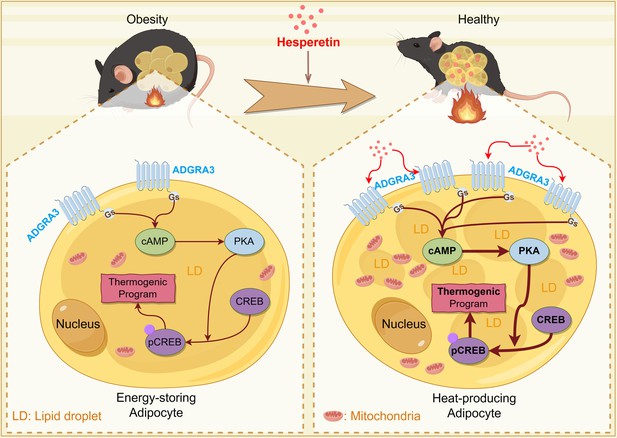
Constitutively active receptor ADGRA3 signaling induces adipose thermogenesis
Figures
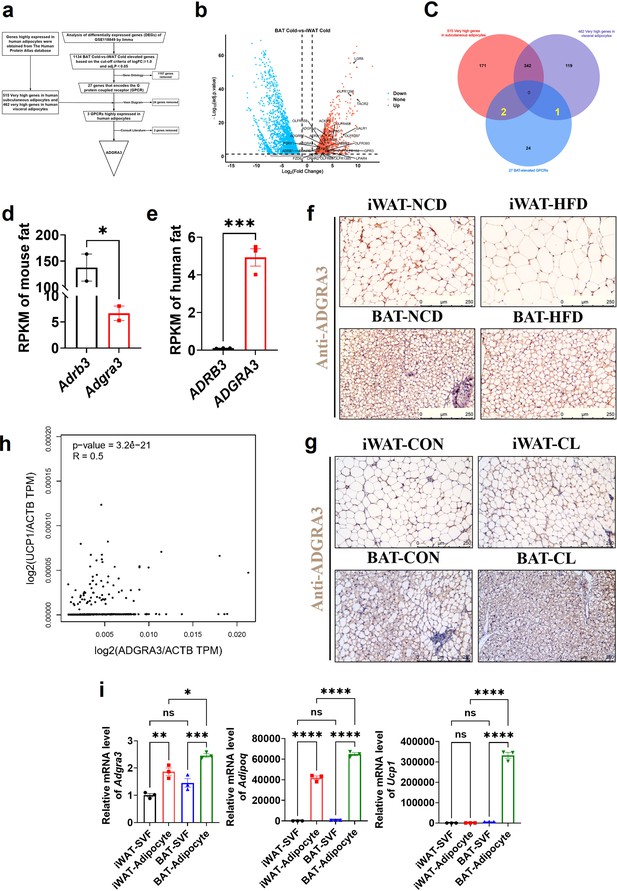
ADGRA3 is a high-expressed GPCR in human adipocytes and mouse brown fat.
(A–F) ADGRA3 screening as a high-expressed GPCR in human adipocytes and mouse brown fat via comprehensive analysis. Brown adipose tissue and subcutaneous WAT were dissected from mice that were treated in cold (4℃) temperature for 72 hr. A total of six samples with three replicates for each adipose tissue were evaluated. The datasets of human subcutaneous adipocytes and human visceral adipocytes were acquired from the human protein atlas database. (A) Flowchart of screening. (B) Volcano plot summarizing the differentially expressed genes (DEGs) between cold temperature BAT group and cold temperature iWAT group. Blue and red shading are used to indicate down-regulation and up-regulation, respectively. (C) 27 BAT-elevated GPCRs from transcriptome, 515 very high genes in subcutaneous adipocytes and 462 very high genes in visceral adipocytes from the human protein atlas database were analyzed by using a Venn diagram. (D–E) The RPKM of ADRB3 and ADGRA3 genes in mouse fat (D) from Mouse ENCODE transcriptome data (PRJNA66167, N=2) and human fat (E) from HPA RNA-seq normal tissues (PRJEB4337, N=3). (F) C57BL/6 J mice fed with a NCD or a HFD for 12 weeks. Representative images of iWAT and BAT stained with ADGRA3. Scale bars, 250 μm. (G) C57BL/6 J mice fed with a HFD for 12 weeks were injected with vehicle or CL (1 mg/kg daily) over 7 days. Representative images of iWAT and BAT stained with ADGRA3. Scale bars, 250 μm. (H) Correlation between UCP1 expression level normalized by ACTB gene and ADGRA3 expression level normalized by ACTB gene in human subcutaneous fat dataset from GTEx Portal database (N=663). (I) qPCR analysis of Adgra3, Adipoq and Ucp1 genes in Stromal Vascular Fraction (SVF) and mature adipocyte isolated from iWAT and BAT (N=3 for each group). iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; RPKM, Reads Per Kilobase per Million mapped reads; TPM, Transcripts Per Kilobase Million; GPCR, G-protein-coupled receptor; NCD, normal chow diet; HFD, high-fat diet; CL, CL-316,243; SVF, Stromal Vascular Fraction. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (D–E), simple linear regression (H) and one-way ANOVA (I).
-
Figure 1—source data 1
Numerical source data for Figure 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig1-data1-v1.zip
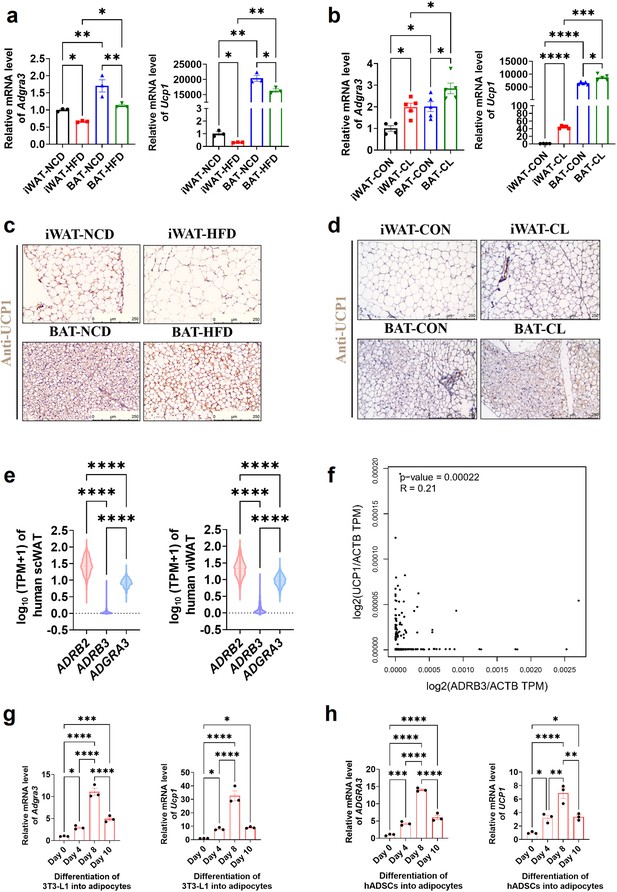
ADGRA3 positively correlated with beige fat.
