Nicotine enhances the stemness and tumorigenicity in intestinal stem cells via Hippo-YAP/TAZ and Notch signal pathway
Figures
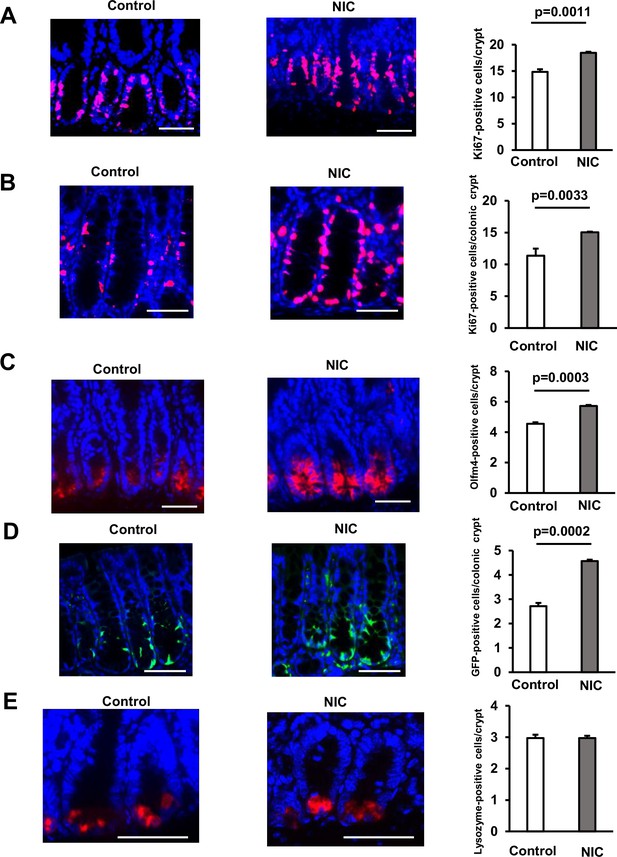
Nicotine (NIC) treatment increases the number of ISCs in the Intestine.
(A and B) Image of Ki67‐positive cells (Red: Ki67, Blue: DAPI) and their quantification at the crypt base of proximal jejunum (A) or colon (B) of NIC-treated and untreated mice (A:3–4 mice per group, B: 3 mice per group). (C) Olfm4 staining image (Red: Olfm4, Blue: DAPI) and the quantification of Olfm4‐positive cells at the crypt base of proximal jejunum with or without NIC treatment (3–4 mice per group). (D) GFP staining image (Green: GFP, Blue: DAPI) and the quantification of LgR5-GFP‐positive cells at the crypt base of the colon in NIC or control-treated Lgr5-EGFP-IRES-CreERT2 mice (3 mice per group). (E) Lysozyme staining image (Red: Lysozyme, Blue: DAPI) and the quantification of Lysozyme‐positive Paneth cells with or without NIC treatment (3–4 mice per group). Original magnifications: 400× (A–E). Scale bar: 50 µm (A–E). Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 1—figure supplement 1.
-
Figure 1—source data 1
The quantification in immunostaining.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig1-data1-v1.xlsx
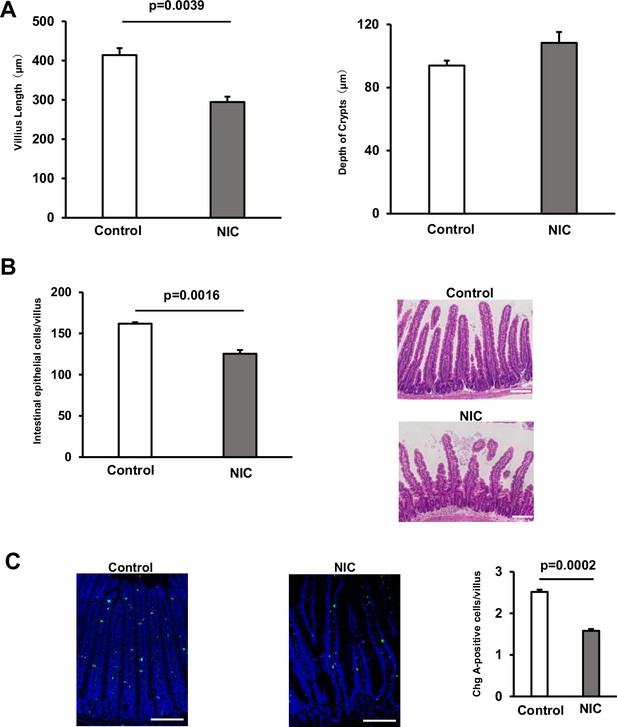
NIC suppresses the differentiation of ISCs.
