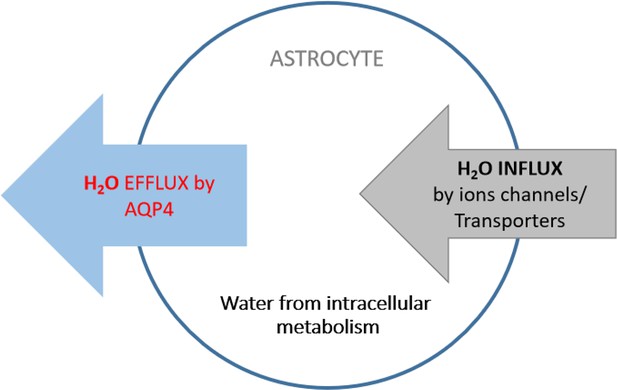
Astrocyte aquaporin mediates a tonic water efflux maintaining brain homeostasis
Figures
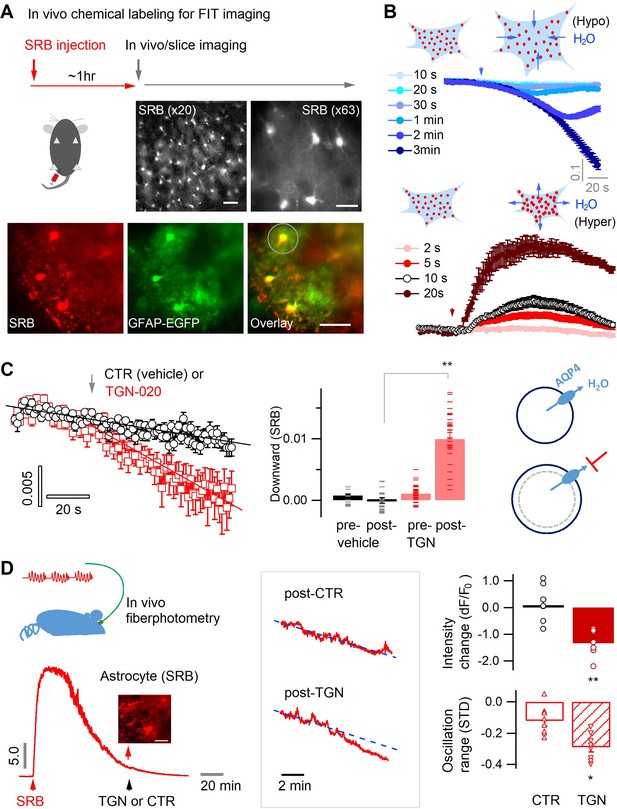
Astrocyte aquaporin mediates a tonic water efflux.
(A) In vivo chemical labeling of astrocytes. Sulforhodamine B (SRB, 10 mg/mL) was intraperitoneally injected in awake mice (10 µL/g). Monochrome images show representative astrocyte labeling in living acute brain slices from cortex under low (×20; scale bar, 50 µm) and high magnification (×63; scale bar, 20 µm) by epifluorescence. Below, SRB labeling was confirmed to be astrocyte-specific in acute brain slices of the astrocyte reporter line GFAP-EGFP, where light sheet imaging was used to gain optical sectioning (Materials and methods; Figure 1—figure supplement 1). The light gray circle indicates the astrocyte regions used for fluorescence analysis. Scale bar, 50 µm. (B) Optical imaging of astrocyte water transport in acute brain slices. Transmembrane water transport was triggered with hypo- and hypertonic solution, inducing water inflow and outflow that were respectively reflected by SRB fluorescence decrease (above) and increase (below; expressed as dF/F0). The hypo- or hypertonic solution was applied to slices over different lengths of duration displayed in colors corresponding to the time courses of SRB fluorescence (n=52 astrocytes, four mice). (C) Acutely blocking astrocyte AQP4 with TGN-020 caused intracellular water accumulation and swelling. The downward change in the SRB fluorescence was respectively calculated for the phases prior and post to vehicle or TGN application. Left, while no effect was seen under CTR condition (vehicle only, n=23 astrocytes, three mice), TGN-020 (20 µM) significantly decreased astrocyte SRB fluorescence (n=30, six mice). Imaging was performed in acute