(A–B) qPCR analysis of Adgra3 and Ucp1 in iWAT and BAT from different treatment mice (A: N=3 for each group; B: N=4 for iWAT-CON, N=5 for each other group). (C) C57BL/6 J mice fed with a NCD or a HFD for 12 weeks. Representative images of iWAT and BAT stained with UCP1. Scale bars, 250 μm. (D) C57BL/6 J mice fed with a HFD for 12 weeks were injected with vehicle or CL (1 mg/kg daily) over 7 days. Representative images of iWAT and BAT stained with UCP1. Scale bars, 250 μm. (E) The TPM of ADRB2, ADRB3 and ADGRA3 genes in human scWAT (left, N=663) and viWAT (right, N=541) from the GTEx database. (F) Correlation between UCP1 expression level normalized by ACTB gene and ADRB3 expression level normalized by ACTB gene in human subcutaneous fat dataset from GTEx Portal database (N=663). (G) qPCR analysis of Adgra3 and Ucp1 during the differentiation of 3T3-L1 into adipocytes (N=3 for each group). (H) qPCR analysis of ADGRA3 and UCP1 during the differentiation of hADSCs into adipocytes (N=3 for each group). iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; scWAT, subcutaneous white adipose tissue; viWAT, visceral white adipose tissue; NCD, normal chow diet; HFD, high-fat diet; CL: CL-316,243; qPCR, quantitative real-time PCR; hADSCs, human adipose-derived mesenchymal stem cells. All data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA (A–B, E and G–H) and simple linear regression (F).
-
Figure 1—figure supplement 1—source data 1
Numerical source data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig1-figsupp1-data1-v1.zip
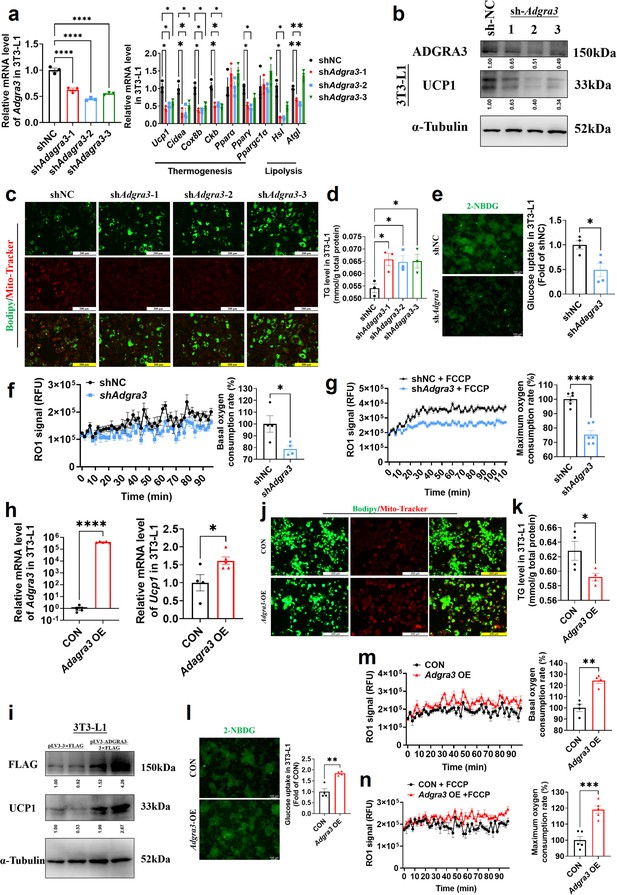
Adgra3 overexpression promotes the biogenesis of beige adipocytes.
(A, H) qPCR analysis of Adgra3, thermogenesis and lipolysis genes in 3T3-L1 mature beige-like adipocytes (A: N=3 for each group; H: N=4 for CON, N=5 for Adgra3 OE). (B, I) Western blot analysis for level of ADGRA3, UCP1 and ADGRA3−3×FLAG protein in 3T3-L1 mature beige-like adipocytes treated with shAdgra3 (pLKO.1-U6-shAdgra3-(1/2/3) plasmid encapsulated in nanomaterials), shNC (pLKO.1-U6-shNC plasmid encapsulated in nanomaterials), Adgra3 OE (pLV3-CMV-Adgra3(mouse)–3×FLAG plasmid encapsulated in nanomaterials) or CON (pLV3-CMV-MCS-3×FLAG plasmid encapsulated in nanomaterials). The ImageJ software was used for gray scanning. (C, J) Bodipy green staining for lipid droplet and Mito-Tracker red staining for mitochondria in 3T3-L1 mature beige-like adipocytes. Scale bars, 200 μm. (D, K) The level of intracellular triglyceride in 3T3-L1 mature beige-like adipocytes (D: N=3 for each group; K: N=4 for each group). (E, I) Glucose uptake assay in 3T3-L1 mature beige-like adipocytes and staining intensity analysis diagram (right, N=4 for each group). (F, M) When 3T3-L1 mature beige-like adipocytes were treated with shNC, shAdgra3, CON or Adgra3 OE, fluorescence of the oxygen probe (RO1) in the cells was monitored and the rate of basal oxygen consumption was analyzed (N=4 for each group). (G, N) When FCCP-treaded 3T3-L1 mature beige-like adipocytes were treated with shNC, shAdgra3, CON or Adgra3 OE, fluorescence of the oxygen probe (RO1) in the cells was monitored and the rate of maximum oxygen consumption was analyzed (G: N=6 for each group; N: N=5 for each group). All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (E–H and K–N) and one-way ANOVA (A and D).
-
Figure 2—source data 1
Raw uncropped blots for Figure 2.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig2-data1-v1.zip
-
Figure 2—source data 2
Uncropped and labeled blots for Figure 2.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig2-data2-v1.zip
-
Figure 2—source data 3
Numerical source data for Figure 2.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig2-data3-v1.zip
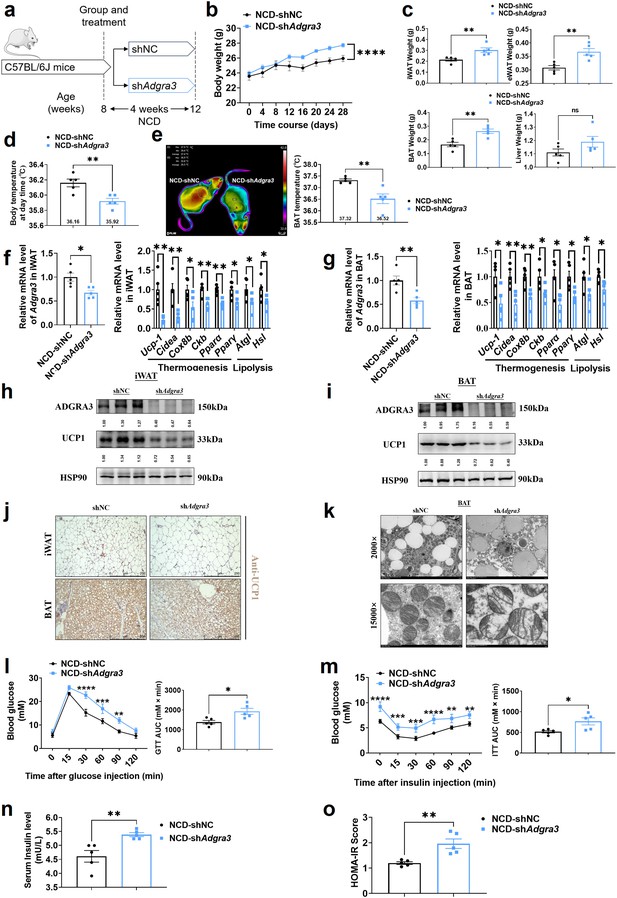
Knockdown of Adgra3 suppressed the adipose thermogenic program and impaired metabolic homeostasis in mice.
