Immune Cells: Growing microglia in the lab
As the global population gets older, age-related diseases are becoming more prevalent, with many linked to dysfunctional immune responses. In the central nervous system, microglia are resident immune cells that maintain homeostasis, respond to injury and regulate inflammation. However, these cells can become dysregulated over time, and the resulting inflammation has been implicated in age-related neurodegenerative diseases, including Alzheimer’s disease (Cai et al., 2022) and Parkinson’s disease (Tansey et al., 2022), as well as diseases that affect the retina, such as glaucoma (Wei et al., 2019; Pan et al., 2023). However, despite extensive research into methods for shifting microglia from a dysfunctional state back into a state that can protect the nervous system, there are currently no drugs that have been approved to do this.
Microglia derived from induced pluripotent stem cells (iPSCs) – adult cells that have been reprogrammed to have the same properties as embryonic stem cells – offer a powerful way to study human microglia in healthy and diseased states. When sourced directly from patients, these cells offer a renewable, patient-specific model with the potential to be used therapeutically to replace dysfunctional microglia with healthier versions without the risk of immune rejection. Now, in eLife, Wai T Wong (Tiresias Bio), Wei Li (National Eye Institute) and colleagues – including Wenxin Ma as first author – report the results of initial efforts to develop iPSC-derived microglia that can be used to treat retinal diseases (Ma et al., 2024).
First, Ma et al. optimized previously reported methods for the production of human microglial cells from iPSCs. This enabled them to continuously produce microglia at a purity (often greater than 95%) and a scale that could support both research and the commercialization of their approach. Testing the method in five distinct stem cell lines showed that it was broadly applicable, increasing the likelihood that it could be used to generate microglia from individual patients.
Gene expression profiles from the resulting microglia closely resembled those of native microglia and were clearly distinct from the progenitor iPSCs. Challenging the iPSC-derived microglia with bacteria revealed that they displayed two hallmark functional activities of native microglia: (i) they secreted proteins associated with inflammatory responses when exposed to bacterial toxins; (ii) they engulfed particles coated with bacteria.
Next, the team optimized a procedure for replacing dysfunctional microglia in the retina with healthy microglia derived from iPSCs. In mice, native microglia in the retina were depleted by inhibiting a receptor required for their survival, and fluorescent human iPSC-derived microglia were then injected into the subretinal space. Over the course of 3–6 months, the human microglial cells successfully repopulated the retina and integrated across all retinal layers (Figure 1). Some native mouse microglia were also present in the retina, but at greatly reduced numbers. The human microglia assumed characteristic shapes that indicated they had taken on a protective and supportive functional state.
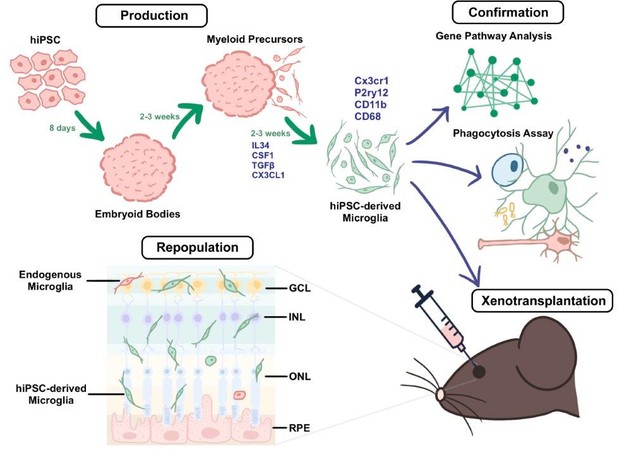
A method for producing human iPSC-derived microglia and transplanting them into mice.
During the production stage (top left), embryoid bodies derived from human iPSCs (induced pluripotent stem cells; pink) develop into myeloid precursors, which then differentiate into microglia (green cells) after exposure to a cocktail of cytokines (IL-34, CSF1, TGFβ, and Cx3cl1). The microglia derived from the iPSCs express typical microglia markers (such as Cx3cr1, P2ry12, CD11b, and CD68). During the confirmation stage (top right), the properties of the microglia are validated through a combination of gene profiling, comparative Gene Pathway Analysis, phagocytosis assays (which probe the ability of the microglia to engulf particles coated with bacterial proteins), and xenotransplantation (which involves transplanting the microglia into the subretinal space of adult mice; bottom right). Xenotransplantation is followed by the repopulation stage (bottom left), in which the microglia derived from the iPSCs become integrated and distributed across the different retinal layers in a pattern consistent with endogenous microglia (red cell). GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer; RPE: retinal pigment epithelium.