brain slices of layer II/III primary somatosensory (S1) cortex. Middle, the downward slope was compared between the periods before and after the application of TGN-020. Right, illustration shows astrocyte aquaporin sustaining a tonic water efflux. Its blockade causes water accumulation and cell swelling. (D) In vivo validation of the effect of TGN-020 application on astrocyte water homeostasis. Left, fiber photometry was used for real-time recording of SRB fluorescence of astrocyte population in S1 cortex in freely moving mice, with saline (CTR) or TGN-020 being intraperitoneally injected when SRB was trapped in astrocytes. Fiber photometry recording shows that in vivo SRB injection resulted in rapid entry into mouse cortex and, in about 1 hr, led to astrocyte labeling (inset scale bar, 50 µm). Middle, example response to saline and TGN-020. The change of SRB fluorescence relative to the photobleaching tendency delineated by line fitting (dotted line) was examined. Right, relative to CTR, TGN administration led to a decrease in astrocyte SRB fluorescence and its oscillation range (n=8 recordings per condition, five mice). Two-sample t-test was performed in Matlab, and error bars represent the standard errors.
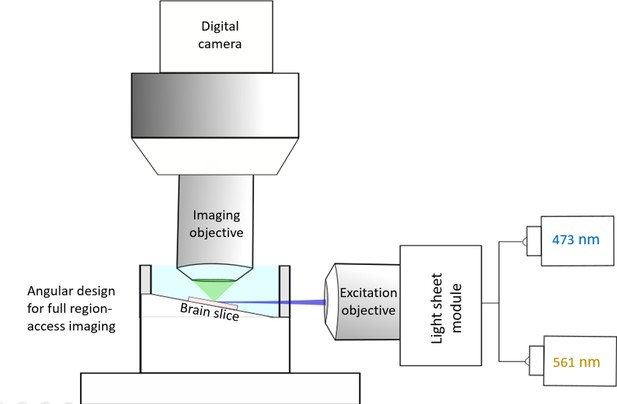
Angular light sheet imaging in acute brain slices.
Brain slice was placed at a slightly tilted (~8–12°) holding chamber so as to ensure the access of the light sheet to any regions of the slice, without interfering with the imaging objective. The light sheet illumination provides wide-field optical sectioning, while fluorescent images are collected by a digital camera via an independent imaging objective. See details in Materials and methods.
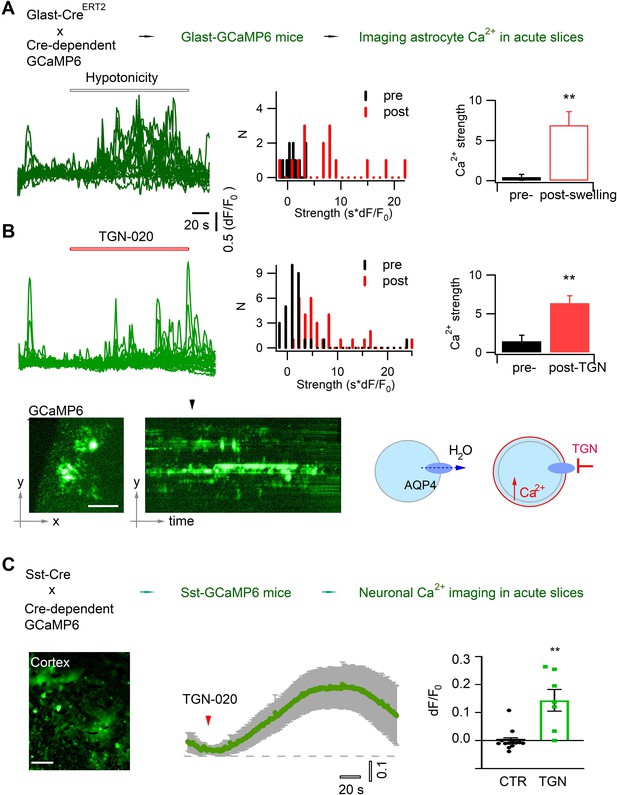
Acutely blocking astrocyte aquaporin induces swelling-associated Ca2+ oscillation.
