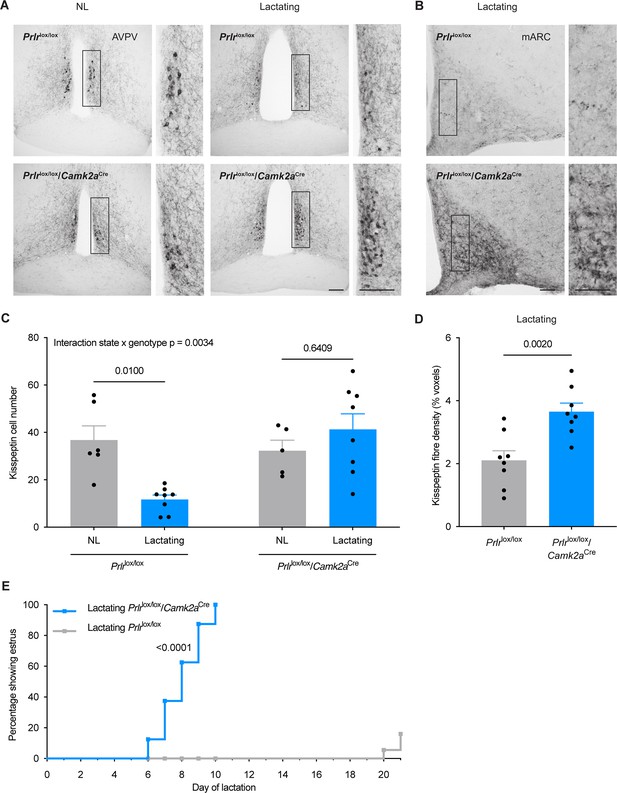
Prlrlox/lox/Camk2aCre mice do not undergo the normal period of lactational infertility and the lactation-induced suppression of kisspeptin immunoreactivity is absent.
(A) Kisspeptin immunoreactivity is shown in representative photomicrographs from diestrus, nulligravid, non-lactating (NL; left), and lactating (right) Prlrlox/lox control and Prlrlox/lox/Camk2aCre mice (from anteroventral periventricular nucleus (AVPV) region of RP3V). (B) Representative photomicrographs showing mid-arcuate nucleus (mARC) of lactating Prlrlox/lox (top) and lactating Prlrlox/lox/Camk2aCre mice (bottom). (C) Total kisspeptin cell number for the RP3V (NL Prlrlox/lox (n=6) versus lactating Prlrlox/lox control (n=8) p=0.0100, NL Prlrlox/lox/Camk2aCre (n=5) versus lactating Prlrlox/lox/Camk2aCre (n=8) p=0.6409). Two-way ANOVA followed by Tukey’s multiple comparisons test. (D) Quantification of kisspeptin fibre density in the arcuate nucleus (Fiji software, measured in percentage voxels per region of interest), showing total kisspeptin fibre density in the arcuate nucleus (lactating Prlrlox/lox control n=8, lactating Prlrlox/lox/Camk2aCre n=7, p=0.0020, unpaired two-tailed t-test). (E) Lactating Prlrlox/lox/Camk2aCre mice (blue, n=8) resume estrous cycles significantly earlier (100% within 6–10 d of lactation) than lactating Prlrlox/lox controls (grey, n=10) (p≤0.0001, Log-rank (Mantel-Cox) test). Scale bar image and insert = 50 μm. Values are shown as mean ± SEM.
-
Figure 1—source data 1
Data for each of the graphs in Figure 1.
- https://cdn.elifesciences.org/articles/94570/elife-94570-fig1-data1-v1.xlsx
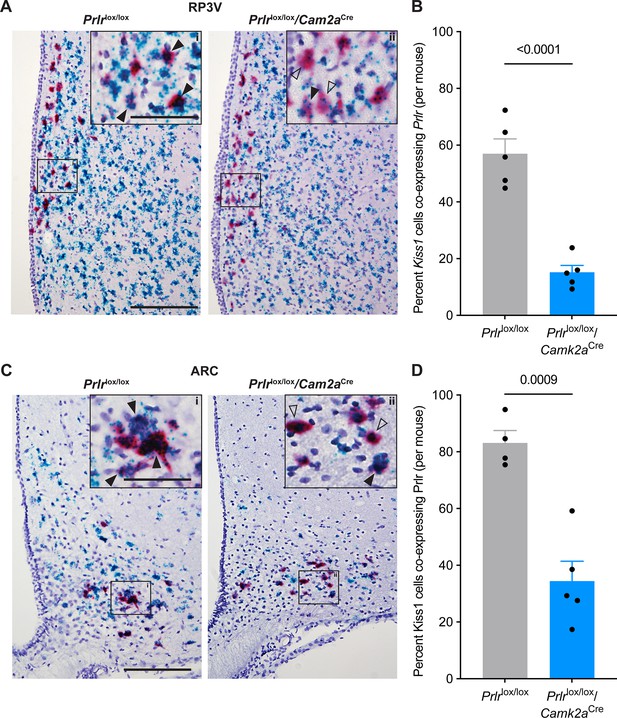
Proportion of kisspeptin neurons showing prolactin receptor (Prlr) deletion using RNAscope in Prlrlox/lox/Camk2aCre mice.