(A) Experimental schematic. C57BL/6 J mice fed with a NCD for eight weeks were injected with shAdgra3 (pLKO.1-U6-shAdgra3-2 plasmid encapsulated in nanomaterials) or shNC (pLKO.1-U6-shNC plasmid encapsulated in nanomaterials) twice a week for four weeks. (B–D) Changes in body mass (B), tissue weight (C) and body temperature (D) in mice injected with shNC or shAdgra3 for 28 days (N=5 for each group). (E) Thermal image and BAT temperature of mice injected with shNC or shAdgra3 for 28 days (N=5 for each group). (F–G) qPCR analysis of Adgra3, genes associated with thermogenesis and lipolysis in iWAT (F) and BAT (G) from different treatment mice (N=5 for each group). (H–I) Western-blot analysis for the level of ADGRA3 and UCP1 protein in iWAT (H) and BAT (I) from differently treated mice. (J) Representative images of iWAT (top) and BAT (bottom) stained with UCP1. Scale bars, 250 μm. (K) Transmission electron microscope photograph of BAT treated with shNC or shAdgra3. (L) Glucose tolerance test (GTT) was conducted by intraperitoneal injection of glucose (2 g/kg) and measurement of blood glucose concentration with a OneTouch Ultra Glucometer at designed time points in 6 hr fasted mice (N=5 for each group). (M) Insulin tolerance test (ITT) was done by intraperitoneal injection of insulin (0.5 U/kg) and measurement of blood glucose concentration by a OneTouch Ultra Glucometer at designed time points in 6 hr fasted mice (N=5 for each group). (N–O) The fasting serum insulin (N) and HOMA-IR (O) in mice injected with either shNC or shAdgra3 for 28 days (N=5 for each group). HOMA-IR=Fasting glucose level (mmol/L) * Fasting insulin level (mIU/L) /22.5. NCD, normal chow diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; GTT, Glucose tolerance test; ITT, Insulin tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (C–G and N–O) and two-way ANOVA (B and L–M).
-
Figure 3—source data 1
Raw uncropped blots for Figure 3.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig3-data1-v1.zip
-
Figure 3—source data 2
Uncropped and labeled blots for Figure 3.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig3-data2-v1.zip
-
Figure 3—source data 3
Numerical source data for Figure 3.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig3-data3-v1.zip
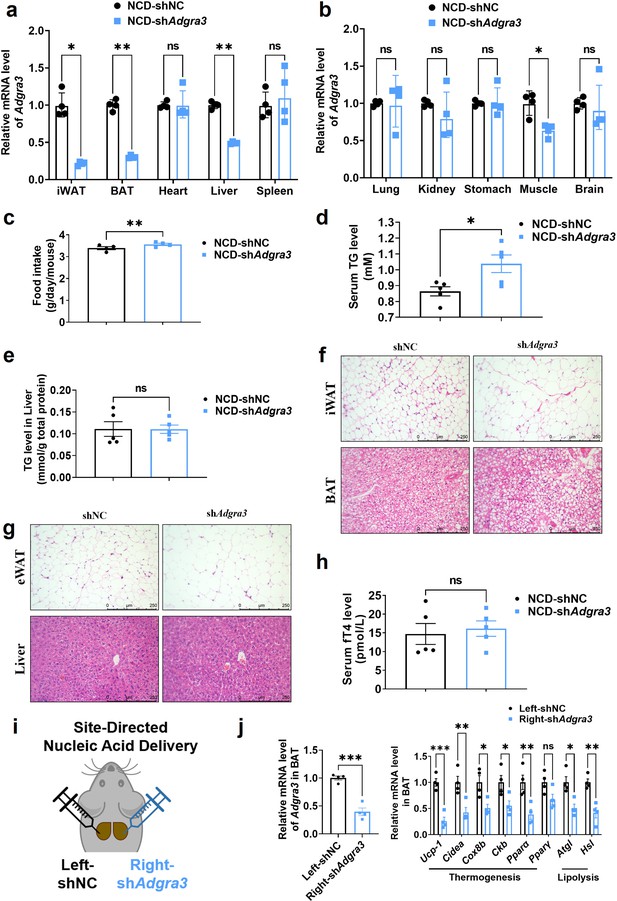
Characterization of wild-type and Adgra3-knockdown mice.
(A–H) C57BL/6 J mice fed with a NCD for eight weeks were injected with shAdgra3 (pLKO.1-U6-shAdgra3-2 plasmid encapsulated in nanomaterials) or shNC (pLKO.1-U6-shNC plasmid encapsulated in nanomaterials) twice a week for 4 weeks. (A) qPCR analysis of Adgra3 gene in iWAT, BAT, heart, liver, and spleen from different treatment mice (N=4 for each group). (B) qPCR analysis of Adgra3 gene in lung, kidney, stomach, skeletal muscle, and brain from different treatment mice (N=4 for each group). (C) Food intake of different treated mice (N=4 for each group). (D–E) The TG level of serum (D) and liver (E) from different treated mice (N=5 for each group). (F) Representative images of iWAT (top) and BAT (bottom) stained with hematoxylin and eosin. Scale bars, 250 μm. (G) Representative images of eWAT (top) and Liver (bottom) stained with hematoxylin and eosin. Scale bars, 250 μm. (H) The fT4 level of serum from different treated mice (N=5 for each group). (I) Schematic depicting the site-directed nanomaterials-encapsulated nucleic acid injections used to knockdown Adgra3 in BAT. (J) qPCR analysis of Adgra3, genes associated with thermogenesis and lipolysis in different-treated BAT (N=4 for each group). shNC, pLKO.1-U6-shNC plasmid encapsulated in nanomaterials; shAdgra3, pLKO.1-U6-shAdgra3-2 plasmid encapsulated in nanomaterials; iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue; BAT, brown adipose tissue; NCD, normal chow diet; fT4, free tetraiodothyronine. All data are presented as mean ± SEM. Statistical significance was determined by paired two-tailed student’s t-test (C) and unpaired two-tailed student’s t-test (A–B, D–E, H and J).