(A) In vivo expression in astrocytes of the genetically encoded fluorescent Ca2+ indicator GCaMP6 for imaging astrocyte Ca2+ in acute brain slices. Angular light sheet microscopy (Figure 1—figure supplement 1) was used to capture transient Ca2+ signals of local regions. As a positive control, astrocyte swelling was induced by hypotonic solution (100 mOsM) that caused Ca2+ changes from their homeostatic level. Left, representative time courses of swelling-induced Ca2+ changes in detected response regions; middle to left, the histogram distribution and bar representation showing the signal strengths that were derived from the temporal integral of individual Ca2+ time courses normalized per minute, before and after cell swelling (n=15 regions of interest [ROIs], from three mice). (B) Astrocyte Ca2+ oscillation caused by acute aquaporin blocking with TGN-020 (20 µM, n=31 ROIs, from five mice), due to the inhibition of the tonic water efflux that led to astrocyte swelling as illustrated. Time scale and Ca2+ rise scale (dF/F0) are the same as in (A). Scale bar, 50 µm. Mann-Whitney U non-parametric test was performed in Matlab (Wilcoxon rank sum test). (C) Intercellular effect on somatostatin (Sst) interneurons of blocking astrocyte aquaporin water efflux. TGN-020 (20 µM) or the equal molar vehicle (CTR) was bath applied to acute cortical slices of Sst-GCaMP6 mice (n=7–12 regional measurements; four mice). Mann-Whitney U non-parametric test was performed in the Prism software. Scale bar, 50 µm.
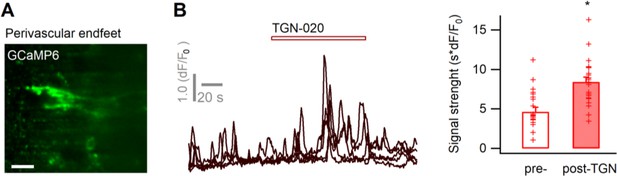
Calcium signals in perivascular astrocyte end feet.
(A) Imaging signals in the perivascular end feet of GCaMP6-expressing astrocytes. Scale bar, 20 µm. (B) The application of TGN-020-induced signal changes in the perivascular end feet (20 µM; n = 22 end feet regions of interest [ROIs] from three mice). On average, the strength of basal Ca2+signals in the end feet is higher than that observed across global astrocyte territories (4.65 ± 0.55 vs. 1.45 ± 0.79 Figure 2B, p<0.01), as does the effect of TGN (8.4 ± 0.62 vs. 6.35 ± 0.97 Figure 2B, p<0.05). Two-sample t-test was performed in Matlab, and error bars represent the standard errors.
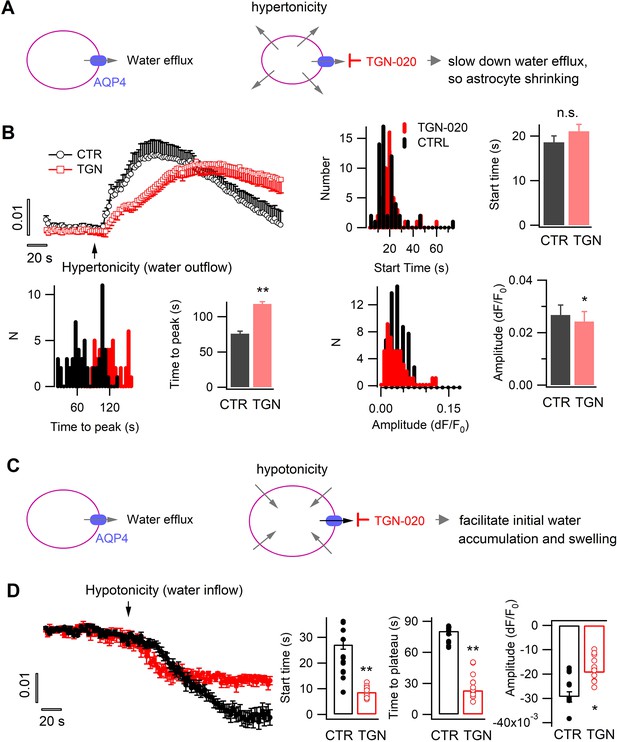
Tonic water efflux via aquaporin modulates phasic transmembrane water transport and astrocyte volume response.
