Stable sequential dynamics in prefrontal cortex represents subjective estimation of time
Figures
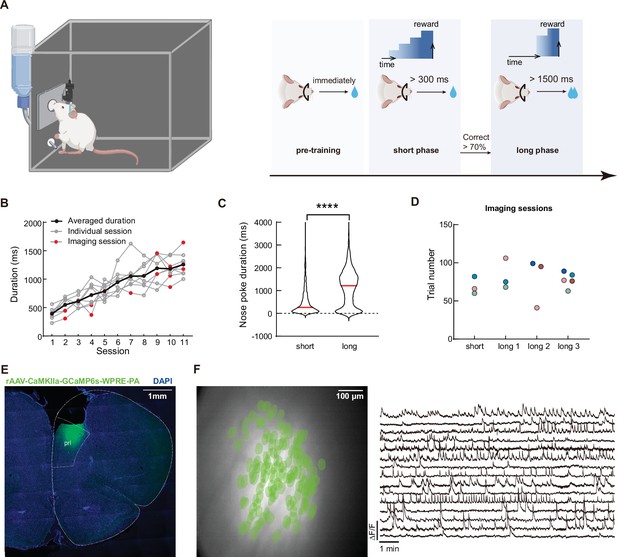
Calcium imaging during the timing task in rat mPFC.
(A) Schematics of the timing tasks. Rats undergo behavior training in three phases. Pre-training phase: rat received water reward immediately after nose poking action. Short phase: rat had to maintain nose poking position for at least 300ms to receive reward. And long phase: rat had to maintain nose poking position for at least 1500ms to receive reward. Additional water reward would be provided for every additional 500ms of holding the nose poke. (B) Quantification of the rats’ average nose poking durations in each session. Gray lines indicate each rat’s performance, and the black line represents the averaged performance. Red dots indicate sessions with calcium imaging. (C) Violin plot for the nose poking duration in two phases of the task. Red lines indicate the median value in each group. Two-tailed t test, ****: p<0.0001, n=5 rats. (D) Sessions with calcium imaging data included in this study. The same color of dots indicated data from the same rat. (E) Representative histological section of mPFC from an imaged rat. The white dotted line indicates borders of brain structures. (F) Representative imaging field of view (left panel) with green masks of extracted cells’ positions and example GCaMP fluorescent traces (right panel).
-
Figure 1—source data 1
Data for generating panels B, C and D.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig1-data1-v1.xlsx
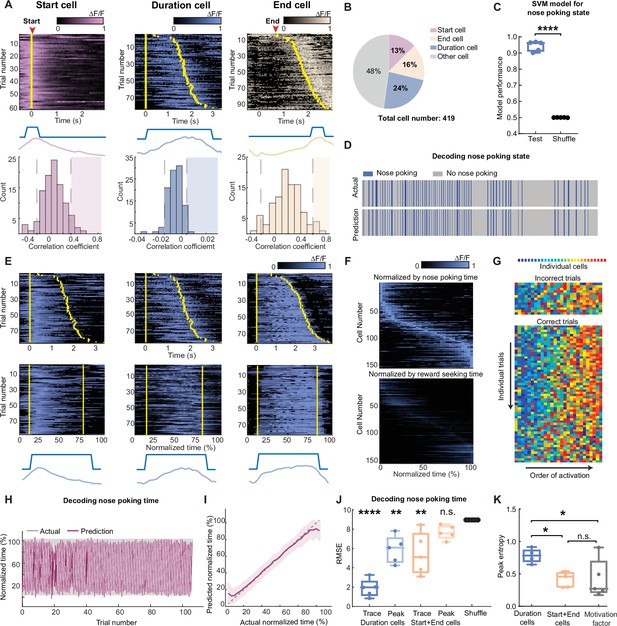
Scaling of the mPFC sequential activity by duration at individual trial resolution.