-
Figure 3—figure supplement 1—source data 1
Numerical source data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig3-figsupp1-data1-v1.zip
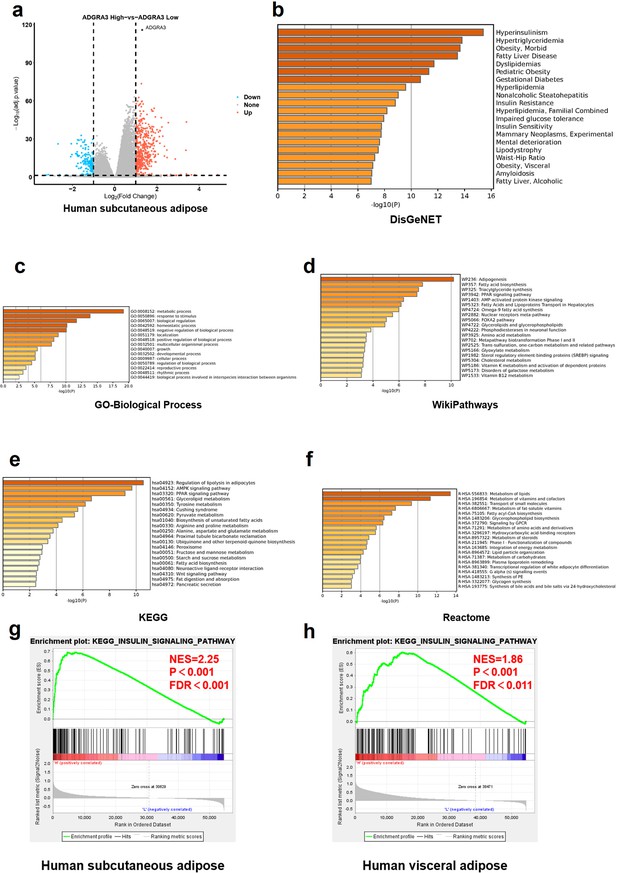
ADGRA3 high expressed gene sets in human subcutaneous fat are enriched to lipid metabolism and adipocyte differentiation.
(A) Volcano plot summarizing the differentially expressed genes (DEGs) between ADGRA3 high-expressed human subcutaneous adipose group and ADGRA3 low-expressed human subcutaneous adipose group. Blue and red shading indicates down-regulation and up-regulation, respectively. (B–F) Enrichment analysis for the high-expressed genes in ADGRA3 high-expressed human subcutaneous adipose in DisGeNET (B), GO-Biological Process (C), WikiPathways (D), KEGG (E) and Reactome (F) databases. (G–H) Gene set enrichment analysis (GSEA) analysis for gene signatures of insulin signaling pathway in human subcutaneous adipose (G) and human visceral adipose (H) from ADGRA3 high-expressed group compared with ADGRA3 low-expressed group. NES, normalized enrichment score. FDR, false discovery rate.
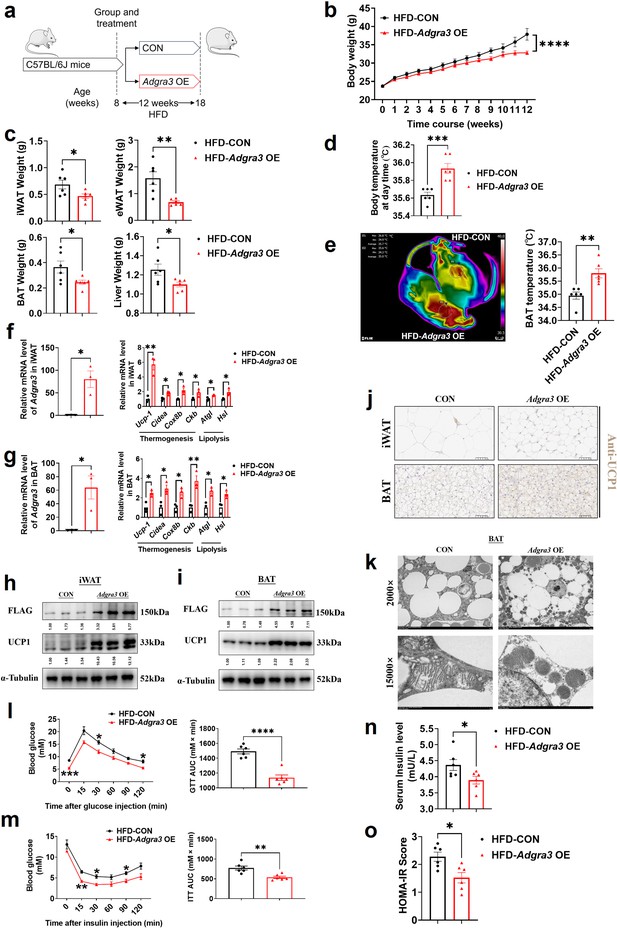
Adgra3 overexpression activated the adipose thermogenic program and facilitated metabolic homeostasis in mice with diet-induced obesity (DIO).
(A) Experimental schematic. C57BL/6 J mice were fed with a HFD and injected with Adgra3 OE (pLV3-CMV-Adgra3 (mouse)–3×FLAG plasmid encapsulated in nanomaterials) or CON (pLV3-CMV-MCS-3×FLAG plasmid encapsulated in nanomaterials) once a week for 12 weeks. (B–D) Changes in body mass (B), tissue weight (C) and body temperature (D) of mice injected with CON or Adgra3 OE (N=6 for each group). (E) Thermal image and BAT temperature in mice injected with CON or Adgra3 OE (N=6 for each group). (F–G) qPCR analysis of Adgra3, genes associated with thermogenesis and lipolysis in iWAT (F) and BAT (G) from different treatment mice (N=3 for each group). (H–I) Western-blot analysis for the level of ADGRA3−3×FLAG and UCP1 protein in iWAT (H) and BAT (I) from differently treated mice. (J) Representative images of iWAT (top; Scale bars, 50 μm.) and BAT (bottom; Scale bars, 50 μm.) stained with UCP1. (K) Transmission electron microscope photograph of BAT treated with CON or Adgra3 OE. (L) Glucose tolerance test (GTT) was conducted by intraperitoneal injection of glucose (1 g/kg) and measurement of blood glucose concentration with a OneTouch Ultra Glucometer at designed time points in six hours fasted mice (N=6 for each group). (M) Insulin tolerance test (ITT) was done by intraperitoneal injection of insulin (1 U/kg) and measurement of blood glucose concentration by a OneTouch Ultra Glucometer at designed time points in six hours fasted mice (N=6 for each group). (N–O) The fasting serum insulin (N) and HOMA-IR (O) in mice injected with CON or Adgra3 OE (N=6 for each group). HOMA-IR=Fasting glucose level (mmol/L) * Fasting insulin level (mIU/L) /22.5. HFD, high-fat diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; GTT, Glucose tolerance test; ITT, Insulin tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (C–G and N–O) and two-way ANOVA (B and L–M).