Lastly, Ma et al. determined whether the transplanted human microglia could respond to retinal damage. Killing photoreceptors (the cells that detect light) by administering high doses of the chemical sodium iodate (Kannan and Hinton, 2014), caused the human microglia to transiently proliferate and migrate into the layer of the retina containing the photoreceptors. Moreover, the human microglia began to consume the cellular debris created by the dying photoreceptors. These responses mirror the actions of native microglia and reflect the critical ability of the iPSC-derived microglia to protect the remaining healthy photoreceptor cells.
With this innovative approach, a biopsy of skin cells from a patient with glaucoma could be transformed into the very cells that might halt or prevent blindness. Even more intriguing, the mouse model developed by Ma et al. to allow transplantation of iPSC-derived human microglia represents a valuable platform for discovering even more therapies for retinal diseases. Taken together, the findings of Ma et al. represent a significant step forward in developing new therapeutic approaches for treating age-related central nervous system disorders where microglial dysfunction plays a central role.
References
-
Sodium iodate induced retinal degeneration: new insights from an old modelNeural Regeneration Research 9:2044–2045.https://doi.org/10.4103/1673-5374.147927
-
Inflammation and immune dysfunction in Parkinson diseaseNature Reviews Immunology 22:657–673.https://doi.org/10.1038/s41577-022-00684-6
-
Neuroinflammation and microglia in glaucoma: time for a paradigm shiftJournal of Neuroscience Research 97:70–76.https://doi.org/10.1002/jnr.24256
Article and author information
Author details
Publication history
Copyright
© 2024, Levenson et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 588
- views
-
- 48
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Stem Cells and Regenerative Medicine
Harnessing the regenerative potential of endogenous stem cells to restore lost neurons is a promising strategy for treating neurodegenerative disorders. Müller glia (MG), the primary glial cell type in the retina, exhibit extraordinary regenerative abilities in zebrafish, proliferating and differentiating into neurons post-injury. However, the regenerative potential of mouse MG is limited by their inherent inability to re-enter the cell cycle, constrained by high levels of the cell cycle inhibitor p27Kip1 and low levels of cyclin D1. Here, we report a method to drive robust MG proliferation by adeno-associated virus (AAV)-mediated cyclin D1 overexpression and p27Kip1 knockdown. MG proliferation induced by this dual targeting vector was self-limiting, as MG re-entered cell cycle only once. As shown by single-cell RNA-sequencing, cell cycle reactivation led to suppression of interferon signaling, activation of reactive gliosis, and downregulation of glial genes in MG. Over time, the majority of the MG daughter cells retained the glial fate, resulting in an expanded MG pool. Interestingly, about 1% MG daughter cells expressed markers for retinal interneurons, suggesting latent neurogenic potential in a small MG subset. By establishing a safe, controlled method to promote MG proliferation in vivo while preserving retinal integrity, this work provides a valuable tool for combinatorial therapies integrating neurogenic stimuli to promote neuron regeneration.
-
- Stem Cells and Regenerative Medicine
Tissue engineering strategies predominantly rely on the production of living substitutes, whereby implanted cells actively participate in the regenerative process. Beyond cost and delayed graft availability, the patient-specific performance of engineered tissues poses serious concerns on their clinical translation ability. A more exciting paradigm consists in exploiting cell-laid, engineered extracellular matrices (eECMs), which can be used as off-the-shelf materials. Here, the regenerative capacity solely relies on the preservation of the eECM structure and embedded signals to instruct an endogenous repair. We recently described the possibility to exploit custom human stem cell lines for eECM manufacturing. In addition to the conferred standardization, the availability of such cell lines opened avenues for the design of tailored eECMs by applying dedicated genetic tools. In this study, we demonstrated the exploitation of CRISPR/Cas9 as a high precision system for editing the composition and function of eECMs. Human mesenchymal stromal/stem cell (hMSC) lines were modified to knock out vascular endothelial growth factor (VEGF) and Runt-related transcription factor 2 (RUNX2) and assessed for their capacity to generate osteoinductive cartilage matrices. We report the successful editing of hMSCs, subsequently leading to targeted VEGF and RUNX2-knockout cartilage eECMs. Despite the absence of VEGF, eECMs retained full capacity to instruct ectopic endochondral ossification. Conversely, RUNX2-edited eECMs exhibited impaired hypertrophy, reduced ectopic ossification, and superior cartilage repair in a rat osteochondral defect. In summary, our approach can be harnessed to identify the necessary eECM factors driving endogenous repair. Our work paves the road toward the compositional eECMs editing and their exploitation in broad regenerative contexts.