(A) The definition and examples of start (pink), duration (blue), and end (beige) -coding cells. To define a cell’s coding identity, we calculated the correlation between its activity and the specific event (middle diagrams), and compared to the null distribution generated from shuffled data from the same cell (bottom graphs). Statistical significance was determined if the actual correlation fell into the right 95th percentiles of the null distribution (shaded areas). Top panels show calcium activities from representative coding cells. Trials were sorted in ascending total durations. Yellow bars indicate the start and end of the nose poking event. (B) The proportion of cell coding types in the observed neurons from five rats in the long3 session. (C) Quantification of SVM classifiers in predicting the nose-poking state using neuron activities from the start and end cells, from the long3 session. Two-tailed t test, ****: p<0.0001, n=5 rats. (D) Representative actual nose poking events and predicted events using SVM models. (E) Three examples of duration cell with their raw traces (top panels) and traces in normalized time (middle panels). Yellow bars indicate the start and end of the nose poking event. Bottom panels show nose poking periods and averaged traces in normalized time scale. (F) Traces of averaged normalized activity of all recorded duration cells in five rats, sorted by the ascending peak position in the normalized time (upper panel) or the water reward-seeking time (lower panel). (G) Representative rainbow plot of the activity peak position ranks from duration cells in one session. Each color indicates an individual duration cell and the x-axis is sorted by the order of each cell’s peak activity appearance on individual trials. (H–I) Representative results of predicted trial progression and actual trial progression by a GPR model fitted from duration cells activity in one session. Results are plotted in individual trials (H) and averaged trial (I). Results are represented as mean ± S.D. (J) Quantification of the GPR model performance. Each dot indicates averaged RMSE from one rat. Models were trained using duration cells or start and end cells activities, with raw calcium traces or extracted peaks. Data are represented as medium±S.D. One-way ANOVA test with Dunnett’s multiple comparisons post-hoc test, ****: p<0.0001, **: p<0.01, n.s.: not significant, n=5 rats. (K) Quantification of the peak entropy on the trial-average calcium traces as a measure of sequentiality. The motivation factor group indicates duration cells’ activities normalized with the water reward-seeking time. One-way ANOVA test with Dunnett’s multiple comparisons post-hoc test, *: p<0.05, n.s.: not significant, n=5 rats.
-
Figure 2—source data 1
Data for generating panels A, C, I, J and K.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig2-data1-v1.xlsx
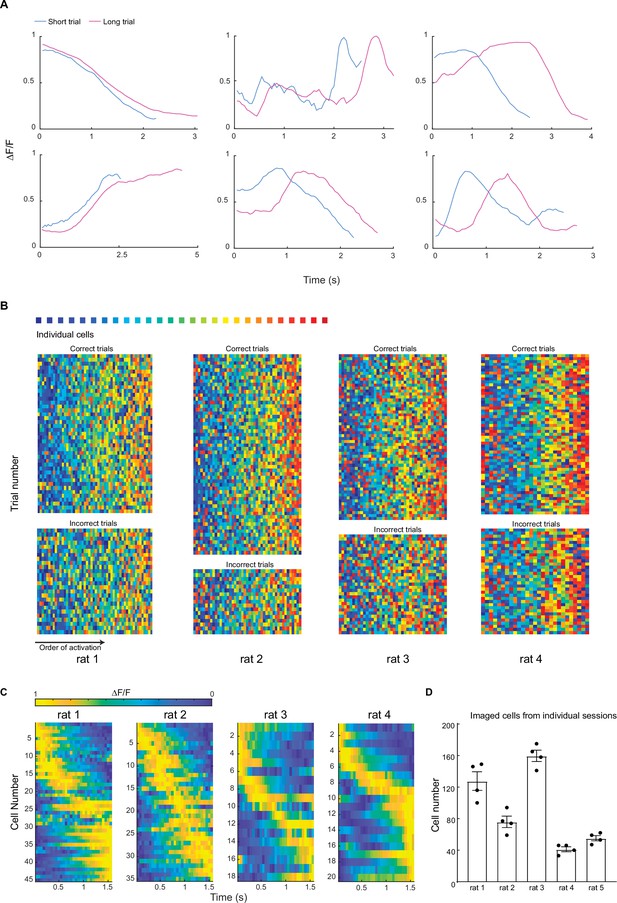
Additional examples of time-related sequential activities in mPFC.
(A) Representative trial-average calcium traces from cells in short phase and long phase sessions. Each graph shows an individual cell’s responses. (B) The rest of the imaged rats of their peak position rank maps in rainbow plot format, similar to Figure 2G. (C) Individual rat’s duration cell activity in trial-average form. Cells were sorted by their peak position in each graph. (D) Number of cells recorded from individual sessions of each rat. Each dot indicates an imaging session.