(A) Images of the H&E-stained crypt and villus and quantification of their length after or without NIC treatment (3–4 mice per group). (B) The quantification of the number of enterocytes per villus in NIC-treated and untreated samples (3 mice per group). (C) Chromogranin A staining image (Green: Chromogranin A, Blue: DAPI) and the quantification of Chromogranin A‐positive cells per villus-crypt unit in NIC-treated and untreated samples (3 mice per group). Original magnifications: ×100 (A), ×200 (C). Scale bar: 100 µm (A and C). Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 1.
-
Figure 1—figure supplement 1—source data 1
The quantification in staining.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig1-figsupp1-data1-v1.xlsx
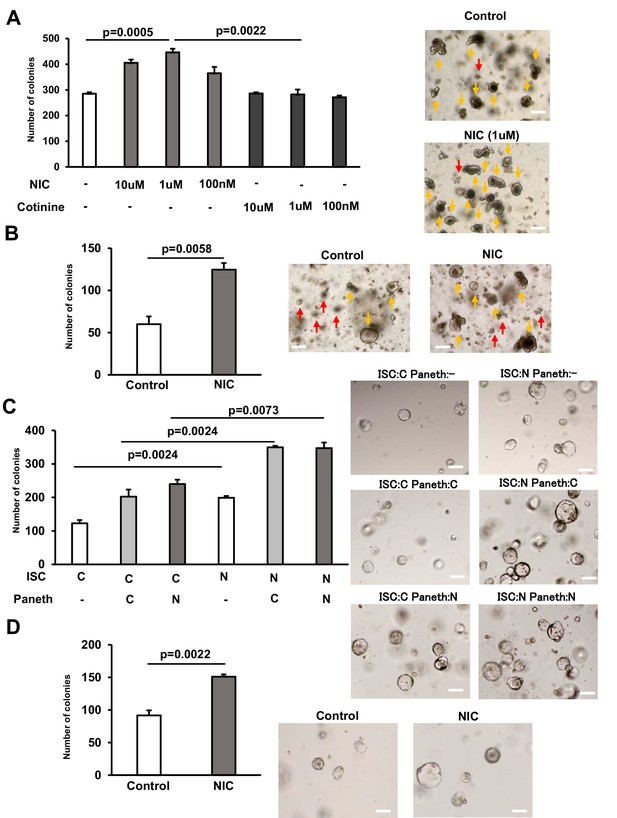
NIC enhances the formation of intestinal organoids from ISCs.
(A) Crypts from the proximal small intestine were cultured with 10 μM, 1 μM, and 100 nM NIC or cotinine to allow ISCs to form organoid colonies; the control set contained no NIC or cotinine. Representative images of the organoids and the quantification of organoids number at day 5 (3 wells/ group) (yellow arrow marks organoids and red arrow indicates aborted crypts). (B) Colonic crypts were cultured with or without 1 μM NIC to allow CSCs to form organoid colonies. Representative images of the organoids and the quantification of organoids number at day 5 are shown (3 wells/ group) (yellow arrow marks organoids and red arrow indicates aborted crypts). (C) ISCs and Paneth cells were isolated from the small intestine of Lgr5‐ EGFP‐IRES‐CreERT2 mice treated with or without NIC; 2×103 cells each were co‐cultured in the medium containing 10 μM CHIR99021. Representative images of the organoids and the frequency of organoids at day 5 (3 wells/ group). (D) ISCs isolated from the small intestine of Lgr5‐EGFP‐IRES‐CreERT2 mice were cultured in the absence of Paneth cells using the medium containing 10 μM CHIR99021, with or without 1 μM NIC. Representative images of the organoids and the frequency of organoids number at day 5 (3 wells/ group). C: control, N: NIC. Original magnification: 40×. Scale bar: 100 µm. Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 3—figure supplement 1.
