Neuromodulation: Transcranial electric stimulation seen from within the brain
The human brain seems well protected, encased within the skull. Yet something as simple as placing a pair of wet sponges onto someone's head and sending a weak electric current between them can actually alter the brain's activity. A refined version of this method – known as transcranial electric stimulation – has attracted considerable interest and is now being used to probe the workings of the brain and develop treatments for medical conditions such as depression, epilepsy or stroke.
Transcranial electric stimulation (or TES for short) has parallels with conventional drug treatments in the sense that delivering an electric field to the brain is analogous to delivering drug molecules into the body. So, just as it is important to know how the human body affects an administered drug (a field of research that is known as pharmacokinetics), in TES we need to know how much of the current applied to the scalp actually enters the brain, and where this current goes.
The 'pharmacokinetics of TES' remains contentious (Underwood, 2016), but is important for several reasons. First, it allows us to relate findings from experiments in which brain tissue from animals is stimulated directly to findings obtained via noninvasive applications in people. Second, it helps researchers optimize the process in order to target specific regions of the brain. Third, it enables researchers to compensate for the differences between individuals, and to standardize the exposure that they receive.
The only established approach for estimating the dose of TES delivered to an individual relies on a three-dimensional model of the subject's head that includes its different tissues and the attached electrodes, which is fed into a computer simulation (Figure 1). Such models have been available for some time (Datta et al., 2009), but they had been validated only partially and indirectly in humans or other primates (Edwards et al., 2013; Lee et al., 2015). Moreover, there are uncertainties about the electric properties of the tissues in these models.
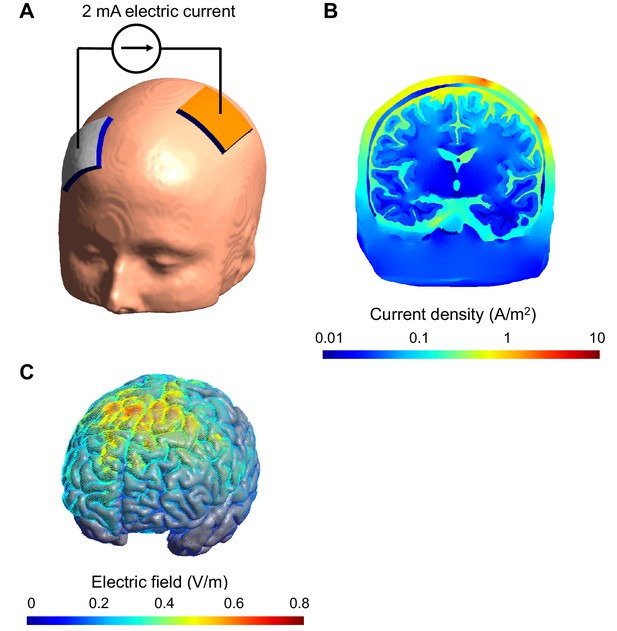
Computational model of the electric field and current produced in an individual's head during transcranial electric stimulation.
(A) Electrodes (white and orange rectangles) are attached to the scalp and electric current is applied; the model of the head shown here is derived from a structural MRI scan. (B) Simulation showing the electric current per unit area (current density) in a section of the brain during transcranial stimulation: this image shows the scalp (outermost layer), skull, cerebrospinal fluid, gray matter and white matter. The highest current density values in the brain (blue) are 100-fold lower than those in the scalp (red). The high resistance of the skull means that the majority of the current is shunted in the scalp. The cerebrospinal fluid is highly conductive and this takes current away from the brain too. (C) Simulation showing the electric field on the surface of the brain. For this configuration, the electric field is strongest between the two electrodes. The model was created and visualized with the free SimNIBS software package (http://simnibs.de; Windhoff et al., 2013).
Now, in eLife, Lucas Parra and colleagues – including Yu Huang and Anli Liu as joint first authors – report how they have addressed these issues by combining elaborate computational modeling with recordings taken within the brains of ten people undergoing surgery for epilepsy (Huang et al., 2017). This sample size markedly exceeds that of other similar measurements (Opitz et al., 2016), and the three-dimensional models used are highly sophisticated too. Leveraging this setup, Huang et al. provide the most extensive and direct estimates of the TES electric field to date. They also confirm that computational models of TES can accurately recreate the electric field generated in a real brain.