-
Figure 2—figure supplement 1—source data 1
Data for generating panel D.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig2-figsupp1-data1-v1.xlsx
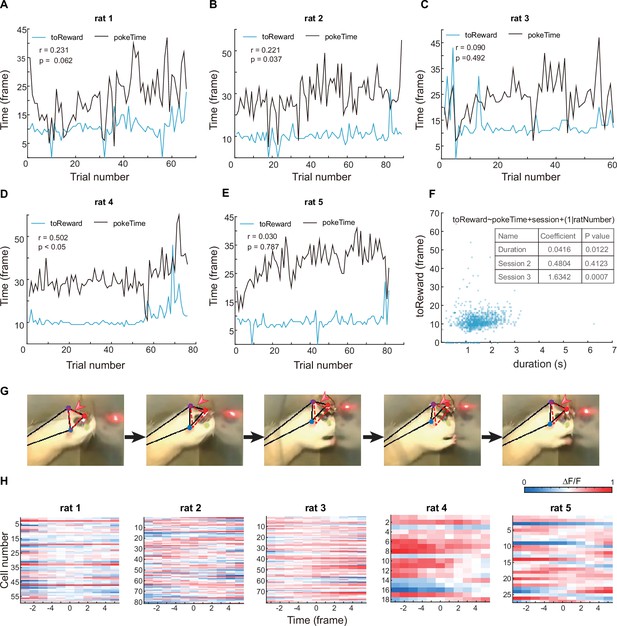
Analyses of rats’ motivation and motions.
(A–E) Plots of each trial’s nose poking duration (black) and interval time between exiting nose poke to licking water reward (blue) for individual rats. Pearson’s correlation analysis was used. (F) A mixed-effect general linear regression model for nose poking duration and reward-seeking time from all data. The formula was indicated above the graph.The coefficient for the ‘Duration’ parameter indicates a significant correlation between nose poke duration and reward-seeking time. (G) Representative images for head motion. Three positions (red arrow head) were identified using DeepLabCut software and used for quantify head motion: left ear (purple dot), right ear (blue dot) and a reflective part on the miniscope (red dot). Red dashed triangle showed the head position at the first time point. (H) Traces of averaged activity of all recorded coding cells in five rats, aligned by the start of head motion (time 0).
-
Figure 2—figure supplement 2—source data 1
Data for generating panel F.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig2-figsupp2-data1-v1.xlsx
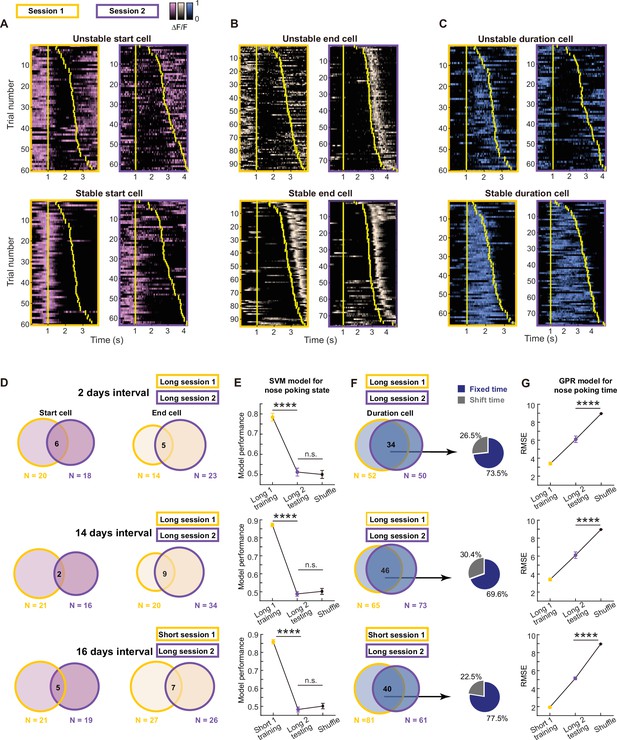
Long-term stable time coding by mPFC duration cell sequences.
(A–C) Example traces of unstable or stable coding start cell pink, (A), end cell beige, (B) and duration cell blue, (C) in two sessions. Yellow bars indicate the onset and offset of the nose poking event. (D) Venn diagrams of dynamics in start cells and end cells across sessions with different intervals. (E) Quantification of within-session and cross-session performance of the SVM models trained by start cells and end cells from one session for classifying nose poking states. For each group, three rats’ data were pooled together. Data are presented as mean ± S.D. One-way ANOVA test with Dunnett’s multiple comparisons post-hoc test, ****: p<0.0001, ***: p<0.001, n.s.: not significant, n=50 random sampling. (F) Venn diagrams of dynamics in start cells and end cells across sessions with different intervals. And pie charts show shifted cell and fixed cell within the stable duration cells. (G) Quantification of within-session and cross-session performance of the GPR models trained by duration cells from one session for predicting normalized nose poking time. For each group, three rats’ data were pooled together. Data are presented as mean ± S.D. Two-tailed t test, ****: p<0.0001, n=50 random sampling.