(A) Left, in basal condition astrocyte aquaporin mediates a tonic water efflux. Right, protocol to induce water outflow from astrocytes, therefore their shrinking, by hypertonic extracellular solution (400 mOsM) in either control condition (CTR) or in the presence of AQP4 inhibitor TGN-020 (20 µM). (B) Time courses of astrocyte sulforhodamine B (SRB) fluorescence increase upon the phasically induced water outflow, reflecting the occurrence of shrinking. The histograms and bar charts compare the start time, namely the delay between hypertonic solution application and rise in SRB fluorescence, the time to reach the peak of shrinking, and the absolute amplitude of water outflow-induced SRB increase (n=58 astrocytes for CTR, and 47 astrocytes for TGN-020, four mice). Mann-Whitney U non-parametric test was performed in Matlab (Wilcoxon rank sum test). (C) Right, protocol to trigger water inflow into astrocytes by hypotonic extracellular solution (100 mOsM) in either CTR solution or in the presence of TGN-020 (20 µM). (D) Time courses of astrocyte SRB fluorescence decrease caused by water inflow, which also reflects concomitant cell swelling. In contrast to the observation with hypertonicity-induced water outflow and astrocyte shrinking, a reduction was observed for both the start time and the time to peak with TGN-020 (n=12 astrocytes for CTR, and 12 astrocytes for TGN-020, four mice). TGN-020 led to a decrease in the absolute amplitude of astrocyte swelling.
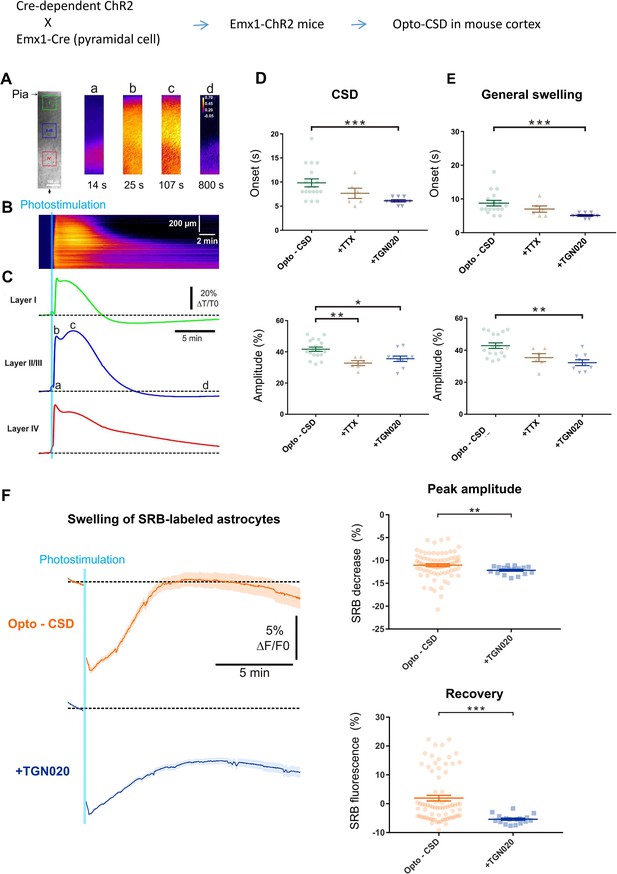
AQP4-mediated tonic water outflow regulates global swelling in cortical parenchyma.