-
Figure 2—source data 1
The quantification of colonies in organoid assay.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig2-data1-v1.xlsx
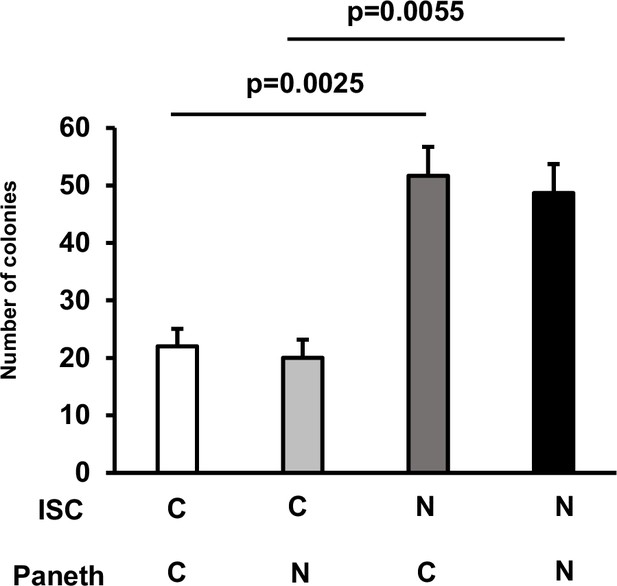
NIC induces the formation of intestinal organoids from ISCs.
ISCs and Paneth cells were isolated from the small intestine of Lgr5‐EGFP‐IRES‐CreERT2 mice (NIC-treated and untreated); 2×103 cells were co-cultured using medium without 10 μM CHIR99021. Representative images of the formed organoids and quantification of the organoid number on day 3 are shown (three wells per group). Values represent the mean ± SEM. Significant differences were denoted by p-values (Student’s t-test). See also Figure 2.
-
Figure 2—figure supplement 1—source data 1
The quantification of colonies in organoid assay.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig2-figsupp1-data1-v1.xlsx
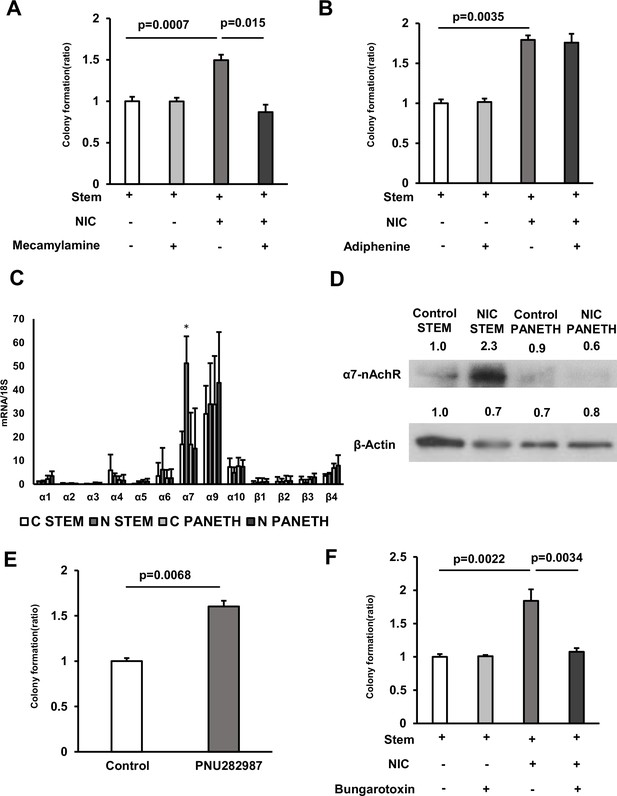
The effect of NIC is mediated via the α7 subunits of α7-nicotinic acetylcholine receptor (nAChR).
(A) Isolated ISCs were cultured in a medium with or without 1 μM NIC and 10 μM Mecamylamine (3 wells/group). (B) Isolated ISCs were cultured in a medium with or without 1 μM NIC and 3 μM Adiphenine hydrochloride (3 wells/group). (C) In ISCs and Paneth cells isolated from control and NIC mice, mRNA levels of nAchR subunits were analyzed using quantitative real-time PCR (n=5 per group; C: control, N: NIC). ∗p < 0.05 (vs C STEM) (Student’s t-test). (D) ISC or Paneth cell lysates prepared from control and NIC mice were immunoblotted with antibodies against α7-nAchR and β-actin. (E) Isolated ISCs were cultured in a medium supplemented with or without 10 μM PNU 282987 (3 wells/group). (F) Isolated ISCs were cultured in a medium with or without 1 μM NIC and 1 μM α-Bungarotoxin (3 wells/group). Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 3—figure supplement 1.
