Decision Making: Unraveling the enigmatic role of the subthalamic nucleus
Pondering whether “to be or not to be”, or impulsively “tilting at windmills”, decision-making comes in many forms, as so eloquently demonstrated in many great works of literature. But what causes us to sometimes come to quick conclusions while we struggle to make up our minds at other times?
Making decisions involves a complex interplay between numerous neurons located in various regions of the brain. The neural circuitry involved in this process remains, however, enigmatic. To study the cognitive processes implicated in making simple two-choice decisions, researchers often employ the Drift Diffusion Model. This mathematical framework assumes that information accumulates over time until a decision threshold is reached, and has three main parameters: the drift rate (the rate information accumulates), the decision threshold (the amount of information needed to make a decision), and the non-decision time (the time taken for processes not directly related to decision-making, such as stimulus encoding and response execution; Ratcliff, 1978; Ratcliff and McKoon, 2008).
Two of the most critical brain areas involved in decision-making are the prefrontal cortex and the hippocampus. More recently, it has been proposed that a region known as the subthalamic nucleus, which is part of the basal ganglia – and is therefore involved in motor control and integration – also has an integral role. Now, in eLife, Kathryn Rogers, Joshua Gold and Long Ding from the University of Pennsylvania report new insights into how the subthalamic nucleus contributes to decision-making (Rogers et al., 2024).
Previous computational models propose three roles for the subthalamic nucleus in decision-making and how it interacts with other parts of the brain (Figure 1): the subthalamic nucleus works with the medial prefrontal cortex to set thresholds for decision-making. These thresholds determine when enough information has been gathered to make a decision, helping control impulsivity (Hypothesis 1). Through its interaction with the external segment of the globus pallidus (a component of the basal ganglia), the subthalamic nucleus helps to calibrate how different options are evaluated, making sure the choices are assessed properly (Hypothesis 2). It helps to implement step-like, all-or-none nonlinear computations to improve the basal ganglia’s efficacy in adjusting decision bounds (Hypothesis 3; Frank, 2006; Cavanagh et al., 2011; Ratcliff and Frank, 2012; Bogacz and Gurney, 2007; Coulthard et al., 2012; Green et al., 2013; Lo and Wang, 2006; Wei et al., 2015).
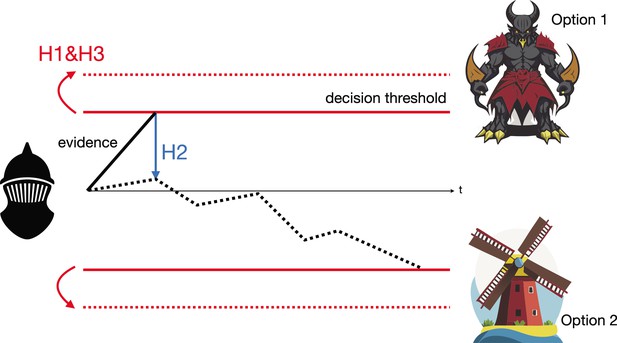
The Drift Diffusion Model illustrated by Don Quixote’s decision-making.
Over time (t), Don Quixote (black mask) accumulates information that allows him to decide whether he is looking at a giant (option 1) or a windmill (option 2), and act accordingly. The subthalamic nucleus is thought to influence this decision-making process in three different ways, per the Drift Diffusion Model. First (H1), by adjusting the amount of information needed to make a decision, known as the decision threshold (represented as the gap between the two red unbroken lines increasing to the gap between the two dashed lines). Second (H2), by calibrating different pieces of evidence so they can be used to evaluate the two possibilities (whether an object is a windmill or a giant). This enables the evidence accumulation process to shift from weighting unreliable perceptual evidence too heavily (represented by the solid black line) to favoring more reliable evidence later in the decision process (represented by the dashed black line). Third (H3), by helping neural pathways sent from the basal ganglia to precisely adjust the boundaries of the decision threshold.
Image credit: Qianli Yang (CC BY 4.0).
Don Quixote’s mistaking of windmills for giants can be explained by all three hypotheses. His decision threshold may have been too low, causing him to react based on initial false evidence that the windmills’ rotating blades are the giants' flailing arms (Hypothesis 1). Similarly, he may have failed to adjust his decision threshold according to his peaceful surroundings (and thus failing to realize that he was surrounded by windmills, rather than giants (Hypothesis 3)), or to properly weigh his unreliable perception of the environment around him, which favored seeing an angry giant over a harmless windmill (Hypothesis 2).
To determine which hypothesis best explains the role of the subthalamic nucleus in decision-making, Rogers et al. recorded the activity of single neurons in the subthalamic nucleus of monkeys during a visual-saccadic decision task. In the experiment, the monkeys undertook a direction-discrimination task, during which they reported the perceived motion direction of a random dot stimulus by making a rapid eye movement (saccade) towards the corresponding choice target.