Cortical spreading depression (CSD)-associated general swelling was induced by photostimulating ChR2-expressing pyramidal cells in acute cortical slices, and recorded by imaging intrinsic optical signal (IOS) with infrared illumination. (A) Representative recording. The pia surface is on the upper side; green, blue, and red squares correspond to regions of interest in layers I, II–III, and IV, respectively. Transmittance signals are represented in pseudocolor images at different time points post photostimulation. Scale bar, 100 µm. (B–C) Kymograph and time courses showing the IOS changes following photoactivaiton, derived from the radial line of interest indicated by a black arrow in (A). The light blue line indicates the 10 s photostimulation that increases the infrared transmittance signal (dT/T0) across cortical layers, as also illustrated by the time courses; a, b, c, and d correspond to the time points depicted in (A). After the onset delay (a), the first (b) and second (c) peak of IOS are characteristic of the CSD and a prolonged general swelling, respectively. (D–E) TGN-020 (20 µM) inhibition of AQP4 reduced the initial onset and the maximal amplitude of both the CSD and general swelling (n=6–18 measurements from 13 mice per condition) in layer II/III cortex. Inhibiting spiking activity with tetrodotoxin (TTX) (1 µM) only affected the amplitude of the initial CSD response. Bonferroni-Holm correction was used for multiplecomparisons. (F) Astrocytes swelling, reflected by sulforhodamine B (SRB) fluorescence decrease, monitored in control condition (n=75 astrocytes) and in the presence of TGN-020 (n=17 astrocytes) in layer II/III cortex. Mann-Whitney U non-parametric test was performed in Statsoft.
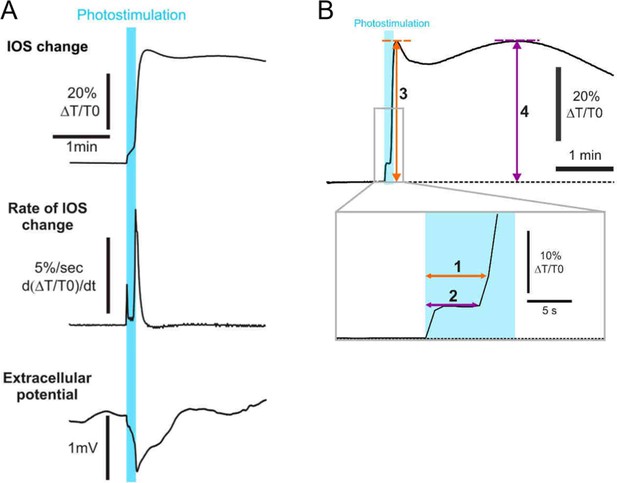
Optogenetic cortical spreading depression (opto-CSD) initiation and parameters analysis.
(A) From the intrinsic optical signal (IOS) change (upper), its temporal derivative was extracted to show the biphasic rate of the initiation of CSD (middle). Its starting, meanwhile, coincides with the extracellular potential drop (bottom; mean = -0.95 ± 0.21 mV, n = 4). (B) Parameter measurements for IOS changes: onset of the CSD (1) and the general swelling (2) relative to the beginning of the photostimulation, maximal IOS changes during CSD (3) and general swelling (4).
