Coevolutionary interplay: Helminths-trained immunity and its impact on the rise of inflammatory diseases
Figures
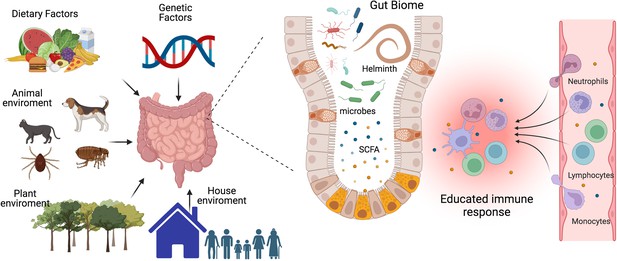
The interplay between gut microbiota, immune system, and environmental factors.
Our immune system’s development, maturation, and response to challenges are significantly influenced by the composition of our gut biome. This complex ecosystem is shaped not only by host genetics but also by critical environmental factors such as diet, lifestyle, and exposure to animals, plants, and other individuals. The micro- and macro-biota play a vital role in maintaining both gut and systemic immune homeostasis by modulating both innate and adaptive immune responses. Helminths can directly or through the microbiota or metabolic changes educate or train our immune system to promote regulatory networks that benefit from a less aggressive inflammatory response. A deeper understanding of the intricate interactions between parasites, hosts, and the microbiome is essential to develop effective strategies for preventing and treating chronic inflammatory diseases. SCFA, a short-chain fatty acid metabolite. This figure was created using BioRender.com.
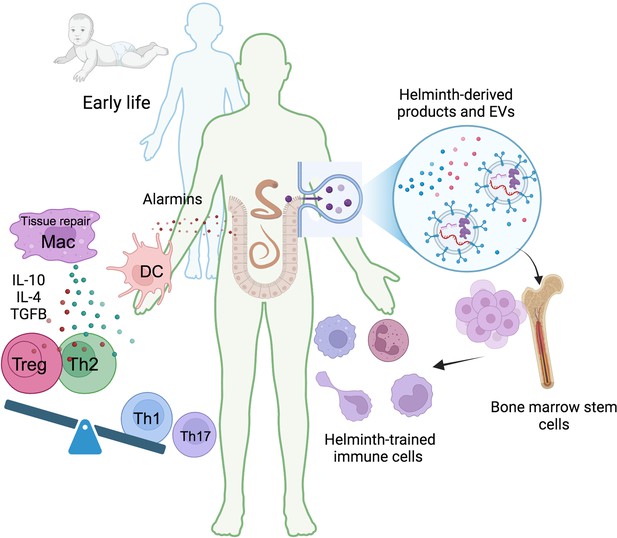
Helminth-induced trained immunity.
Early life immune education is crucial for developing a robust and well-regulated immune system. Humans have evolved a symbiotic relationship with commensal microbiota, and disruptions to this balance can lead to immune dysregulation. Helminths, with their complex life cycles and prolonged host interactions, can significantly influence host immunity. During infection, tissue damage caused by migrating helminths triggers the release of alarmins like TSLP, IL-25, and IL-33. These alarmins recruit monocytes and DC, and the Th2 environment promotes the differentiation of anti-inflammatory and tissue repair macrophages. These trained macrophages produce higher levels of IL-10, IL-4, or TGFβ promoting the differentiation of regulatory T cells (Tregs) and suppressing pro-inflammatory Th1 and Th17 responses. Helminth-derived products, including small peptides, enzymes, lipids, and EVs carrying various molecules like RNA and proteins, can induce central anti-inflammatory trained immunity. This leads to the generation of long-lasting anti-inflammatory myeloid cells, suggesting a potential impact on bone marrow hematopoietic progenitors. TSLP, thymic stromal lymphopoietin; DC, dendritic cells; Mac, macrophages; Treg, regulatory T cells; EVs, extracellular vesicles. This figure was created using BioRender.com.
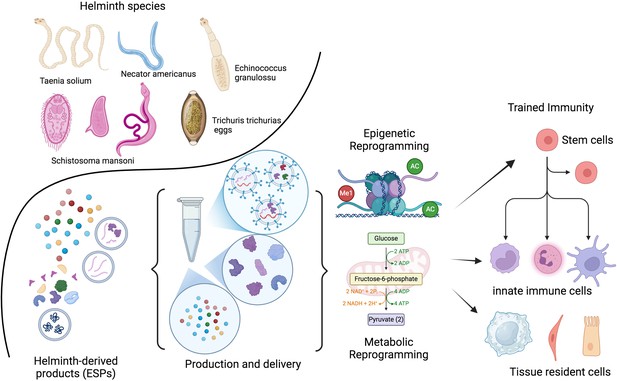
Therapeutic potential of helminths and their products.
The therapeutic potential of helminths and their products depends on various factors, including the specific helminth species, the dosage, timing of inoculation, and the parasite’s life stage. Helminths have evolved sophisticated mechanisms to modulate the host immune system. Their products (ESPs) contain a diverse range of molecules, including proteins, peptides, enzymes, lipids, glycans, and EVs. These EVs can deliver fragile cargo, like RNA, to distant cells. Identifying and characterizing these molecules and their target pathways presents a unique opportunity to develop novel, safe, and effective therapeutic strategies inspired by nature. Recent research suggests that the adaptation of the developing immune system to helminths involves epigenetic and metabolic changes. These adaptations may be lost after a few generations without helminth exposure. The goal is to identify the optimal combination of patient, genetic factors, disease, and helminth products or their metabolic byproducts to train the immune system both locally and at the stem cell level. ESPs, excretory/secretory products; EVs, extracellular vesicles. This figure was created using BioRender.com.