This revealed three distinct neuronal subpopulations in the subthalamic nucleus, each corresponding to one of the hypothetical models: The first subpopulation showed choice- and coherence-dependent activity that ramped up during motion viewing, consistent with the theory that these neurons pool and normalize evidence-related signals (Hypothesis 2). The second subpopulation exhibited an early, sharp rise in activity that was independent of choice and coherence during motion viewing, and gradually decreased toward saccade onset. This matches the prediction that the neurons provide an early signal to suppress immature choices (Hypothesis 1). The third subpopulation showed choice- and coherence-dependent ramping activity during motion viewing and a short burst of activity for one choice just before saccade onset. This aligns with the prediction that the subthalamic nucleus balances evidence-related signals until decision time (Hypothesis 3). This heterogeneity suggests that the subthalamic nucleus influences perceptual decision-making (where sensory information is used to guide behavior) in multiple ways.
Rogers et al. then applied electrical microstimulation (weak electric currents that affect neurons near the electrode) to the subthalamic nucleus during the task to perturb its activity. This caused the biases influencing the monkeys’ choice biases to change, reduced the influence of motion strength (i.e., the speed of the moving dot) on choices, and decreased response times. Fitting the data with a Drift Diffusion Model revealed that microstimulation of the subthalamic nucleus affected the decision threshold, evidence accumulation, as well as processes not involved in decision-making.
This groundbreaking study provides new insights into the causal roles of the subthalamic nucleus in perceptual decision-making, highlighting its involvement in modulating various aspects of the decision process through distinct neural subpopulations. These findings advance our understanding of how the basal ganglia contribute to decision-making and cognitive function.
However, it remains unclear if the subthalamic nucleus interacts with other brain regions, and if potential anatomical and/or functional alterations to this region could be implicated in neurological and psychiatric disorders. Future research could explore these issues and investigate therapy avenues that target the subthalamic nucleus for conditions involving impaired decision-making, potentially offering hope for individuals like Don Quixote.
References
-
Subthalamic nucleus stimulation reverses mediofrontal influence over decision thresholdNature Neuroscience 14:1462–1467.https://doi.org/10.1038/nn.2925
-
A theory of memory retrievalPsychological Review 85:59–108.https://doi.org/10.1037/0033-295X.85.2.59
-
Role of the indirect pathway of the basal ganglia in perceptual decision makingThe Journal of Neuroscience 35:4052–4064.https://doi.org/10.1523/JNEUROSCI.3611-14.2015
Article and author information
Author details
Publication history
Copyright
© 2024, Yang
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 536
- views
-
- 59
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Computational and Systems Biology
- Neuroscience
Accumulating evidence to make decisions is a core cognitive function. Previous studies have tended to estimate accumulation using either neural or behavioral data alone. Here, we develop a unified framework for modeling stimulus-driven behavior and multi-neuron activity simultaneously. We applied our method to choices and neural recordings from three rat brain regions—the posterior parietal cortex (PPC), the frontal orienting fields (FOF), and the anterior-dorsal striatum (ADS)—while subjects performed a pulse-based accumulation task. Each region was best described by a distinct accumulation model, which all differed from the model that best described the animal’s choices. FOF activity was consistent with an accumulator where early evidence was favored while the ADS reflected near perfect accumulation. Neural responses within an accumulation framework unveiled a distinct association between each brain region and choice. Choices were better predicted from all regions using a comprehensive, accumulation-based framework and different brain regions were found to differentially reflect choice-related accumulation signals: FOF and ADS both reflected choice but ADS showed more instances of decision vacillation. Previous studies relating neural data to behaviorally inferred accumulation dynamics have implicitly assumed that individual brain regions reflect the whole-animal level accumulator. Our results suggest that different brain regions represent accumulated evidence in dramatically different ways and that accumulation at the whole-animal level may be constructed from a variety of neural-level accumulators.
-
- Genetics and Genomics
- Neuroscience
The central complex (CX) plays a key role in many higher-order functions of the insect brain including navigation and activity regulation. Genetic tools for manipulating individual cell types, and knowledge of what neurotransmitters and neuromodulators they express, will be required to gain mechanistic understanding of how these functions are implemented. We generated and characterized split-GAL4 driver lines that express in individual or small subsets of about half of CX cell types. We surveyed neuropeptide and neuropeptide receptor expression in the central brain using fluorescent in situ hybridization. About half of the neuropeptides we examined were expressed in only a few cells, while the rest were expressed in dozens to hundreds of cells. Neuropeptide receptors were expressed more broadly and at lower levels. Using our GAL4 drivers to mark individual cell types, we found that 51 of the 85 CX cell types we examined expressed at least one neuropeptide and 21 expressed multiple neuropeptides. Surprisingly, all co-expressed a small molecule neurotransmitter. Finally, we used our driver lines to identify CX cell types whose activation affects sleep, and identified other central brain cell types that link the circadian clock to the CX. The well-characterized genetic tools and information on neuropeptide and neurotransmitter expression we provide should enhance studies of the CX.