-
Figure 4—source data 1
Raw uncropped blots for Figure 4.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-data1-v1.zip
-
Figure 4—source data 2
Uncropped and labeled blots for Figure 4.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-data2-v1.zip
-
Figure 4—source data 3
Numerical source data for Figure 4.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-data3-v1.zip
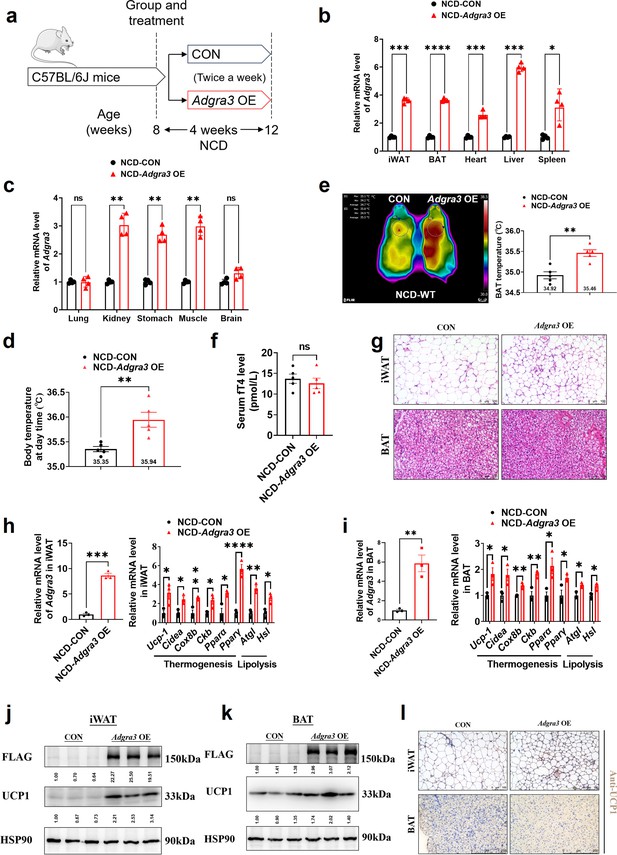
Adgra3 overexpression activated the adipose thermogenic program in mice.
(A) Experimental schematic. C57BL/6 J mice fed with a NCD for 8 weeks were injected with Adgra3 OE (pLV3-CMV-Adgra3(mouse)–3×FLAG plasmid encapsulated in nanomaterials) or CON (pLV3-CMV-MCS-3×FLAG plasmid encapsulated in nanomaterials) twice a week for 4 weeks. (B) qPCR analysis of Adgra3 gene in iWAT, BAT, heart, liver and spleen from different treatment mice (N=4 for each group). (C) qPCR analysis of Adgra3 gene in lung, kidney, stomach, skeletal muscle, and brain from different treatment mice (N=4 for each group). (D) Body temperature of mice injected with CON or Adgra3 OE for 28 days (N=5 for each group). (E) Thermal image and BAT temperature in mice injected with CON or Adgra3 OE for 28 days (N=5 for each group). (F) The fT4 level of serum from different treated mice (N=5 for each group). (G) Representative images of iWAT (top) and BAT (bottom) stained with HE. Scale bars, 100 μm. (H–I) qPCR analysis of Adgra3, genes associated with thermogenesis and lipolysis in iWAT (H) and BAT (I) from different treatment mice (N=3 for each group). (J–K) Western-blot analysis for the level of ADGRA3−3×FLAG and UCP1 protein in iWAT (J) and BAT (K) from differently treated mice. The ImageJ software was used for gray scanning. (L) Representative images of iWAT (top; Scale bars, 100 μm.) and BAT (bottom; Scale bars, 250 μm.) stained with UCP1. NCD, normal chow diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; fT4, free tetraiodothyronine. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (B–F and H–I).
-
Figure 4—figure supplement 1—source data 1
Raw uncropped blots for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Uncropped and labeled blots for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-figsupp1-data2-v1.zip
-
Figure 4—figure supplement 1—source data 3
Numerical source data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-figsupp1-data3-v1.zip
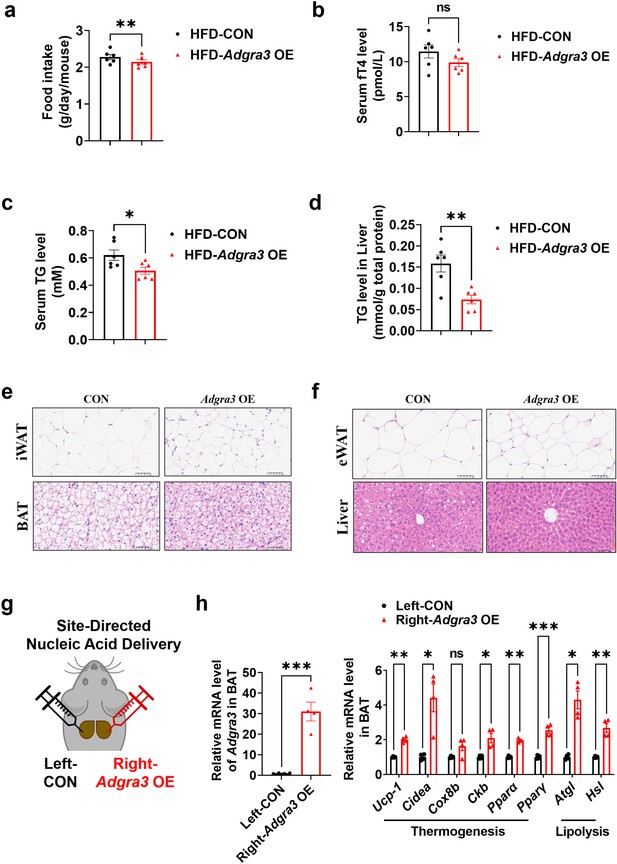
Characterization of wild-type and Adgra3-overexpressed mice.
(A–F) C57BL/6 J mice were fed with a HFD and injected with Adgra3 OE (pLV3-CMV-Adgra3 (mouse)–3×FLAG plasmid encapsulated in nanomaterials) or CON (pLV3-CMV-MCS-3×FLAG plasmid encapsulated in nanomaterials) once a week for 12 weeks. (A) Food intake of different treated mice (N=6 for each group). (B) The fT4 level of serum from different treated mice (N=6 for each group). (C–D) The TG level of serum (C) and liver (D) from different treated mice (N=6 for each group). (E) Representative images of iWAT (top) and BAT (bottom) stained with hematoxylin and eosin. Scale bars, 50 μm. (F) Representative images of eWAT (top) and Liver (bottom) stained with hematoxylin and eosin. Scale bars, 50 μm. (G) Schematic depicting the site-directed nanomaterials-encapsulated nucleic acid injections used to overexpress Adgra3 in BAT. (H) qPCR analysis of Adgra3, genes associated with thermogenesis and lipolysis in different-treated BAT (N=4 for each group). HFD, high-fat diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; fT4, free tetraiodothyronine. All data are presented as mean ± SEM. Statistical significance was determined by paired two-tailed student’s t-test (A) and unpaired two-tailed student’s t-test (B–D and H).