-
Figure 3—source data 1
Colony quantification and qRT-PCR.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Immunoblotting data with labeling.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig3-data2-v1.zip
-
Figure 3—source data 3
Immunoblotting raw data.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig3-data3-v1.zip
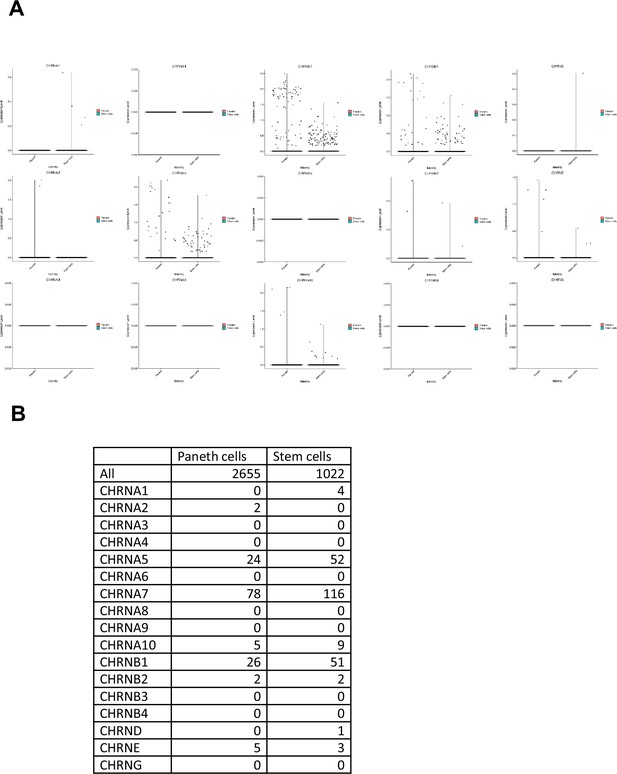
The distribution of human nAchR subunits in ISCs and Pante cells.
(A) Violin plots for nAchR subunits in ISCs and Panth cells by the analysis of human gut single-cell datasets (Elmentaite et al., 2021). (B) The number of positive cells for each nAchR in ISCs and Panth cells are shown. See also Figure 3.
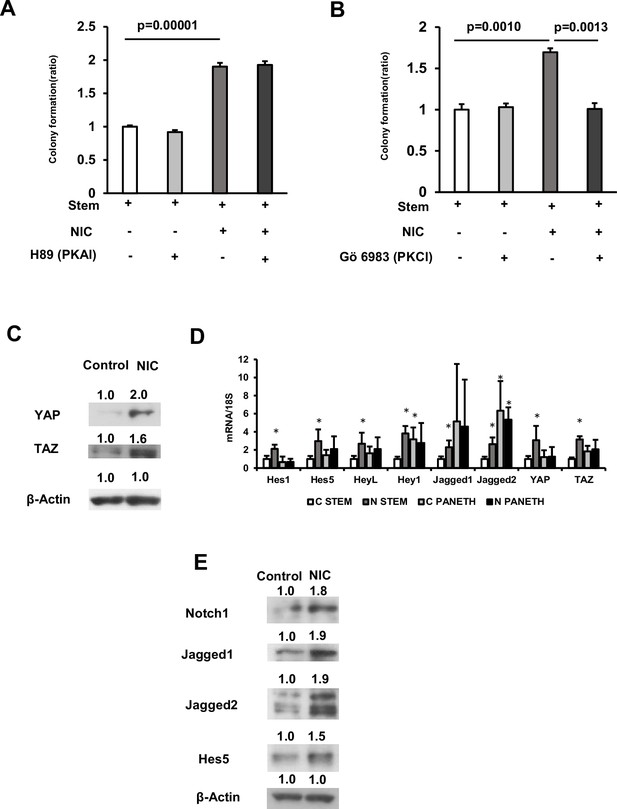
NIC induces Hippo-YAP/TAZ and notch signaling in ISCs.
(A and B) Isolated ISCs cultured using a medium supplemented with or without 1 μM NIC combined with either (A) 1 μM H89 dihydrochloride (PKA inhibitor) or (B) 10 nM Gö 6983 (PKC inhibitor; 3 wells/group). (C) Crypt lysates isolated from control and NIC-treated mice were immunoblotted using antibodies against YAP, TAZ, and β-actin. (D) In ISCs or Paneth cells (n=5 per group) isolated from control or NIC mice, mRNA levels of genes associated with Hippo-YAP/TAZ and Notch signaling were determined through quantitative real-time PCR. ∗p < 0.05 (vs C STEM) (Student’s t-test). (E) Crypt lysates obtained from control and NIC-treated mice were immunoblotted using antibodies against Notch1, Jagged1, Jagged2, Hes5, and β-actin. Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 4—figure supplement 1.