Representative photomicrographs showing RNAscope labelling for Prlr (blue) and Kiss1 (red) in the rostral periventricular region of the third ventricle (RP3V, A-B, intact; Prlrlox/lox control n=5, Prlrlox/lox/Camk2aCre, n=5) or arcuate nucleus (ARC, C-D, ovariectomised (OVX); Prlrlox/lox control n=4, Prlrlox/lox/Camk2aCre n=5). Compared to Prlrlox/lox control mice, Prlrlox/lox/Camk2aCre mice show a significant decrease in the percentage of Kiss1-expressing cells co-expressing Prlr in both the RP3V (B; p≤0.0001) and arcuate nucleus (D; p=0.0009) (unpaired two-tailed t-tests). Solid black arrows = doubled labelled cells expressing both Kiss1 and Prlr; black outlined arrows = Kiss1 cells with sparse or no co-labelling for Prlr. Scale bar = 150 μm, insert = 60 μm. Values are shown as mean ± SEM.
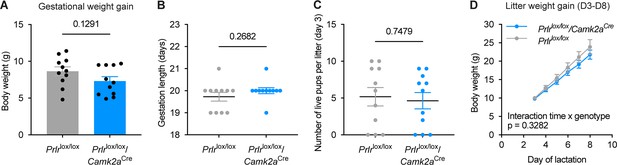
Gestational and maternal phenotyping of Prlrlox/lox/Camk2aCre mice and respective Prlrlox/lox controls.
(A–D) Data show; gestational weight gain (A; Prlrlox/lox n=11, Prlrlox/lox/Camk2aCre n=11), gestation length (B; Prlrlox/lox n=11, Prlrlox/lox/Camk2aCre n=11), number of live pups on day 3 of lactation (C; Prlrlox/lox n=11; Prlrlox/lox/Camk2aCre n=11), and litter weight gain between day 3–8 of lactation (D; Prlrlox/lox n=8; Prlrlox/lox/Camk2aCre n=8). There were no differences in any of these parameters (A, C) unpaired two-tailed t-test; B, Mann Whitney test; D, repeated measures mixed effect analysis, fixed effects type III with Šídák’s multiple comparisons test. Grey = Prlrlox/lox; blue = Prlrlox/lox/Camk2aCre. Values are shown as mean ± SEM.
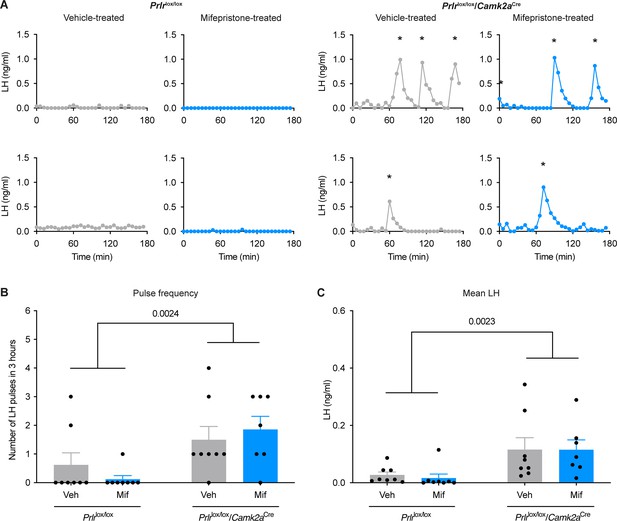
Prolactin action in the brain during lactation is necessary for the suppression of pulsatile luteinizing hormone (LH) secretion.