-
Figure 4—figure supplement 2—source data 1
Numerical source data for Figure 4—figure supplement 2.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig4-figsupp2-data1-v1.zip
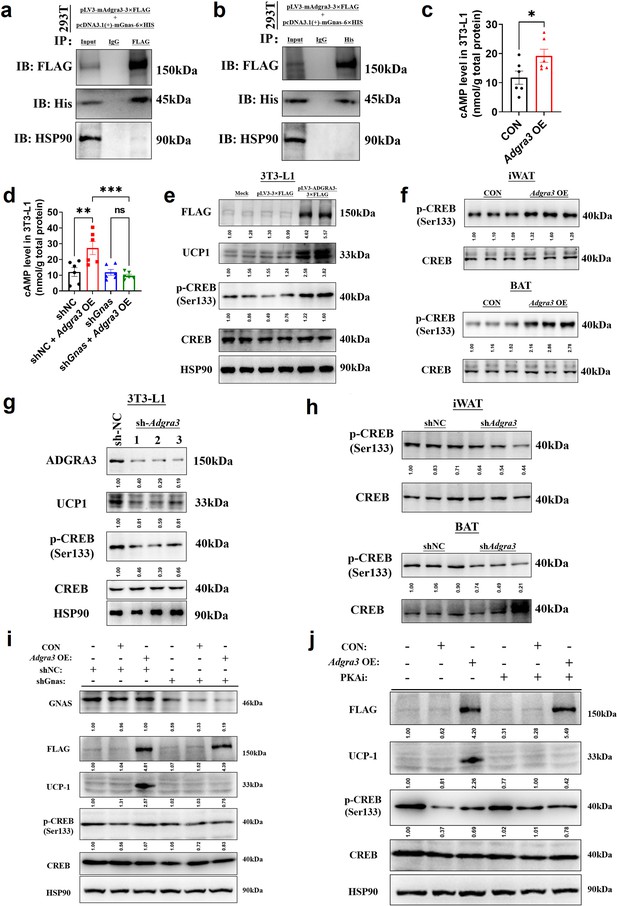
ADGRA3 promotes the biogenesis of beige adipocytes via the Gs-PKA-CREB axis.
(A–B) Western-blot analysis for level of ADGRA3−3×FLAG, GNAS-6 ×HIS and HSP90 proteins in 293T transfected with different plasmids. (C–D) The level of cAMP in 3T3-L1. An ELISA kit was used to measure the level of cAMP (N=6 for each group). (E, G and I–J) Western-blot analysis for level of ADGRA3, ADGRA3−3×FLAG, UCP1, p-CREB and CREB protein in 3T3-L1 mature beige-like adipocytes. (F and H) Western-blot analysis for level of p-CREB and CREB proteins in iWAT and BAT from differently treated mice. PKAi, protein kinase A inhibitor, 20 μM H-89. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (C) and one-way ANOVA (D).
-
Figure 5—source data 1
Raw uncropped blots for Figure 5.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-data1-v1.zip
-
Figure 5—source data 2
Uncropped and labeled blots for Figure 5.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-data2-v1.zip
-
Figure 5—source data 3
Numerical source data for Figure 5.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-data3-v1.zip
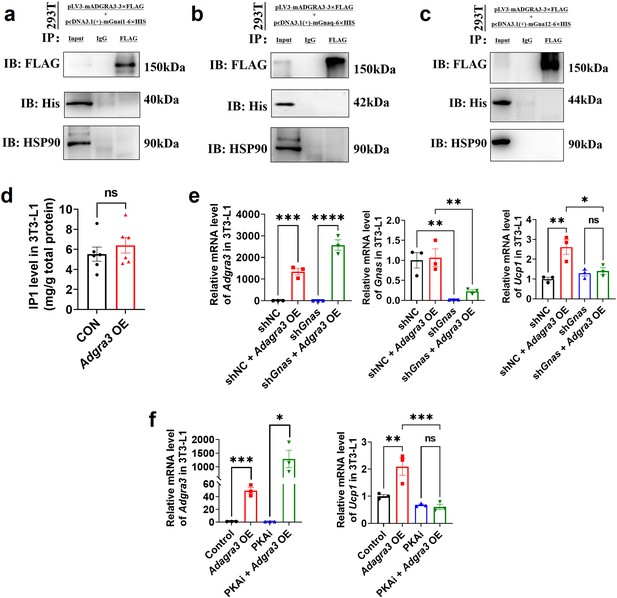
ADGRA3 was not observed to bind to Gi, Gq and G12.
(A–C) Western-blot analysis for level of ADGRA3−3×FLAG, GNAI1−6×HIS (A), GNAQ-6 ×HIS (B), GNA12−6×HIS (C) and HSP90 proteins in 293T transfected with different plasmids. (D) The level of IP1 in 3T3-L1 (N=6 for each group). An ELISA kits was used to measure the level of IP1. (E–F) qPCR analysis of Gnas, Adgra3 and Ucp1 in 3T3-L1 mature beige-like adipocytes (N=3 for each group). PKAi, protein kinase A inhibitor, 20 μM H-89. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (D) and one-way ANOVA (E–F).
-
Figure 5—figure supplement 1—source data 1
Raw uncropped blots for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-figsupp1-data1-v1.zip
-
Figure 5—figure supplement 1—source data 2
Uncropped and labeled blots for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-figsupp1-data2-v1.zip
-
Figure 5—figure supplement 1—source data 3
Numerical source data for Figure 5—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig5-figsupp1-data3-v1.zip
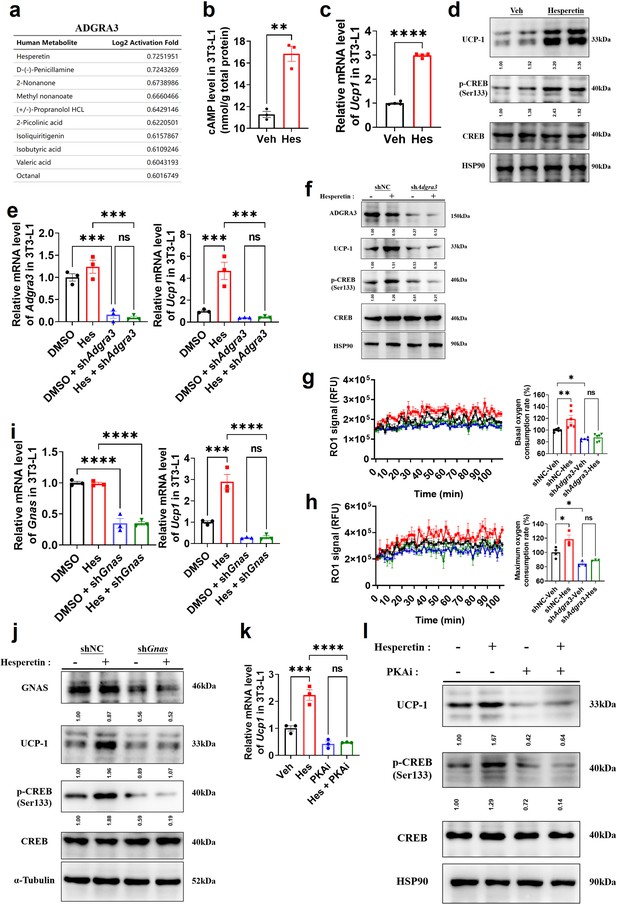
Hesperetin promotes the biogenesis of beige adipocytes via ADGRA3-Gs-PKA-CREB axis.