-
Figure 4—source data 1
Colony quantification and qRT-PCR.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-data1-v1.xlsx
-
Figure 4—source data 2
Immunoblotting data with labeling.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-data2-v1.zip
-
Figure 4—source data 3
Immunoblotting raw data.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-data3-v1.zip
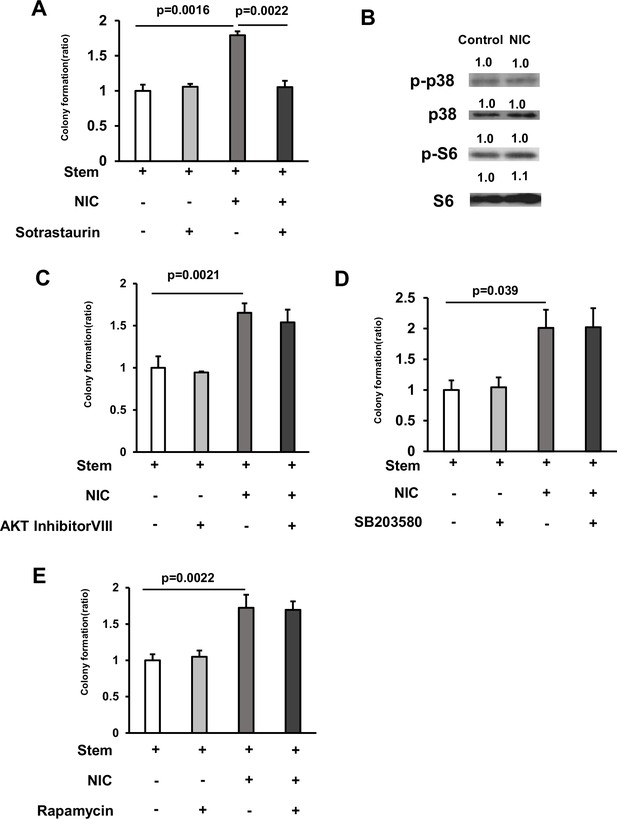
PI3K/AKT, mTORC1, and p38 /MAPK signaling cascades do not mediate the effect of NIC.
(A) Isolated ISCs cultured using a medium supplemented with or without 1 μM NIC combined with 5 nM Sotrastaurin (PKC inhibitor) (3 wells/group). (B) Immunoblotting analyses of p38, p-p38, p-S6, or S6 in ISCs isolated from mice treated with vehicle or NIC. (C) Isolated ISCs were cultured in a medium with or without 1 μM NIC and 1 μM AKT inhibitor VIII (3 wells/group). (D) Isolated ISCs were cultured in a medium with or without 1 μM Nicotine and 5 μM SB 203580 (3 wells/group). (E) Isolated ISCs were cultured in a medium with or without 1 μM NIC and 1 μM Rapamycin (3 wells/group). Significant differences are denoted by p values (Student’s t-test). See also Figure 4.
-
Figure 4—figure supplement 1—source data 1
The quantification of colonies in organoid assay.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-figsupp1-data1-v1.xlsx
-
Figure 4—figure supplement 1—source data 2
Immunoblotting data with labeling.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-figsupp1-data2-v1.zip
-
Figure 4—figure supplement 1—source data 3
Immunoblotting raw data.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig4-figsupp1-data3-v1.zip
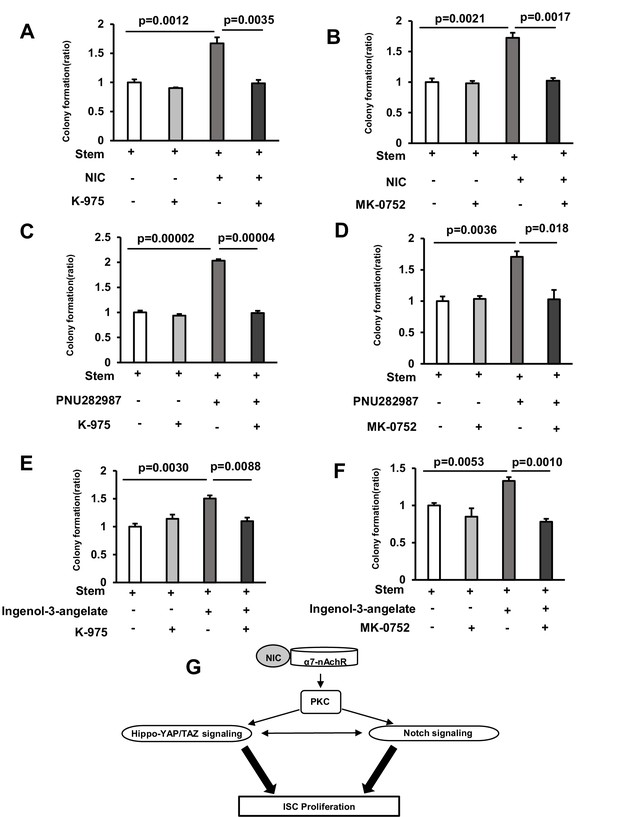
Inactivation of Hippo-YAP/TAZ and Notch signaling suppresses NIC-induced Colony Formation in mice.
