Gasdermin D-mediated neutrophil pyroptosis drives inflammation in psoriasis
Figures
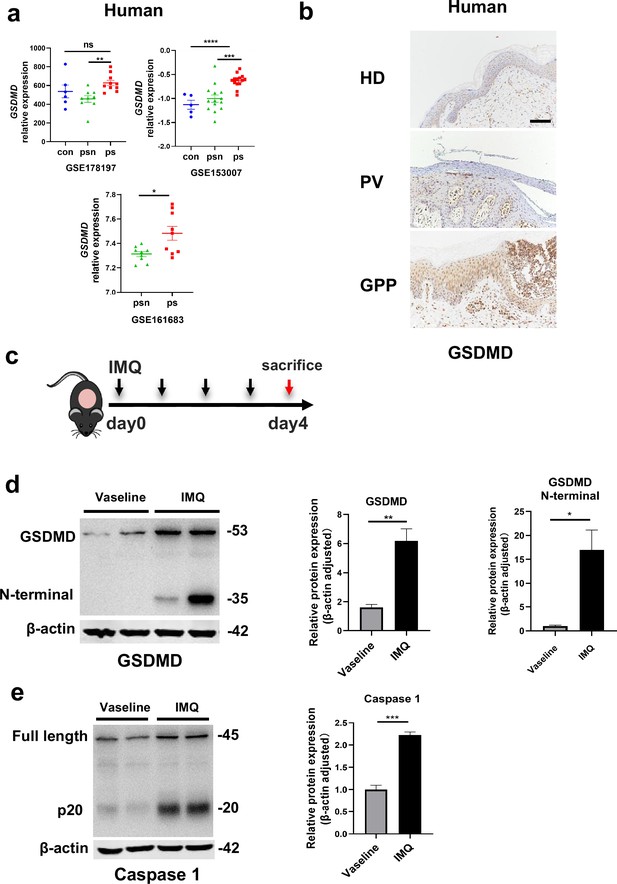
The GSDMD-mediated pyroptosis is activated in psoriasis.
(a) Expression of GSDMD in patients with psoriasis patients and healthy people from GSE178197, GSE161683, and GSE153007. (b) Representative immunohistochemical staining of GSDMD in sections of skin tissue from healthy individuals and patients with psoriasis (n=3–5, each group). Scale bar = 100 µm. (c) Schematic representation of the IMQ-induced psoriasis mouse model. (d) Representative images and statistical analysis of western blot analysis showing the expression of GSDMD and its N-terminal fragments in dorsal skin of WT mice treated with vehicle or IMQ at day 4 (n=4). (e) Representative images and statistical analysis of western blot analysis showing the expression level of pro-caspase 1 and caspase 1 in dorsal skin of WT mice treated with vehicle or IMQ at day 4. psn, psoriasis non-lesional skin; ps, psoriasis skin; con, control; HD, healthy donor; GPP, generalized pustular psoriasis; IMQ, imiquimod; WT, wild-type. Error bars show mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ***p≤0.0001. Data are representative of three independent experiments for (b, d, e).
-
Figure 1—source data 1
PDF file containing original western blots for Figure 1d and e, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig1-data1-v1.pdf
-
Figure 1—source data 2
Original files for western blot analysis displayed in Figure 1d and e.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig1-data2-v1.zip
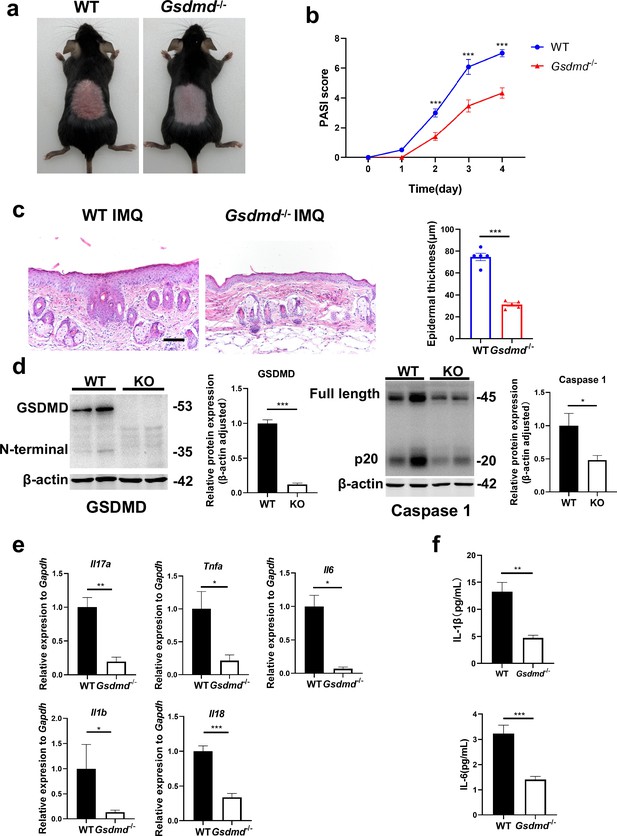
GSDMD deficiency mitigates psoriasis-like inflammation in mice.