-
Figure 3—source data 1
Data for generating panels E and G.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig3-data1-v1.xlsx
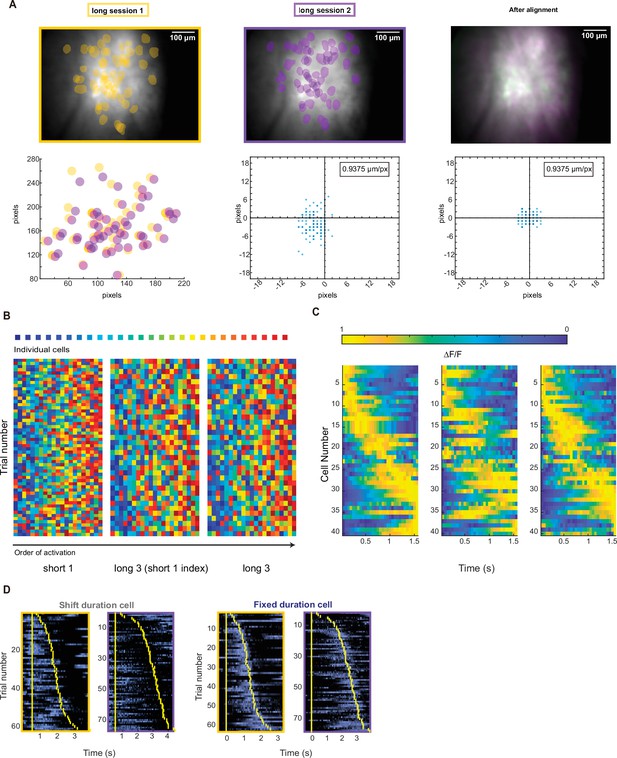
Additional information related to cross-session alignment.
(A) Representative field of view for two sessions from the same rat. Yellow and purple shades indicate extracted cells’ position masks from each session. The right panel shows the matched cells’ positions after alignment. The scatter plot in the left bottom corner shows the cell masks coordinates in two movies. The scatter plots on the right side show matched cell’s distances between session before (middle) and after (right) alignment. (B) Representative rainbow plot of the same rat’s duration cells of their peak position rank maps in different sessions. Left panel shows data from short1 session sorted by the peak order in short1 session. Right panel shows data from long3 session sorted by the peak order in long3 session. Middle panels show data from long3 session sorted by the peak order in short1 session. (C) Representative trial-averaged activities in normalized time scale from the same rat’s two sessions, with the same sorting scheme described in B. (D) Example traces of stable duration cells that show shifted preferred position and fixed preferred duration. Yellow bars indicate the onset and offset of the nose poking event.
-
Figure 3—figure supplement 1—source data 1
Data for generating panel A.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig3-figsupp1-data1-v1.xlsx
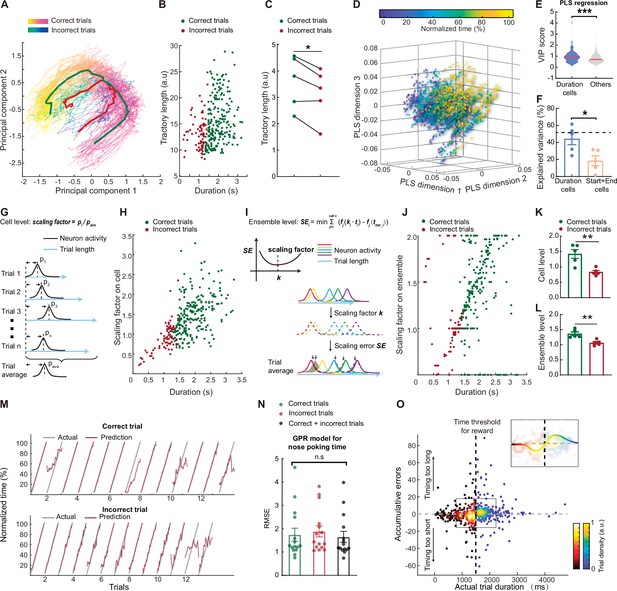
Behavioral errors in time estimation can be attributed to miscalculation in scaling the mPFC sequences.