(A) Isolated ISCs were cultured using a medium with or without 1 μM Nicotine and 5 nM K-975 (3 wells/group). (B) Isolated ISCs were cultured using a medium with or without 1 μM Nicotine and 1 μM MK-0752 (3 wells/group). (C) Isolated ISCs were cultured using a medium with or without 10 μM PNU282987 and 5 nM K-975 (3 wells/group). (D) Isolated ISCs were cultured using a medium with or without 10 μM PNU282987 and 1 μM MK-0752 (3 wells/group). (E) Isolated ISCs were cultured in a medium with or without 1 nM Ingenol-3-angelate and 5 nM K-975 (3 wells/group). (F) Isolated ISCs were cultured in a medium with or without 1 nM Ingenol-3-angelate and 1 μM MK-0752 (3 wells/group). (G) Schematic model of NIC-associated signaling pathway in ISCs. The model traces a signaling cascade via α7-nAchR, PKC, Hippo-YAP/TAZ and Notch signaling in ISCs. Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test).
-
Figure 5—source data 1
The quantification of colonies in organoid assay.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig5-data1-v1.xlsx
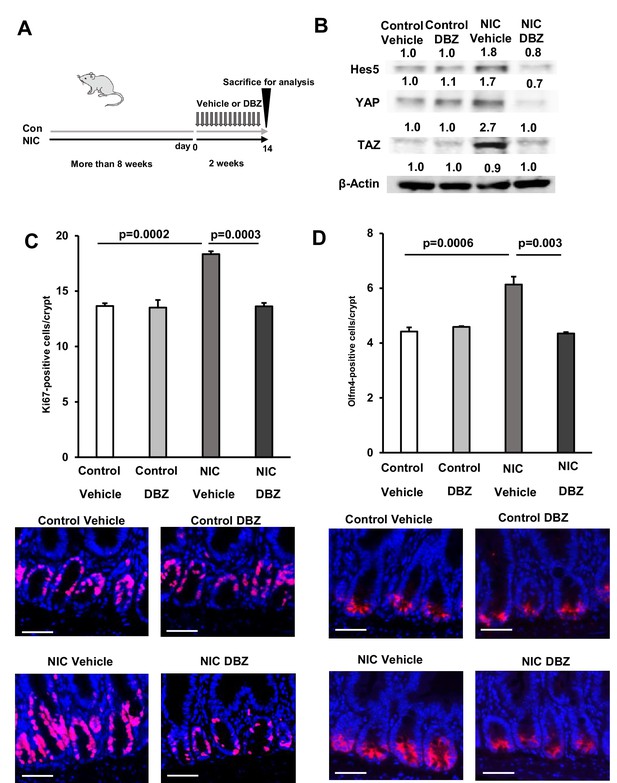
Dibenzazepine (DBZ) treatment suppresses the NIC-induced expansion of ISCs in vivo.
(A) Schematic representation of the treatment showing daily injection of DBZ (1 mg/kg body weight) for 2 weeks. (B) Immunoblotting analysis of crypt lysate isolated from DBZ- and vehicle-treated mice in control and NIC-treatment groups using Hes5, YAP, TAZ, and β-actin antibodies. (C and D) Immunostained Ki67-positive and (C) (Red, Ki67; Blue, DAPI) Olfm4-positive cells (D) (Red, Olfm4; blue, DAPI) and their quantification in the proximal jejunum of DBZ- or vehicle-treated mice (NIC-treated and untreated) (n=3 per group). Original magnifications: ×400 (C and D). Scale bar: 50 µm (C and D). Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 6—figure supplement 1.