(a) Macroscopic phenotypic representation of the dorsal skin in WT and GSDMD-KO mice treated with IMQ at day 4. (b) Disease severity of psoriasis induced by IMQ in mice as assessed by PASI score (n=10–12). (c) Representative images and statistical analysis of hematoxylin and eosin staining of the dorsal skin in WT and GSDMD-KO mice treated with IMQ at day 4 (n=5). Scale bar = 100 µm. (d) Representative images and statistical analysis of western blot analysis showing the expression level of GSDMD and caspase 1 in dorsal skin of WT and GSDMD-KO mice treated with IMQ at day 4 (n=4). (e) Quantitative PCR analysis of the relative mRNA expression of proinflammatory cytokines in the dorsal skin of WT and GSDMD-KO mice treated with IMQ at day 4 (n=4). Data were normalized to a reference gene, GAPDH. (f) ELISA analysis of IL-6 and IL-1β per 1 mg of the dorsal skin from WT and GSDMD-KO mice treated with IMQ at day 4 (n=5). ELISA, enzyme-linked immunosorbent assay; IMQ, imiquimod; WT, wild-type; KO, knockout; PASI, psoriasis area and severity index. Error bars show mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Data are representative of three independent experiments for (a, c, d).
-
Figure 2—source data 1
PDF file containing original western blots for Figure 2d, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig2-data1-v1.pdf
-
Figure 2—source data 2
Original files for western blot analysis displayed in Figure 2d.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig2-data2-v1.zip
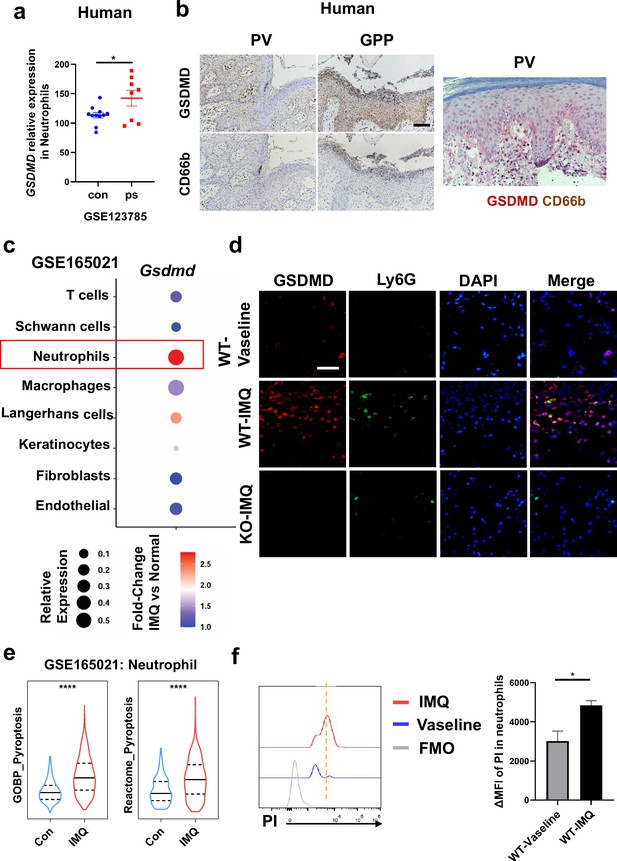
Neutrophils undergo GSDMD-mediated pyroptosis in psoriasis.
(a) Expression of GSDMD in neutrophils in psoriasis patients and healthy people from GSE123785. (b) Representative immunohistochemical staining of CD66b and GSDMD in two consecutive sections of skin tissue from patients with psoriasis vulgaris or generalized pustular psoriasis (left); representative immunohistochemical staining of CD66b (brown) and GSDMD (red) in sections of skin tissue from patients with psoriasis vulgaris (n=3–5, each group). Scale bar = 100 µm. (c) Dot plot of Gsdmd expression in each cell type in the skin of IMQ-induced psoriasis-like mice (GSE165021). (d) Representative immunofluorescence of GSDMD (red), Ly6G (green), and nuclear (blue) in dorsal skin of WT mice treated with vehicle, WT mice treated with IMQ, and GSDMD-KO mice treated with IMQ at day 5. Scale bar = 50 µm. (e) ssGSEA showed the expression of pyroptosis-related genes in neutrophils infiltrating in control and IMQ-induced psoriasis-like tissue (GSE165021). (f) Representative and statistical graphs of the mean fluorescence intensity of propidium iodide in neutrophils from skin of WT mice as detected by flow cytometry (n=3). ps, psoriasis skin; con, control; PV, psoriasis vulgaris; GPP, generalized pustular psoriasis; IMQ, imiquimod; WT, wild-type; MFI, mean fluorescence intensity; PI, propidium iodide; MFO, fluorescence minus one. Error bars show mean ± SEM. *p<0.05, ****p<0.0001. Data are representative of three independent experiments for (b, d–e).
