Spatial Attention: Time to get deep
As you read this text, you are probably focusing on a screen or piece of paper directly in front of you. This ability to focus on a specific object relies on the brain filtering out visual distractions from the surrounding area (Desimone and Duncan, 1995). However, if you wanted, you could suddenly shift your attention to the left or right, without moving your eyes. Understanding more about the relationship between attention and the anatomy of the brain is fundamental for research in neuroscience.
Previous studies have shown that attention is controlled by the cortex, the outer layer of the brain, which is thought to implement cognition, language, reasoning and other higher-order brain functions. However, much less is known about how structures deep within the brain affect this process. This is partly because most of the techniques commonly used to image brain activity are good at measuring the activity of neurons in the cortex but less so in deeper subcortical structures. Now, in eLife, Ole Jensen and colleagues at the University of Birmingham, University of New South Wales, and CERMEP-Imagerie du Vivant – including Tara Ghafari as first author – report how they used two brain imaging techniques to investigate the role of subcortical structures in spatial attention (Ghafari et al., 2024).
First, the team used a technique called magnetoencephalography (MEG) to measure magnetic fields generated by the electrical activity of neurons in the cortical layer. This method was applied to the brains of 33 individuals as they performed a task that required them to shift their attention between faces on the left and right of a computer screen (Gutteling et al., 2022). The same group of people were also placed in a magnetic resonance imaging (MRI) scanner to assess the size of two brain structures, the thalamus and basal ganglia, both of which sit beneath the cortex.
When our attention shifts to the left or right, the balance of waves in the left versus the right hemisphere of the brain changes. This is particularly true for a pattern of electrical activity known as the alpha wave which repeats roughly ten times per second (first discovered by Berger, 1929). It is believed that this asymmetry reflects one side of the brain focusing on the relevant input, while the other suppresses distractions from the surrounding environment (Schneider et al., 2022).
However, the human brain is not perfectly symmetrical. For instance, structures which are present on both sides of the brain (such as the thalamus) might be larger in the right hemisphere in some individuals, but larger in the left in others. On top of this, the response of alpha waves to attention is also asymmetrical: a shift in attention might lead to a larger change in the alpha waves in one hemisphere for some individuals, and the opposite hemisphere for others (Mazzetti et al., 2019).
Ghafari et al. set out to find whether the size of subcortical structures in the left and right hemispheres correlates with the asymmetry in alpha modulations. They found that whichever brain hemisphere had the larger caudate nucleus or globus pallidus (two structures that make up the basal ganglia) also displayed a higher level of alpha wave modulation. However, this effect was reversed for the thalamus, with higher levels of modulation happening in the hemisphere with the smaller thalamus (Figure 1). Moreover, Ghafari et al. also report what happens to these relationships when various features of the relevant stimuli and irrelevant distractors used in the experiments are changed.
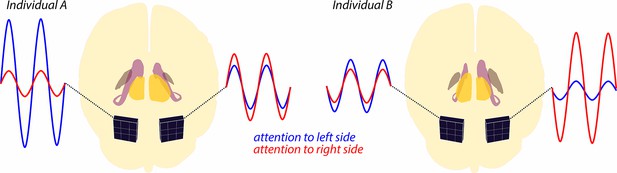
Alpha wave modulation during a spatial attention task.
The brains of two individuals are shown schematically from the back, with subcortical structures (thalamus, caudate nucleus and globus pallidus) highlighted in different colors. Note that for individual A, the thalamus (yellow) in the left hemisphere is slightly smaller than the thalamus in the right hemisphere. There are also asymmetries in the sizes of the caudate nucleus (purple) and globus pallidus (brown), and also for all three structures in individual B. MEG sensors (black squares) attached to the posterior cortex record alpha waves as the individuals shift their attention to the left or right side of their visual field. For both individuals, shifting attention to the left leads to increased alpha waves in the left hemisphere (blue lines), and shifting attention to the right leads to increased alpha waves in the right hemisphere (red lines). The extent of this modulation of the alpha waves is related to the size of various subcortical structures: a larger thalamus on one side of the brain – the right hemisphere of individual A, the left hemisphere of individual B – correlates with a stronger alpha modulation in the opposite hemisphere. Conversely, a larger caudate nucleus or globus pallidus correlates with stronger alpha modulation in the same hemisphere.
Image Credit: Shapes of the brain and subcortical structures adapted from an interactive brain model powered by the Wellcome Trust and developed by Matt Wimsatt and Jack Simpson, Society for Neuroscience (2017).