(A) Table of human metabolites with the ability to activate ADGRA3, from the PRESTO-Salsa database. (B) The level of cAMP in 3T3-L1. ELISA kit was used to measure the level of cAMP (N=3 for each group). (C, E, I and K) qPCR analysis of Adgra3, Gnas and Ucp1 in 3T3-L1 mature beige-like adipocytes (N=3 for each group). (D, F, J and L) Western-blot analysis for level of ADGRA3, GNAS, UCP1, p-CREB and CREB protein in 3T3-L1 mature beige-like adipocytes. (G) When 3T3-L1 mature beige-like adipocytes were treated with shNC, shAdgra3, or Hesperetin, fluorescence of the oxygen probe (RO1) in the cells was monitored and the rate of basal oxygen consumption was analyzed (N=5 for shAdgra3-Veh; N=6 for each other group). (H) When FCCP-treaded 3T3-L1 mature beige-like adipocytes were treated with shNC, shAdgra3, or Hesperetin, fluorescence of the oxygen probe (RO1) in the cells was monitored and the rate of maximum oxygen consumption was analyzed (N=4 for shNC-Veh; N=3 for each other group). Hes, 10 μM Hesperetin; PKAi, protein kinase A inhibitor, 20 μM H-89. All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (B–C) and one-way ANOVA (E, G–I and K).
-
Figure 6—source data 1
Raw uncropped blots for Figure 6.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig6-data1-v1.zip
-
Figure 6—source data 2
Uncropped and labeled blots for Figure 6.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig6-data2-v1.zip
-
Figure 6—source data 3
Numerical source data for Figure 6.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig6-data3-v1.zip
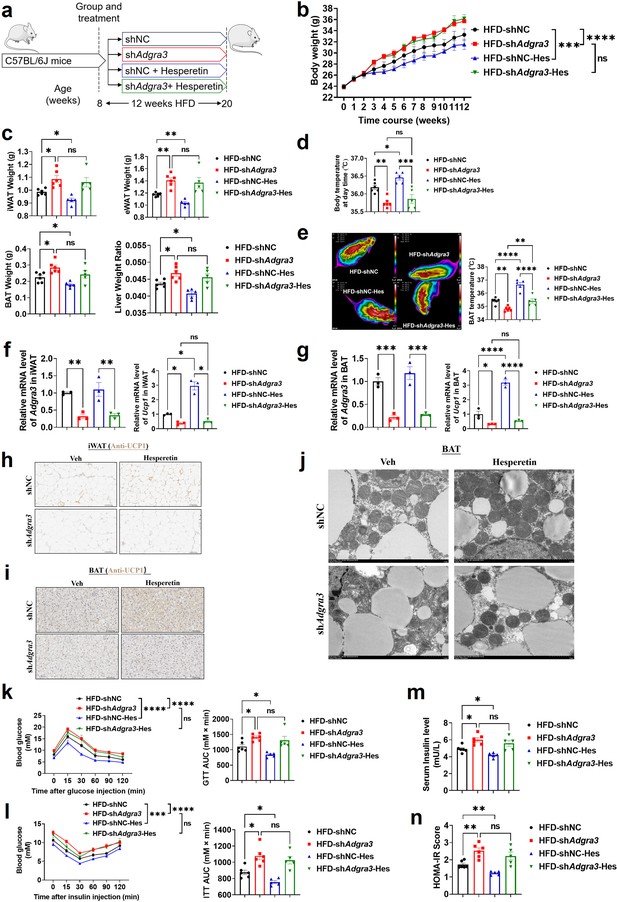
Hesperetin activated the adipose thermogenic program and facilitated metabolic homeostasis in mice with diet-induced obesity (DIO) dependent on ADGRA3.
(A) Experimental schematic. Different treated C57BL/6 J mice were fed with a HFD for 12 weeks. (B–D) Changes in body mass (B), tissue weight (C) and body temperature (D) of different treated mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (E) Thermal image and BAT temperature of different treated mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (F–G) qPCR analysis of Adgra3 and Ucp1 in iWAT (F) and BAT (G) from different treated mice (N=3 for each group). (H–I) Representative images of iWAT (H; Scale bars, 50 μm.) and BAT (I; Scale bars, 50 μm.) stained with UCP1. (J) Transmission electron microscope photograph of BAT from different treated mice (Scale bars, 2 μm.). (K) Glucose tolerance test (GTT) was conducted by intraperitoneal injection of glucose (1 g/kg) and measurement of blood glucose concentration with a OneTouch Ultra Glucometer at designed time points in six hours fasted mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (L) Insulin tolerance test (ITT) was done by intraperitoneal injection of insulin (1 U/kg) and measurement of blood glucose concentration by a OneTouch Ultra Glucometer at designed time points in six hours fasted mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (M–N) The fasting serum insulin (M) and HOMA-IR (N) in different treated mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). HOMA-IR=Fasting glucose level (mmol/L) * Fasting insulin level (mIU/L) /22.5. HFD, high-fat diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; GTT, Glucose tolerance test; ITT, Insulin tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance; Hes, Hesperetin. All data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA (C–G and M–N) and two-way ANOVA (B and K–L).
-
Figure 7—source data 1
Numerical source data for Figure 7.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig7-data1-v1.zip
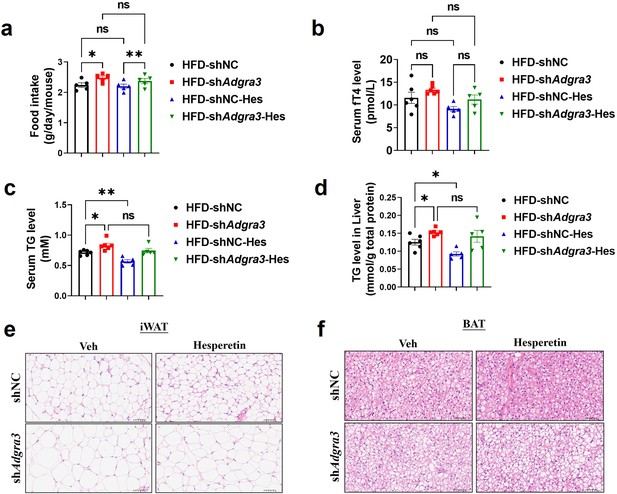
Characterization of wild-type and Adgra3-knockdown mice after hesperetin treatment.