Huang et al. – who are based at City College of the City University of New York, New York University School of Medicine and the Mayo Clinic – provide practical insights that should help others to implement the models as well. For accurate results, the individual scan should capture the entire head, from neck to crown. This is not the convention in clinical imaging, which currently only focuses on the brain, but Huang et al. get round this limitation by splicing the bottom portion of a standard model of a head onto the individual scans. To do this, the images must be properly cropped and morphed, though this feature has yet to be added to publicly available electric field modeling software.
Including a compartment for the cerebrospinal fluid (the colorless liquid that surrounds the brain) also makes the models more accurate. Appropriate imaging and image analysis methods are required to capture this layer as well as the skull, which are both quite thin (see Figure 1B). However, modelers can breathe a sigh of relief, because the data suggest that the different layers within the skull can be omitted from the models without significantly impacting their accuracy. The way that conductivity changes depending on the orientation of the current in the brain's white matter can similarly be ignored, at least for the mostly outer regions of the brain explored so far by Huang et al.
This work also underscores the present limitations of modeling. It is still uncertain exactly what values for tissue conductivity should be used, and whether it is acceptable to use the same values for everyone. Addressing this question requires further studies likely involving a range of techniques. For example, there are promising efforts to measure tissue conductivities directly during surgery (Koessler et al., 2017), or with other noninvasive techniques (Chauhan et al., 2017).
Even without making the absolute electric field estimates more accurate, existing modeling approaches and software appear suitable for measuring the relative strength of stimulation across brain regions, and predicting how an individual's anatomy might affect this. Indeed, the National Institutes of Health now requires that researchers applying for certain grants "use realistic head modeling" to characterize what electric field is delivered across the brain (NIH, 2017). All in all, it seems that the time is now right for wider adoption of 'pharmacokinetics' of transcranial brain stimulation.
References
-
Electric field model of transcranial electric stimulation in nonhuman primates: correspondence to individual motor thresholdIEEE Transactions on Biomedical Engineering 62:2095–2105.https://doi.org/10.1109/TBME.2015.2425406
Article and author information
Author details
Publication history
Copyright
© 2017, Peterchev
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,809
- views
-
- 259
- downloads
-
- 8
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Cerebellar dysfunction leads to postural instability. Recent work in freely moving rodents has transformed investigations of cerebellar contributions to posture. However, the combined complexity of terrestrial locomotion and the rodent cerebellum motivate new approaches to perturb cerebellar function in simpler vertebrates. Here, we adapted a validated chemogenetic tool (TRPV1/capsaicin) to describe the role of Purkinje cells — the output neurons of the cerebellar cortex — as larval zebrafish swam freely in depth. We achieved both bidirectional control (activation and ablation) of Purkinje cells while performing quantitative high-throughput assessment of posture and locomotion. Activation modified postural control in the pitch (nose-up/nose-down) axis. Similarly, ablations disrupted pitch-axis posture and fin-body coordination responsible for climbs. Postural disruption was more widespread in older larvae, offering a window into emergent roles for the developing cerebellum in the control of posture. Finally, we found that activity in Purkinje cells could individually and collectively encode tilt direction, a key feature of postural control neurons. Our findings delineate an expected role for the cerebellum in postural control and vestibular sensation in larval zebrafish, establishing the validity of TRPV1/capsaicin-mediated perturbations in a simple, genetically tractable vertebrate. Moreover, by comparing the contributions of Purkinje cell ablations to posture in time, we uncover signatures of emerging cerebellar control of posture across early development. This work takes a major step towards understanding an ancestral role of the cerebellum in regulating postural maturation.
-
- Neuroscience
When holding visual information temporarily in working memory (WM), the neural representation of the memorandum is distributed across various cortical regions, including visual and frontal cortices. However, the role of stimulus representation in visual and frontal cortices during WM has been controversial. Here, we tested the hypothesis that stimulus representation persists in the frontal cortex to facilitate flexible control demands in WM. During functional MRI, participants flexibly switched between simple WM maintenance of visual stimulus or more complex rule-based categorization of maintained stimulus on a trial-by-trial basis. Our results demonstrated enhanced stimulus representation in the frontal cortex that tracked demands for active WM control and enhanced stimulus representation in the visual cortex that tracked demands for precise WM maintenance. This differential frontal stimulus representation traded off with the newly-generated category representation with varying control demands. Simulation using multi-module recurrent neural networks replicated human neural patterns when stimulus information was preserved for network readout. Altogether, these findings help reconcile the long-standing debate in WM research, and provide empirical and computational evidence that flexible stimulus representation in the frontal cortex during WM serves as a potential neural coding scheme to accommodate the ever-changing environment.