(A) Representative trajecotries of neuronal activities from correct (spring colored) and incorrect (winter colored) trials. The red and green curves indicate averaged trajectories from incorrect and correct trials, respectively. (B) Scatter plot of the trajectories lengths from individual trials, plotted against trial durations. (C) Quantification of averaged correct and incorrect trajectory lengths. N=5 rats. Paired t test, *: p<0.05. (D) Scatter plots of the first 3 dimensions of a partial least-square (PLS) regression model between neuron activities and normalized trial time. (E) Violin plot of the variable importance for projection (VIP) scores derived from the PLS regression in A. Two-tailed t test, ***: p<0.0001. (F). Quantification of the explained variance in the normalized time by neuron activities from duration cells or start and end cells. Dashed line indicated explained variance from all cells. Data are represented as mean ± S.D. Two-tailed t test, *: p<0.05. n=5 rats. (G) Diagram of the scaling factor calculation process based on individual cell activities. (H) Scatter plot of the single-cell level scaling factor plotted against trial durations. (I) Diagram of the scaling factor calculation process based on population cell activities. (J) Scatter plot of the population level scaling factor plotted against trial durations. (K and L) Quantification of the averaged scaling factors on single-cell (K) or population (L) level. N=5 rats. Two-tailed t test, **: p<0.01. (M) Representative model prediction of normalized trial time for correct trials and incorrect trials. Each segment indicated on trial. The GPR model was trained on balanced correct and incorrect trials. (N) Quantification of GPR model performance for predicting different types of trials. In each group the model was trained on one type of trials and tested on the same trial type. Data are presented as mean ± S.D. One-way ANOVA test, n.s., not significant. (O) Scatter plots of cumulative prediction errors and actual trial duration. Correct and incorrect trials were colored differently but both by trial densities. There is a cluster of incorrect trials with below 0 cumulative errors and one for correct trials above 0. The insert shows the same data from the region in the box with a trend line color-coded with the trial densities.
-
Figure 4—source data 1
Data for generating panels F, H, J, K, L, N and O.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig4-data1-v1.xlsx
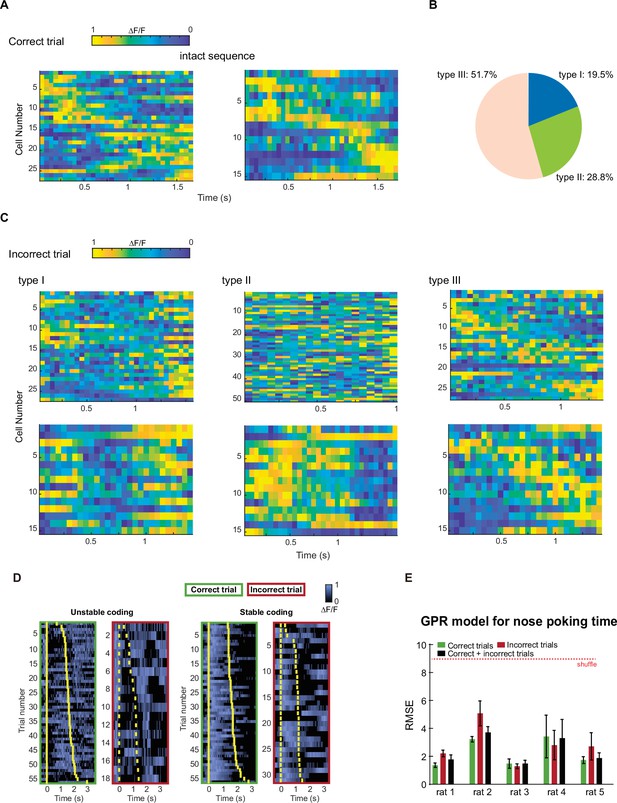
Different types of incorrect types based on changes in mPFC time sequences.
(A) Representative activities of duration cells in correct trials. (B) Pie chart of the prevalence of the three types of incorrect trials. (C) Example duration cell activities in the three types of incorrect trials. (D) Example traces of duration cells that show correctness-modulated (left) or correctness-independent coding (right). Yellow bars indicate the onset and offset of the nose poking event. (E) Quantification of GPR model performance for predicting different types of trials for individual rats. In each group, the model was trained on one type of trials and tested on the same trial type. Data are presented as mean ± S.D. Red dotted line indicates errors derived from a randomly shuffled dataset.
-
Figure 4—figure supplement 1—source data 1
Data for generating panel E.
- https://cdn.elifesciences.org/articles/96603/elife-96603-fig4-figsupp1-data1-v1.xlsx
Graphic summary of the findings in current study.
We described a group of neurons in the rat mPFC that represent time with activity sequences. The sequential procession can be rapidly stretched or compressed to track the total time durations on individual trial level. The identity of these time-coding cells and their sequential code remain stable over weeks, which is not seen in neurons coding for trial start or end.