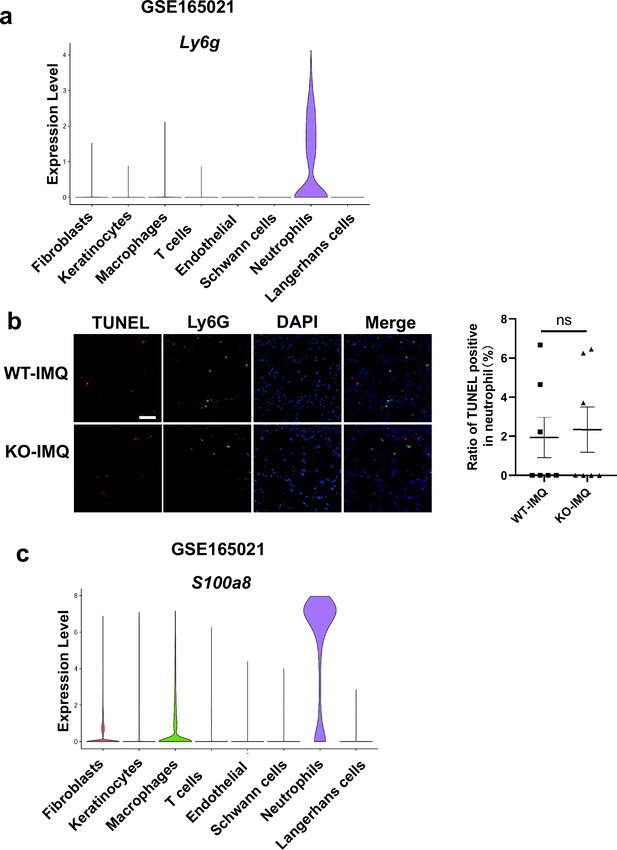
The expression of Ly6g and S100a8 in the skin of IMQ-induced psoriasis-like mice, and TUNEL assay in neutrophils.
(a) Expression level of Ly6g in each cell type in the skin of imiquimod (IMQ)-induced psoriasis-like mice (GSE165021). (b) Representative immunofluorescence of TUNEL (red), Ly6G (green), and nuclear (blue) in dorsal skin of WT mice treated with IMQ and GSDMD-KO mice treated with IMQ at day 4. Scale bar = 50 µm. Statistical analysis of ratio of TUNEL positive cells in neutrophils. (n=7). (c) Expression level of S100a8 in each cell type in the skin of IMQ-induced psoriasis-like mice (GSE165021). Error bars show mean ± SEM. ns, p≥0.05. Data are representative of three independent experiments for (b).
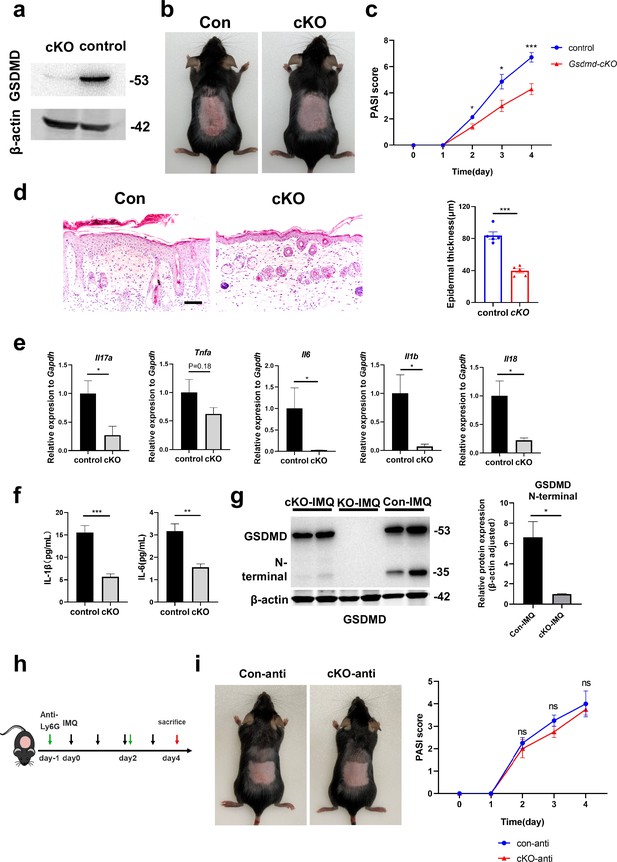
GSDMD depletion in neutrophils attenuates the development of skin inflammation in psoriasis.