(A) Food intake of different treated mice (N=5 for each group). (B) The fT4 level of serum from different treated mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (C–D) The TG level of serum (C) and liver (D) from different treated mice (N=6 for HFD-shNC and HFD-shAdgra3; N=5 for HFD-shNC-Hes and HFD-shAdgra3-Hes). (E–F) Representative images of iWAT (E) and BAT (F) stained with hematoxylin and eosin. Scale bars, 50 μm. HFD, high-fat diet; iWAT, inguinal white adipose tissue; BAT, brown adipose tissue; fT4, free tetraiodothyronine, Hes, Hesperetin. All data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA (A–D).
-
Figure 7—figure supplement 1—source data 1
Numerical source data for Figure 7—figure supplement 1.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig7-figsupp1-data1-v1.zip
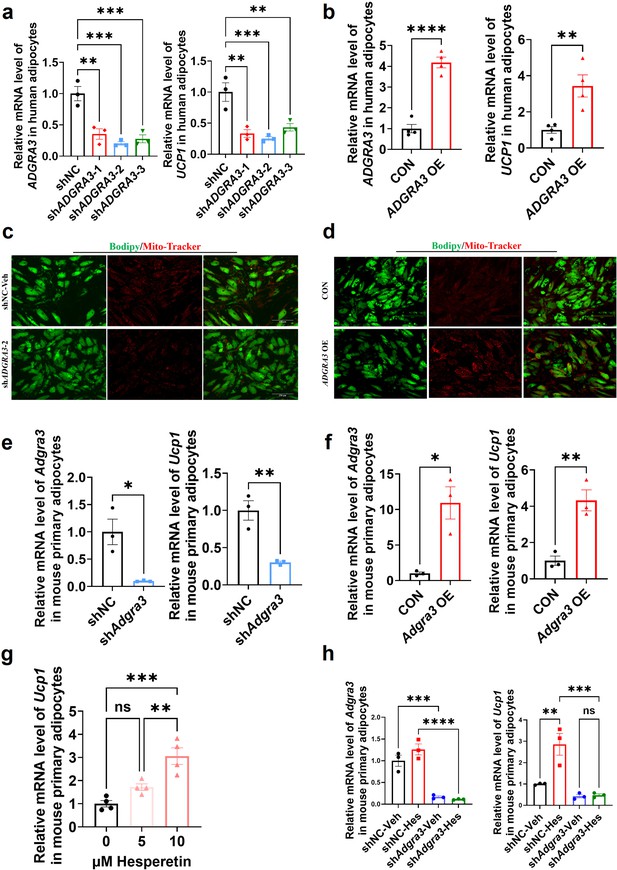
ADGRA3 overexpression induces the beiging of human adipocytes.
(A–B) qPCR analysis of ADGRA3 and UCP1 genes in adipocytes induced from human adipose-derived mesenchymal stem cells (hADSCs) (A: N=3 for each group; B: N=4 for each group). (C–D) Bodipy green staining for lipid droplet and Mito-Tracker red staining for mitochondria in adipocytes induced from hADSCs. Scale bars, 150 μm. (E–H) qPCR analysis of Adgra3 and Ucp1 genes in mouse primary adipocytes induced from stromal vascular fraction (SVF) of WAT (E-H: N=3 for each group). shADGRA3 (pLKO.1-U6-shADGRA3-(1/2/3) plasmid encapsulated in nanomaterials), shNC (pLKO.1-U6-shNC plasmid encapsulated in nanomaterials), ADGRA3 OE (pLV3-CMV-ADGRA3(human)–3×FLAG plasmid encapsulated in nanomaterials) or CON (pLV3-CMV-MCS-3×FLAG plasmid encapsulated in nanomaterials). All data are presented as mean ± SEM. Statistical significance was determined by unpaired two-tailed student’s t-test (B and E–F) and one-way ANOVA (A and G–H).
-
Figure 8—source data 1
Numerical source data for Figure 8.
- https://cdn.elifesciences.org/articles/100205/elife-100205-fig8-data1-v1.zip
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo-sapiens) | 293T | Cell Bank of the Chinese Academy of Sciences in Shanghai | SCSP-502 | |
| Cell line (M. musculus) | 3T3-L1 | Cell Bank of the Chinese Academy of Sciences in Shanghai | SCSP-5038 | |
| Antibody | anti-HSP90 (Rabbit monoclonal) | Cell Signaling Technology | Cell Signaling Technology Cat# 4877, RRID:AB_2233307 | WB (1:1000) |
| Antibody | anti-α-tubulin (Mouse monoclonal) | Proteintech | Proteintech Cat# 66031-1-Ig, RRID:AB_11042766 | WB (1:10000) |
| Antibody | anti-ADGRA3 (Rabbit polyclonal) | Proteintech | Proteintech Cat# 11912-1-AP, RRID:AB_2877804 | WB (1:1000) |
| Antibody | anti-UCP1 (Rabbit polyclonal) | Abcam | Abcam Cat# ab10983, RRID:AB_2241462 | WB (1:1000 for iWAT and cells or 1:10000 for BAT) |
| Antibody | anti-FLAG-tag (Mouse monoclonal) | Beyotime | Beyotime Cat# AF2855, RRID:AB_3674126 | WB (1:2000) |
| Antibody | anti-HIS-tag (Mouse monoclonal) | Beyotime | Beyotime Cat# AF2879, RRID:AB_3674127 | WB (1:2000) |
| Antibody | anti-p-CREB (Rabbit polyclonal) | Beyotime | Beyotime Cat# AF5785, RRID:AB_3674128 | WB (1:1000) |
| Antibody | anti-CREB (Rabbit polyclonal) | Beyotime | Beyotime Cat# AF6566, RRID:AB_3674129 | WB (1:1000) |
| Antibody | anti-FLAG-tag (Mouse monoclonal) | Servicebio | ServiceBio Cat# GB15938-100, RRID:AB_3674125 | IP (1:50) |
| Antibody | anti-HIS-tag (Mouse monoclonal) | Servicebio | ServiceBio Cat# GB151251, RRID:AB_3665294 | IP (1:50) |
| Biological sample (Homo-sapiens) | hADSCs | National Stem Cell Translational Resource Center | ZB10DGAC | |
| Biological sample (M. musculus) | SVF | Center of Laboratory Animal at Sun Yat-sen University | Freshly isolated from C57BL/6 J (Male) | |
| Other | Lipo8000 | Beyotime | C0533 | The nanomaterials mentioned in this article refer to Lipo8000 reagent, a highly efficient transfection reagent based on nanomaterials |
Additional files
-
Supplementary file 1
Primer sequences used for qPCR.
- https://cdn.elifesciences.org/articles/100205/elife-100205-supp1-v1.docx
-
Supplementary file 2
Gene annotation for screened genes.
The 1134 screened genes were annotated by David database and 27 of these genes were identified as G-protein coupled receptor encoding gene.
- https://cdn.elifesciences.org/articles/100205/elife-100205-supp2-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/100205/elife-100205-mdarchecklist1-v1.docx