Vision: The inner workings of a miniature eye
How small can an organ get while remaining functional? Parasitic wasps from the Megaphragma genus are ideal organisms in which to investigate this question. Using a range of senses, including vision, they locate minuscule insects, known as thrips, inside which they lay their eggs (Polaszek et al., 2022). Upon hatching, the wasp larvae eat the insides of their hosts, pupate and emerge as adults from the remains, ready to repeat the cycle. This parasitic lifestyle imposes severe selective pressures; in particular, much like matryoshka dolls, the parasites generally need to be smaller than the animals they take advantage of. Since thrips are less than a millimeter long, Megaphragma wasps are minuscule, often barely reaching a fifth of a millimeter. Such extreme miniaturization requires drastic space-saving adaptations; the brain of Megaphragma viggianii wasps, for example, has fewer neurons which also lack nuclei (Polilov, 2012). Still, this reduction process can only go so far – especially for structures like the eye, which have strict size limitations due to the laws of physics and the nature of light.
Insects possess compound eyes formed of discrete, elongated units known as ommatidia. Like pixels in a camera sensor, each ommatidium samples a portion of the visual space and provides a fraction of the overall perceived image. To do so, ommatidia feature a lens that focuses and directs light onto the rhabdom, a structure stemming from photosensitive cells that convert light packets (photons) into electrical signals which are then relayed to brain neurons. To function properly, these building blocks must meet certain size requirements: in particular, the lenses must be large enough to focus an adequate amount of light from a certain direction without distortion, and the rhabdom must be sufficiently thick to guide the incoming photons.
With only 29 ommatidia, the eyes of M. viggianii wasps are one the smallest amongst animals that exhibit complex behaviors and spatial navigation (Figure 1A). Previous work has started to shed light on how their visual system can accommodate such extreme miniaturization (Chua et al., 2023). Now, in eLife, Alexey Polilov and colleagues – including Anastasia Makarova as first author – report the first complete 3D map of the compound eye of adult M. viggianii wasps with unprecedented detail (Makarova et al., 2025).
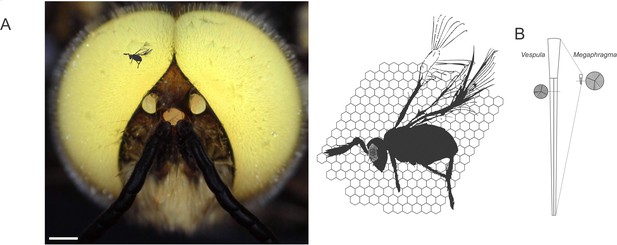
Size scaling of hymenopteran eyes, from Megaphragma viggianii to common wasps and honeybee drones.
(A) M. viggianii (black silhouette), honeybees (head of an albino drone with white eyes; left) and other bee-sized insects such as the common wasp (Vespula vulgaris) all rely on their vision to navigate their environment. Despite striking size differences, these species possess similar compound eyes consisting of individual units, or ommatidia (right; ommatidial facets of V. vulgaris represented as hexagonal shapes behind the full body of M. viggianii, whose ommatidia are depicted as small circular shapes in the eye). Scale bar, 0.5 mm. (B) The work by Makarova et al. sheds light on the organization of the eye in M. viggianii wasps. As in most insects, ommatidia in V. vulgaris (left) and M. viggianii (right) wasps are shaped like slightly tapered columns with a lens near their surface (top), and photosensitive structures (known as rhabdom; circular insets) further down inside. In M. viggianii, each ommatidium is only 20 µm long (compared to 350 µm in V. vulgaris), and the diameter of its lens reaches around 8 µm (25 µm in V. vulgaris). Yet the width of the rhabdom remains similar between the two species (1.5 µm diameter in V. vulgaris vs. 2 µm in M. viggianii).
Image credit: Honeybee drone picture by Gregor Belušič (CC BY 4.0); M. viggianii silhouette adapted from Figure 1B of Polilov, 2017 (CC BY 4.0); ommatidia silhouette adapted from Figure 4A–B of Gutiérrez et al., 2024 (CC BY 4.0).
The team, which is based at Lomonosov Moscow State University and various institutions in the United States, exploited one of the advantages conferred by the small size of the insect; namely, that its entire body could be sectioned and imaged via electron microscopy at once (Polilov, 2017). Imaging revealed that the eye is formed of 478 cells, with a single ommatidium bringing together nine photosensitive cells, four cone cells (that focus light), and two primary pigment cells containing granules full of opaque compounds.
Further analyses showed that the diameter of the lens in an ommatidium is only 8 µm at most, which is hardly sufficient to focus light onto photoreceptors without distortion. However, the underlying rhabdom has retained a relatively broad cross-section, 2 µm, which is similar to that found in large diurnal insects (Figure 1B). Together, the lens and rhabdom are therefore able to efficiently capture enough light for the eyes to perform during the day. In addition, the sides of the ommatidia are coated with very dense layers of pigment granules that optically isolate the rhabdoms, blocking stray light and therefore contributing to image formation.