-
Figure 6—source data 1
The quantification in immunostaining.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig6-data1-v1.xlsx
-
Figure 6—source data 2
Immunoblotting data with labeling.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig6-data2-v1.zip
-
Figure 6—source data 3
Immunoblotting raw data.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig6-data3-v1.zip
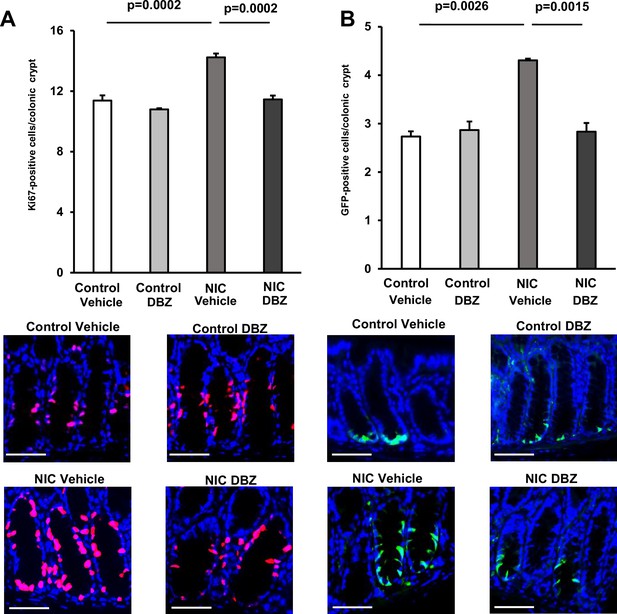
DBZ treatment suppresses the NIC-induced expansion of CSCs in vivo.
(A and B) Immuno-stained Ki67-positive cells (A) (Red, Ki67; Blue, DAPI) and LgR5-GFP-positive cells (B) (Red, Olfm4; blue, DAPI) and quantification of their abundance in the colon of DBZ or vehicle-treated Lgr5-EGFP-IRES-CreERT2 mice (NIC-treated and untreated; n=3 per group). Original magnifications: ×400. Scale bar: 50 µm. Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test). See also Figure 6.
-
Figure 6—figure supplement 1—source data 1
The quantification in immunostaining.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig6-figsupp1-data1-v1.xlsx

BZ inhibits intestinal tumor growth by NIC.
(A) Schematic representation of Apcflox/flox; Lgr5-EGFP-IRES-CreERT2 (Lgr5CreERT2 Apcfl/fl)tumor initiation. Mice were treated with control or NIC more than 8 weeks before a single Tamoxifen injection (30 mg/kg body weight), continued for 4 weeks before tissue collection. (B) Macroscopic quantification of the number and area of polyps in the entire intestine of control or NIC-treated Lgr5CreERT2 Apcfl/fl mice. (C) Representative images (Red: β-catenin, Blue: DAPI) and the quantification of the number of β-catenin positive adenomatous lesions in the entire intestine of control or NIC-treated Lgr5CreERT2 Apcfl/fl mice. (D) Schematic presentation of Lgr5CreERT2 Apcfl/fl tumor initiation. Control or NIC-treated Lgr5CreERT2 Apcfl/fl mice were subjected to a single Tamoxifen injection (30 mg/kg body weight), followed by daily DBZ or vehicle injections continued for 4 weeks before tissue collection. (E) Macroscopic quantification of the number of polyps in the entire intestine of DBZ or vehicle-treated Lgr5CreERT2 Apcfl/fl mice (NIC-treated and untreated). (F) Representative images (Red: β-catenin, Blue: DAPI) and the quantification of the number of β-catenin positive adenomatous lesions in the entire intestine of DBZ or vehicle-treated Lgr5CreERT2 Apcfl/fl mice (NIC-treated and untreated). Original magnifications: ×200 (C, and F). Scale bar: 50 µm (C, and F). Values represent the mean ± SEM. Significant differences are denoted by p values (Student’s t-test).
-
Figure 7—source data 1
The quantification of polyps and β-catenin positive lesions.