(a) Representative images of western blot showing the expression of GSDMD in bone marrow-derived neutrophils of cKO and isotype control mice. (b) Macroscopic phenotypic representation of the dorsal skin in control and GSDMD-cKO mice treated with IMQ at day 4. (c) Disease severity of psoriasis induced by IMQ in mice as assessed by PASI score (n=7). (d) Representative images and statistical analysis of hematoxylin and eosin staining of the dorsal skin in control and GSDMD-cKO mice treated with IMQ at day 4 (n=5). Scale bar = 100 µm. (e) Quantitative PCR analysis of the relative mRNA expression of proinflammatory cytokines in the dorsal skin of control and GSDMD-cKO mice treated with IMQ at day 4 (n=4). Data were normalized to a reference gene, GAPDH. (f) ELISA analysis of IL-6 and IL-1β per 1 mg of the dorsal skin from control and GSDMD-cKO mice treated with IMQ at day 4 (n=5). (g) Representative images and statistical analysis of western blot analysis showing the expression level of GSDMD and its N-terminal fragments in dorsal skin of WT, cKO, and Gsdmd-/- mice treated with IMQ at day 4 (n=4). (h) Schematic representation of the use of anti-Ly6G antibody in the IMQ-induced psoriasis mouse model. (i) Macroscopic phenotypic representation and PASI score of cKO mice and control mice treated with IMQ and Ly6G antibody. cKO, conditional knockout; ELISA, enzyme-linked immunosorbent assay; IMQ, imiquimod; PASI, psoriasis area and severity index, WT, wild-type. Error bars show mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Data are representative of three independent experiments for (a, b, d, g, i).
-
Figure 4—source data 1
PDF file containing original western blots for Figure 4a and g, indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig4-data1-v1.pdf
-
Figure 4—source data 2
Original files for western blot analysis displayed in Figure 4a and g.
- https://cdn.elifesciences.org/articles/101248/elife-101248-fig4-data2-v1.zip
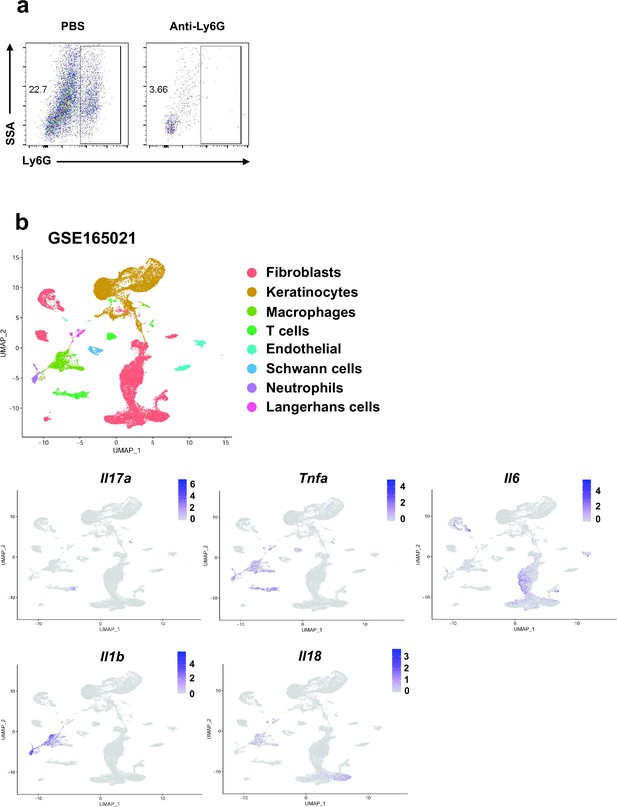
Flow cytometry plots of neutrophil, and data analysis in single-cell RNA sequencing from the skin of IMQ-induced psoriasis-like mice.
(a) Flow cytometry plots of neutrophil, isolated from dorsal skin of wild-type (WT) mice treated with imiquimod (IMQ). (b) Uniform Manifold Approximation and Projection (UMAP) of single cells isolated from the skin of IMQ-induced psoriasis-like mice (GSE165021), and feature plots showing expression of Il17a, Il6, Tnfa, Il1b, and Il18 (n=2). Data are representative of three independent experiments for (a).