Examples of pulsatile LH levels in the blood from lactating Prlrlox/lox control and lactating Prlrlox/lox/Camk2aCre mice that have either been treated with vehicle (sesame oil, s.c., grey, veh) or 4 mg/kg mifepristone (in sesame oil, s.c., blue, mif). Graphs show LH pulse frequency (B; interaction p=0.2807, genotype p=0.0024, state p=0.8558), and mean LH levels (C; interaction p=0.8572, genotype p=0.0023, state p=0.8457). Lactating vehicle-treated Prlrlox/lox (n=8), lactating mifepristone-treated Prlrlox/lox (n=8), lactating vehicle-treated Prlrlox/lox/Camk2aCre (n=8), lactating mifepristone-treated Prlrlox/lox/Camk2aCre (n=7). Asterisks indicate LH pulse peaks as detected by PULSAR Otago analysis. Two-way ANOVA followed by Tukey’s multiple comparisons test. Values are shown as mean ± SEM.
-
Figure 2—source data 1
Spreadsheet containing LH data from each individual sample.
- https://cdn.elifesciences.org/articles/94570/elife-94570-fig2-data1-v1.xlsx
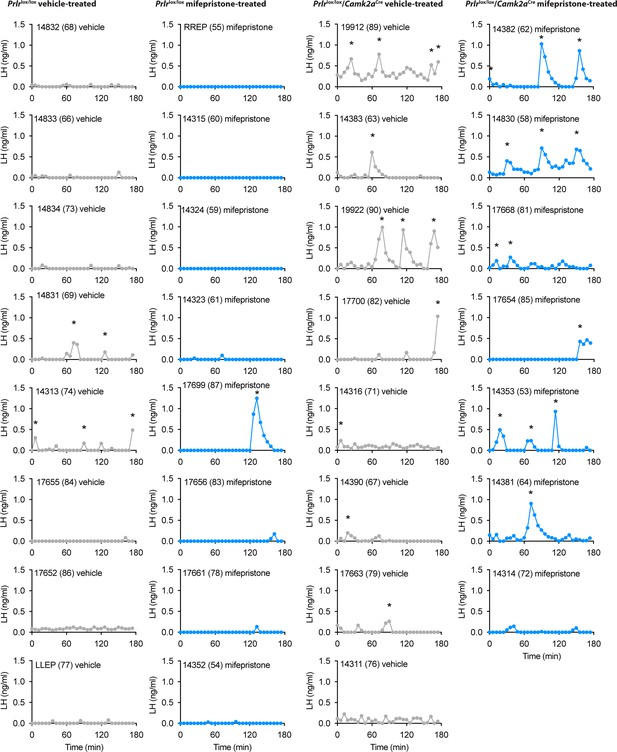
Pulsatile luteinizing hormone (LH) secretion profiles of Prlrlox/lox/Camk2aCre mice and Prlrlox/lox controls following vehicle or mifepristone treatment.
Individual LH pulse data from lactating Prlrlox/lox control and Prlrlox/lox/Camk2aCre mice treated with either vehicle (sesame oil, s.c., grey) or 4 mg/kg mifepristone (in sesame oil, s.c., blue). Lactating vehicle-treated Prlrlox/lox (n=8), lactating mifepristone-treated Prlrlox/lox (n=8), lactating vehicle-treated Prlrlox/lox/Camk2aCre (n=8), lactating mifepristone-treated Prlrlox/lox/Camk2aCre (n=7). Asterisks indicate LH pulse peaks as detected by PULSAR Otago analysis.
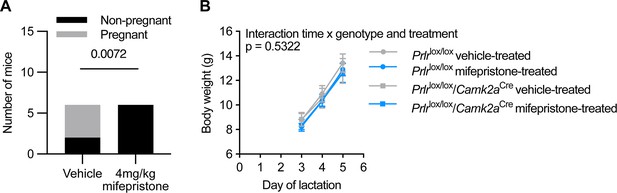
Mifepristone dose pilot study and effect on litter weight gain.
(A) Mifepristone dose pilot study showing a dose of 4 mg/kg was sufficient to terminate pregnancy in all mice (p=0.0072, chi-squared test, n=6 both in groups). (B) Mifepristone or vehicle treatment had no effect on litter weight gain from day 3 to day 5 of lactation (interaction of time x genotype & treatment p=0.5322; time p≤0.0001; genotype and treatment p=0.8811; subject p≤0.0001; two-way ANOVA). Values are shown as mean ± SEM.