- https://cdn.elifesciences.org/articles/95267/elife-95267-fig7-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | Lgr5-EGFP-IRES-CreERT2 mice | Jackson Laboratory | #008875 | |
| Strain, strain background (Mus musculus) | Apc CKO mice | National Cancer Institute | #01XAA | |
| Strain, strain background (Mus musculus) | Rosa26-CAG-lsl-tdTomato mice | Jackson Laboratory | #007909 | |
| Antibody | rabbit monoclonal anti-Ki67 | Cell Signaling Technology | #12202 | IHC (1:200) |
| Antibody | rabbit monoclonal anti-Olfm4 | Cell Signaling Technology | #39141 | IHC (1:400) |
| Antibody | mouse monoclonal anti-GFP | Santa Cruz Biotechnology | #sc-9996 | IHC (1:50) |
| Antibody | rabbit polyclonal anti-Lysozyme | Thermo Fisher Schientific | #PA5-16668 | IHC (1:50) |
| Antibody | mouse monoclonal anti-chromogranin A | Santa Cruz Biotechnology | #sc-393941 | IHC (1:50) |
| Antibody | rabbit polyclonal anti-β-catenin | Cell Signaling Technology | #9562 | IHC (1:200) |
| Antibody | goat polyclonal anti-mouse IgG H&L (Alexa Fluor 488) | Abcam | #ab150113 | IHC (1:200) |
| Antibody | mouse monoclonal anti-β-actin | Santa Cruz | #sc-47778 | WB (1:200) |
| Antibody | mouse monoclonal anti-YAP | Santa Cruz | #sc-101199 | WB (1:200) |
| Antibody | mouse monoclonal anti-TAZ | Santa Cruz | #sc-293183 | WB (1:200) |
| Antibody | mouse monoclonal anti-α7-AchR | Santa Cruz | #sc-58607 | WB (1:200) |
| Antibody | mouse monoclonal anti-Notch1 | Santa Cruz | #sc-376403 | WB (1:100) |
| Antibody | mouse monoclonal anti-Jagged1 | Santa Cruz | #sc-390177 | WB (1:100) |
| Antibody | mouse monoclonal anti-Jagged2 | Santa Cruz | #sc-515725 | WB (1:100) |
| Antibody | mouse monoclonal anti-Hes5 | Santa Cruz | #sc-293445 | WB (1:200) |
| Antibody | mouse monoclonal anti-p38 | Santa Cruz | #sc-81621 | WB (1:200) |
| Antibody | mouse monoclonal anti-phospho-p38 | Santa Cruz | #sc-166182 | WB (1:100) |
| Antibody | rabbit monoclonal anti-S6 | Cell Signaling | #2217 | WB (1:1000) |
| Antibody | rabbit monoclonal anti-phospho-S6 Ser235/236 | Cell Signaling | #4858 | WB (1:2000) |
| Antibody | anti-Mouse IgG, HRP-Linked Whole Ab Sheep | Cytiva | NA931 | WB (1:5000) |
| Antibody | anti-Rabbit IgG, HRP-Linked Whole Ab Donkey | Cytiva | NA934 | WB (1:5000) |
| Antibody | rat monoclonal APC-conjugated anti-mouse CD24 Antibody | Biolegend | #101814 | FCY (1:500) |
| Commercial assay or kit | TSA Plus Cyanine 3 System | Akoya Biosciences | #NEL744001KT | |
| Chemical compound, drug | Mecamylamine | Cayman Chemical | #14602 | |
| Chemical compound, drug | Adiphenine hydrochloride | MedChemExpress | #HY-B0379A | |
| Chemical compound, drug | PNU282987 | MedChemExpress and Cayman Chemical | #17424 #HY-12560A | |
| Chemical compound, drug | α-Bungarotoxin | R&D | #2133/1 | |
| Chemical compound, drug | H-89 dihydrochloride | MedChemExpress | #HY-15979A | |
| Chemical compound, drug | Gö 6983 | Cayman Chemical | #13311 | |
| Chemical compound, drug | Sotrastaurin | MedChemExpress | #HY-10343 | |
| Chemical compound, drug | K-975 | MedChemExpress | #HY-138565 | |
| Chemical compound, drug | MK-0752 | MedChemExpress | #HY-10974 | |
| Chemical compound, drug | Ingenol-3-angelate | Cayman Chemical | #16207 | |
| Chemical compound, drug | AKT Inhibitor VIII | MedChemExpress | #HY-10355 | |
| Chemical compound, drug | SB203580 | Tokyo Chemical Industry | #F0864 | |
| Chemical compound, drug | Rapamycin | LKT Laboratories, Inc | #R0161 | |
| Chemical compound, drug | Collagenase Type IV | Worthington Biochemical Corporation | #CLS4 | |
| Chemical compound, drug | Valproic acid sodium salt | FUJIFILM Wako Pure Chemical Corporation | #2815/100 | |
| Chemical compound, drug | [-]-Cotinine | Sigma-Aldrich | #C-016 | |
| Chemical compound, drug | Nicotine hemisulfate salt | Sigma-Aldrich | #N1019 | |
| Chemical compound, drug | DBZ (Dibenzazepine) | Cayman Chemical | #14627 | |
| Chemical compound, drug | Tamoxifen | Cayman Chemical | #13258 | |
| Chemical compound, drug | Mounting medium With DAPI Aqueous Fluoroshield | Abcam | #ab104139 | |
| Chemical compound, drug | Can Get Signal Immunoreaction Enhancer Solution | TOYOBO | NKB-101 |