These new findings will help us understand the underlying subcortical circuitry that controls how spatial attention is allocated in humans. Not least, this work paves the way for further research on how changes in the subcortical regions in neurological disorders such as Alzheimer’s disease or dementia alter the behavior of brain waves in the cortex.
Since the results of the present research are purely correlational, it remains unclear whether the asymmetry of subcortical structures is responsible for the asymmetry in alpha wave modulation. Furthermore, attention is just one of many perceptual and cognitive processes that modulate alpha waves (Clayton et al., 2018). It will thus be important for future studies to test if the results are specific to spatial attention or apply more generally to other cognitive processes and to other types of brain waves.
References
-
Über das Elektrenkephalogramm des MenschenArchiv für Psychiatrie und Nervenkrankheiten 87:527–570.https://doi.org/10.1007/BF01797193
-
The many characters of visual alpha oscillationsThe European Journal of Neuroscience 48:2498–2508.https://doi.org/10.1111/ejn.13747
-
Neural mechanisms of selective visual attentionAnnual Review of Neuroscience 18:193–222.https://doi.org/10.1146/annurev.ne.18.030195.001205
-
Alpha oscillations reflect suppression of distractors with increased perceptual loadProgress in Neurobiology 214:102285.https://doi.org/10.1016/j.pneurobio.2022.102285
-
Hemispheric asymmetry of globus pallidus relates to alpha modulation in reward-related attentional tasksThe Journal of Neuroscience 39:9221–9236.https://doi.org/10.1523/JNEUROSCI.0610-19.2019
-
Target enhancement or distractor suppression? Functionally distinct alpha oscillations form the basis of attentionThe European Journal of Neuroscience 55:3256–3265.https://doi.org/10.1111/ejn.15309
Article and author information
Author details
Publication history
Copyright
© 2024, Schulz and Wöstmann
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 429
- views
-
- 34
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Computational and Systems Biology
- Neuroscience
Accumulating evidence to make decisions is a core cognitive function. Previous studies have tended to estimate accumulation using either neural or behavioral data alone. Here, we develop a unified framework for modeling stimulus-driven behavior and multi-neuron activity simultaneously. We applied our method to choices and neural recordings from three rat brain regions—the posterior parietal cortex (PPC), the frontal orienting fields (FOF), and the anterior-dorsal striatum (ADS)—while subjects performed a pulse-based accumulation task. Each region was best described by a distinct accumulation model, which all differed from the model that best described the animal’s choices. FOF activity was consistent with an accumulator where early evidence was favored while the ADS reflected near perfect accumulation. Neural responses within an accumulation framework unveiled a distinct association between each brain region and choice. Choices were better predicted from all regions using a comprehensive, accumulation-based framework and different brain regions were found to differentially reflect choice-related accumulation signals: FOF and ADS both reflected choice but ADS showed more instances of decision vacillation. Previous studies relating neural data to behaviorally inferred accumulation dynamics have implicitly assumed that individual brain regions reflect the whole-animal level accumulator. Our results suggest that different brain regions represent accumulated evidence in dramatically different ways and that accumulation at the whole-animal level may be constructed from a variety of neural-level accumulators.
-
- Genetics and Genomics
- Neuroscience
The central complex (CX) plays a key role in many higher-order functions of the insect brain including navigation and activity regulation. Genetic tools for manipulating individual cell types, and knowledge of what neurotransmitters and neuromodulators they express, will be required to gain mechanistic understanding of how these functions are implemented. We generated and characterized split-GAL4 driver lines that express in individual or small subsets of about half of CX cell types. We surveyed neuropeptide and neuropeptide receptor expression in the central brain using fluorescent in situ hybridization. About half of the neuropeptides we examined were expressed in only a few cells, while the rest were expressed in dozens to hundreds of cells. Neuropeptide receptors were expressed more broadly and at lower levels. Using our GAL4 drivers to mark individual cell types, we found that 51 of the 85 CX cell types we examined expressed at least one neuropeptide and 21 expressed multiple neuropeptides. Surprisingly, all co-expressed a small molecule neurotransmitter. Finally, we used our driver lines to identify CX cell types whose activation affects sleep, and identified other central brain cell types that link the circadian clock to the CX. The well-characterized genetic tools and information on neuropeptide and neurotransmitter expression we provide should enhance studies of the CX.