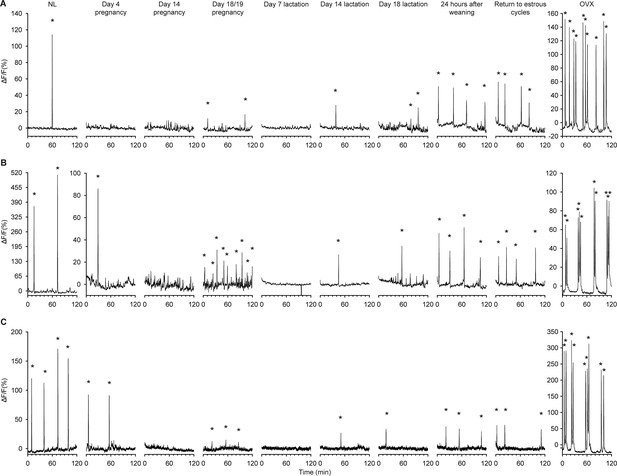
Longitudinal recordings of arcuate kisspeptin neuron GCaMP population activity throughout different reproductive states in the same Kiss1Cre mice.
Representative neuronal activity from three Kiss1Cre mice throughout the NL (diestrus, nulligravid, non-lactating), pregnant, lactating, and post-weaning states. The time points monitored in order were: diestrus NL, day 4 pregnancy, day 14 pregnancy, day 18/19 pregnancy (overnight), day 7 lactation, day 14 lactation, day 18 lactation, 24 hr after weaning (day 22 postpartum), return to normal cycling following weaning (return to estrous cycles), and 10 d post ovariectomy (OVX). Asterisks indicate SEs. Note: change in y-axes scale on all three OVX timepoints and mouse shown in dataset (B) from day 4 of pregnancy onwards.
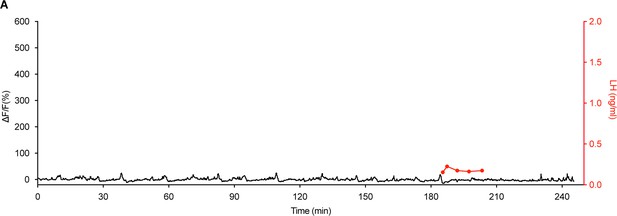
Miniature synchronised event (SE)-like activity on day 14 of pregnancy in Kiss1Cre mice does not result in pulsatile luteinizing hormone (LH) secretion.
(A) Paired fibre photometry and blood sampling from the representative mouse on day 14 of pregnancy showed miniature SE-like activity have no significant effect on pulsatile LH secretion (red).
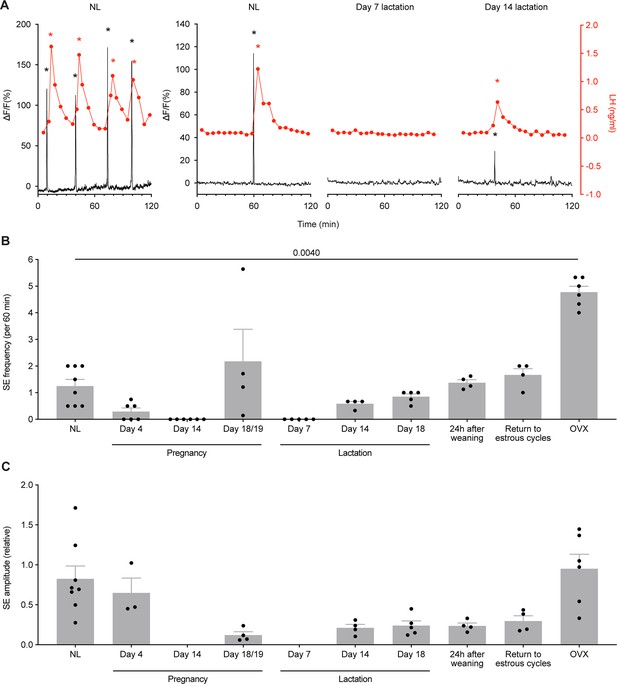
Synchronised Ca2+ events (SE) are consistently correlated with pulsatile luteinizing hormone (LH) secretion across different reproductive states in Kiss1Cre mice.
(A) When fibre photometry was paired with serial blood sampling for pulsatile LH secretion, the relationship between SEs and LH pulses was examined. Each time an SE was seen during a recording with blood sampling, pulsatile secretion of LH was also observed, with 100% correlation (p≤0.0001, chi-squared test; 73 out of 73 SEs observed led to an LH pulse). Representative examples of paired photometry and blood sampling are shown from the NL (diestrus, nulligravid, non-lactating) state, from day 7 lactation, and from day 14 lactation. (B) Quantitative analysis of SE frequency per hour across different reproductive states in Kiss1Cre mice (p=0.0040, mixed effect analysis (fixed type III) with Tukey’s multiple comparisons tests). (C) Relative SE amplitude of normalised ΔF/F across different reproductive states (unable to undertake statistical analysis). Black asterisks indicate SEs, and red asterisks indicate LH pulse peaks as detected by PULSAR Otago analysis. Values are shown as mean ± SEM.