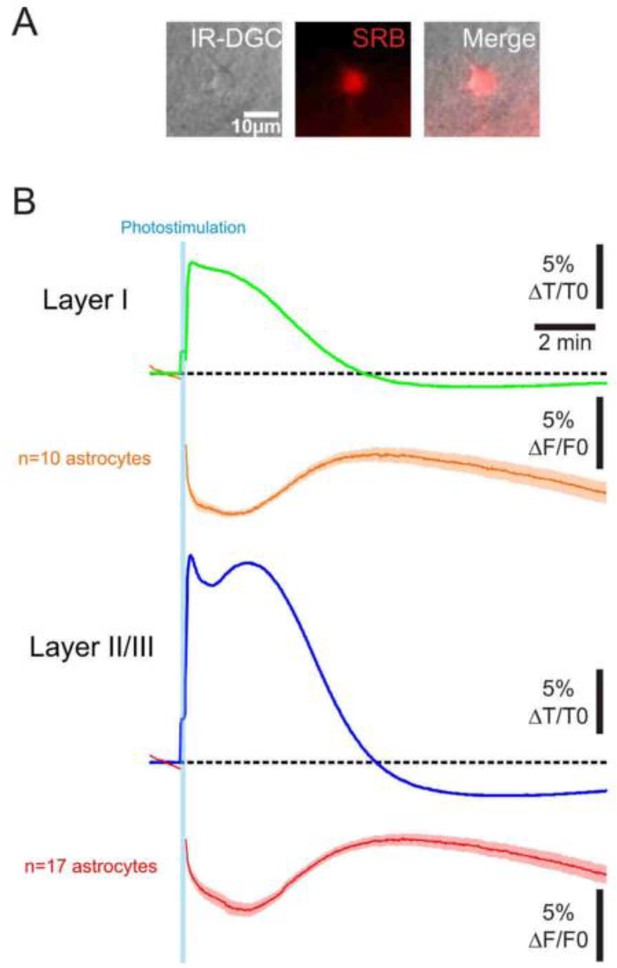
Astrocytic swelling during an optogenetic cortical spreading depression (opto-CSD) across cortical layers.
(A) Representative astrocyte sulforhodamine B (SRB) labeling observed under IR-DGC and epifluorescence. (B) Intrinsic optical signal (IOS) response (green and blue traces) and astrocytes swelling, reflected by SRB fluorescence decrease (orange and red traces), co-imaged in acute cortical slices. The dotted lines represent baseline and fluorescence imaging in fluorescent intensity. Astrocyte swelling spans over both the initial CSD response and the subsequent general cellular swelling of the parenchyma.
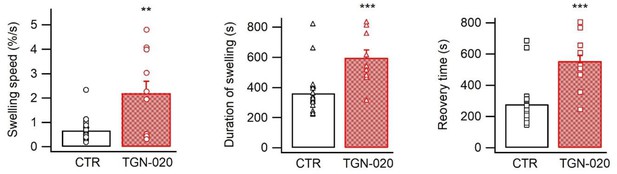
The swelling speed, duration of swelling, and recovery time during optogenetic cortical spreading depression (opto-CSD) (n=18 measurements from five mice for CTR, 10 measurements from four mice for TGN-020 for each parameter).
Mann-Whitney U non-parametric test was performed in Matlab.
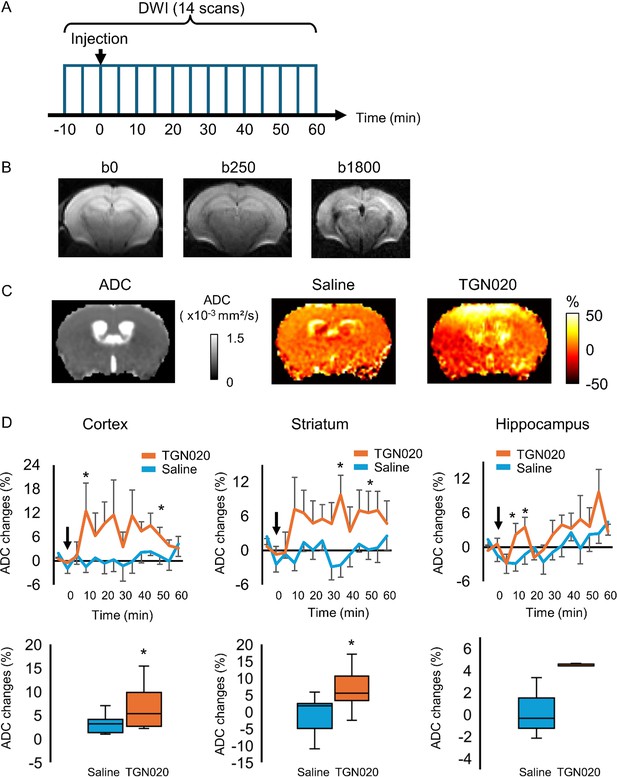
Perturbing the tonic water efflux via astrocyte aquaporin alters brain water homeostasis.