Examining cell structure revealed that, unlike neurons in the brain of M. viggianii, eye cells have nuclei; probably due to optical constraints, these cells remained large enough to escape extreme miniaturization and retain an essential organelle lost elsewhere in the visual pathway. Finally, Makarova et al. show that photoreceptor cells are packed with mitochondria, suggesting that vision is metabolically costly to the wasps.
In many insects, an eye region known as the dorsal rim area is part of a visual system used to analyze polarized light from the sky, which serves as a stable spatial reference when animals travel in arbitrary directions or across longer distances (Labhart and Meyer, 1999). Surprisingly, in M. viggiani wasps, about a third of the available ommatidia, located dorsally, show specializations for the detection of polarized light; this includes a special rhabdom geometry, smaller optical parts and even cell nuclei inserted into the optical pathway that may play an optical role, similar to eye structures facilitating polarized light navigation in honeybees (Meyer and Labhart, 1981). The compound eye thus shows a remarkable resource allocation, with ~30% dedicated to navigation and ~60% to image formation. Lastly, the analysis revealed the presence of three photoreceptors disconnected from the eye optics behind the first ommatidia in the dorsal margin; these structures possibly contribute to other biological processes requiring light detection, such as the regulation of circadian rhythms.
Overall, the work by Makarova et al. represents a valuable quantitative morphological reference for the future studies of compound eyes and miniaturized organs in visual physiology and cell biology. It will inform the design of miniaturized imaging sensors, enable the numerical modelling of small compound eyes, and support the framework for the understanding of cellular architecture in eukaryotic organisms.
References
-
A complete reconstruction of the early visual system of an adult insectCurrent Biology 33:4611–4623.https://doi.org/10.1016/j.cub.2023.09.021
-
Spatial resolution and optical sensitivity in the compound eyes of two common European wasps, Vespula germanica and Vespula vulgarisThe Journal of Experimental Biology 227:jeb246670.https://doi.org/10.1242/jeb.246670
-
Pore canals in the cornea of a functionally specialized area of the honey bee’s compound eyeCell and Tissue Research 216:491–501.https://doi.org/10.1007/BF00238646
-
The smallest insects evolve anucleate neuronsArthropod Structure & Development 41:29–34.https://doi.org/10.1016/j.asd.2011.09.001
Article and author information
Author details
Publication history
Copyright
© 2025, Belušič
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 305
- views
-
- 25
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
Cichlid fishes inhabiting the East African Great Lakes, Victoria, Malawi, and Tanganyika, are textbook examples of parallel evolution, as they have acquired similar traits independently in each of the three lakes during the process of adaptive radiation. In particular, ‘hypertrophied lip’ has been highlighted as a prominent example of parallel evolution. However, the underlying molecular mechanisms remain poorly understood. In this study, we conducted an integrated comparative analysis between the hypertrophied and normal lips of cichlids across three lakes based on histology, proteomics, and transcriptomics. Histological and proteomic analyses revealed that the hypertrophied lips were characterized by enlargement of the proteoglycan-rich layer, in which versican and periostin proteins were abundant. Transcriptome analysis revealed that the expression of extracellular matrix-related genes, including collagens, glycoproteins, and proteoglycans, was higher in hypertrophied lips, regardless of their phylogenetic relationships. In addition, the genes in Wnt signaling pathway, which is involved in promoting proteoglycan expression, was highly expressed in both the juvenile and adult stages of hypertrophied lips. Our comprehensive analyses showed that hypertrophied lips of the three different phylogenetic origins can be explained by similar proteomic and transcriptomic profiles, which may provide important clues into the molecular mechanisms underlying phenotypic parallelisms in East African cichlids.
-
- Evolutionary Biology
A major question in animal evolution is how genotypic and phenotypic changes are related, and another is when and whether ancient gene order is conserved in living clades. Chitons, the molluscan class Polyplacophora, retain a body plan and general morphology apparently little changed since the Palaeozoic. We present a comparative analysis of five reference quality genomes, including four de novo assemblies, covering all major chiton clades, and an updated phylogeny for the phylum. We constructed 20 ancient molluscan linkage groups (MLGs) and show that these are relatively conserved in bivalve karyotypes, but in chitons they are subject to re-ordering, rearrangement, fusion, or partial duplication and vary even between congeneric species. The largest number of novel fusions is in the most plesiomorphic clade Lepidopleurida, and the chitonid Liolophura japonica has a partial genome duplication, extending the occurrence of large-scale gene duplication within Mollusca. The extreme and dynamic genome rearrangements in this class stands in contrast to most other animals, demonstrating that chitons have overcome evolutionary constraints acting on other animal groups. The apparently conservative phenome of chitons belies rapid and extensive changes in genome.