-
Figure 4—source data 1
Data for LH pulse analysis in Figure 4B and C.
- https://cdn.elifesciences.org/articles/94570/elife-94570-fig4-data1-v1.xlsx
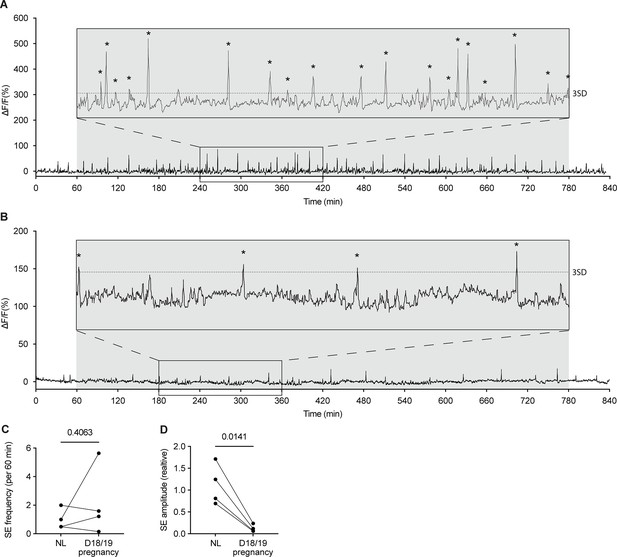
Activity of arcuate kisspeptin neurons on days 18 and 19 of pregnancy in Kiss1Cre mice.
Fibre photometry recordings of mice on the evening of day 18 of pregnancy (1800 hr) to the morning of day 19 of pregnancy (0800 hr) show low amplitude synchronised events (SEs). 3 hr section blown up for ease of viewing. (C) No difference was seen between the frequency of SEs (per 60 min) in the NL (diestrus, nulligravid, non-lactating) versus day 18/19 pregnancy (p=0.4063, paired two-tailed t-test), however, a significant decrease in relative SE amplitude is seen (D; p=0.0141, paired two-tailed t test). Asterisks indicate SEs. Dotted line in the insert of (A) and (B) indicates three standard deviations (3SD). Grey shaded region = lights off.
-
Figure 5—source data 1
Data for graphs Figure 5C and D.
- https://cdn.elifesciences.org/articles/94570/elife-94570-fig5-data1-v1.xlsx
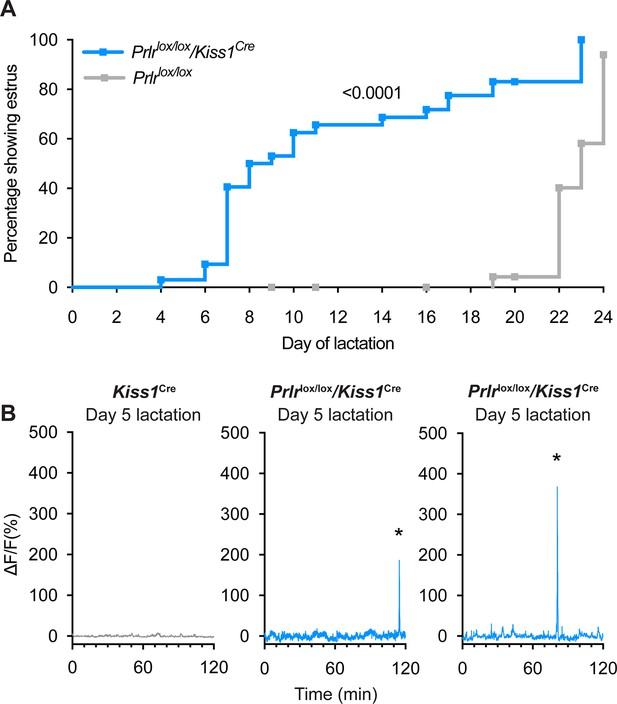
Prlrlox/lox/Kiss1Cre mice do not undergo the normal period of lactational infertility and show early reactivation of arcuate kisspeptin neurons prior to estrus in lactation.