In vivo diffusion-weighted magnetic resonance imaging (DW-MRI) (7 T) was employed to map water diffusion in the entire brain scale following the acute inhibition of astrocytic AQP4 (TGN-020, intraperitoneal injection, 200 mg/kg), with paralleled control experiments performed with saline injection. (A) Experimental protocol for DW-MRI. Saline or TGN-020 was injected at 10 min after the start of acquisition. Diffusion-weighted image (DWI) was acquired every 5 min. (B) Representative image obtained at three different b-values to derive the water diffusion rates. (C) Left, brain water diffusion rate was mapped by the calculation of apparent diffusion coefficient (ADC). Right, representative images illustrating the relative changes of ADC at 60 min after injection of saline or TGN-020. (D) Upper panel, time courses depicting the temporal changes in ADC in the cortex, striatum, and hippocampus, revealing the regional heterogeneity. Arrowhead indicates the injection of saline or TGN-020 (n=7 mice for saline injection, 7 mice for TGN-020). *p<0.05 vs baseline at each time point following the two-way repeated measures ANOVA. Lower panel, box-plot of the averaged ADC between 30 and 50 min after the injection. *p<0.05 by paired t-test.
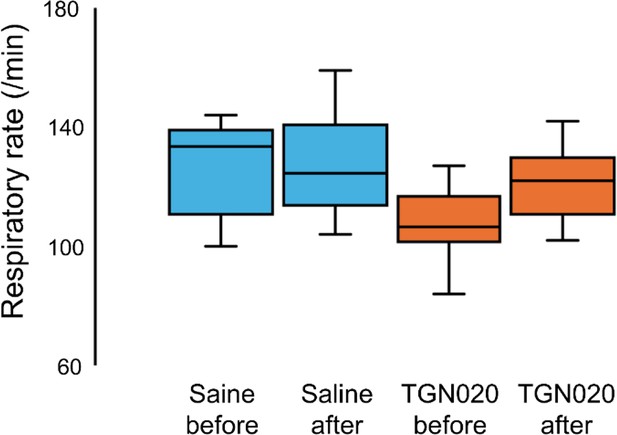
Box-car plot of averaged respiratory rate before and after injection of saline or TGN-020.
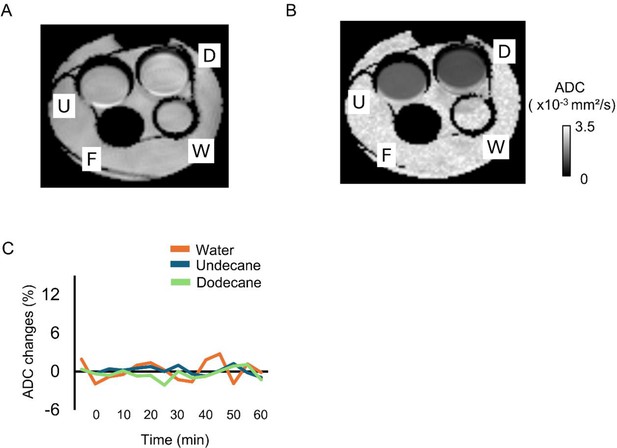
Apparent diffusion coefficient (ADC) in water, undecane, dodecane, and fluorine (proton-free liquid).
(A, B) Representative image obtained of (A) b = 0 s/mm² and (B) apparent diffusion coefficient (ADC) map. D, n-dodecane; F, fluorine; U, undecane; W, water. (C) Time courses of water diffusion change in each solution was mapped by the calculation of ADC.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/95873/elife-95873-mdarchecklist1-v1.pdf
-
Source code 1
Matlab scripts used for signal analysis.
Calcium signal analysis by spatial-temporal correlation screening. The method principle has been described in Pham et al., 2020, which identified the active regions of interest (ROIs) of astrocytes displaying dynamic Ca2+ signals.
- https://cdn.elifesciences.org/articles/95873/elife-95873-code1-v1.zip