(A) Prlrlox/lox/Kiss1Cre mice resume estrous cycles significantly earlier (63% by day 10 of lactation, n=32, blue) than Prlrlox/lox controls (4% by day 19 of lactation, n=30, grey) (p≤0.0001, Log-rank (Mantel-Cox) test). (B) Representative fibre photometry traces from day 5 of lactation from either a Kiss1Cre control mouse or Prlrlox/lox/Kiss1Cre mice. Mice with prolactin receptor (Prlr) knocked out of arcuate kisspeptin neurons (Prlrlox/lox/Kiss1Cre) show synchronised events (SEs) early in lactation. Asterisks indicate SEs.
-
Figure 6—source data 1
Data for each of the graphs in Figure 6.
- https://cdn.elifesciences.org/articles/94570/elife-94570-fig6-data1-v1.xlsx
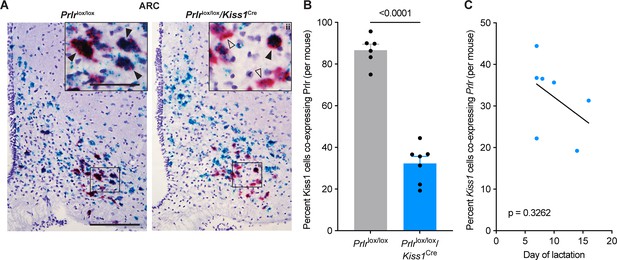
Proportion of kisspeptin neurons showing prolactin receptor (Prlr) deletion using RNAscope in Prlrlox/lox/Kiss1Cre mice.
Representative photomicrographs showing RNAscope labelling for Prlr (blue) and Kiss1 (red) in the arcuate nucleus (ARC), in ovariectomised (OVX; Prlrlox/lox control n=6, Prlrlox/lox/Kiss1Cre n=7) mice. (B) A significant decrease in the percentage of Kiss1-expressing cells co-expressing Prlr was seen in Prlrlox/lox/Kiss1Cre compared to Prlrlox/lox controls (p≤0.0001, unpaired two-tailed t-test). (C) No correlation was found between the percentage of Kiss1 cells co-expressing Prlr and the day of estrus return during lactation (p=0.3262, simple linear regression). Solid black arrows = doubled labelled cells expressing both Kiss1 and Prlr; black outlined arrows = Kiss1 cells with sparse or no co-labelling for Prlr. Scale bar = 150 μm, insert = 60 μm. Values are shown as mean ± SEM.
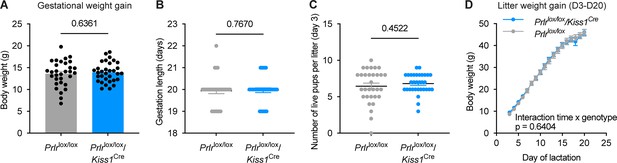
Gestational and maternal phenotyping of Prlrlox/lox/Kiss1Cre mice and respective Prlrlox/lox controls.
(A–D) Data show; gestational weight gain (A; Prlrlox/lox n=31, Prlrlox/lox/Kiss1Cre n=27), gestation length (B; Prlrlox/lox n=31, Prlrlox/lox/Kiss1Cre n=27), number of live pups on day 3 of lactation (C; Prlrlox/lox n=31, Prlrlox/lox/Kiss1Cre n=27), and day 3–20 of lactation (D; Prlrlox/lox n=22, Prlrlox/lox/Kiss1Cre n=20). There were no differences in any of these parameters (A, C) unpaired two-tailed t-test; B, Mann Whitney test; D, repeated measures mixed effect analysis, fixed effects (type III) with Šídák’s multiple comparisons test. Grey = Prlrlox/lox; blue = Prlrlox/lox/Kiss1Cre. N numbers of Prlrlox/lox/Kiss1Cre mice and respective Prlrlox/lox controls changed due to: Prlrlox/lox (n=5), and Prlrlox/lox/Kiss1Cre (n=5) mice euthanised prior to day 20 lactation due to COVID-19 lockdown; Prlrlox/lox/Kiss1Cre (n=2) showed estrus and, therefore, underwent blood sampling (with respective Prlrlox/lox controls (n=2)). All these mice were euthanised 2 hr following blood sampling; a Prlrlox/lox dams’ (n=1) litter losing weight and being excluded from subsequent analysis. Values are shown as mean ± SEM.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/94570/elife-94570-mdarchecklist1-v1.docx
-
Supplementary file 1
Table containing description of each statistical comparison.
- https://cdn.elifesciences.org/articles/94570/elife-94570-supp1-v1.docx





