Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo
Figures
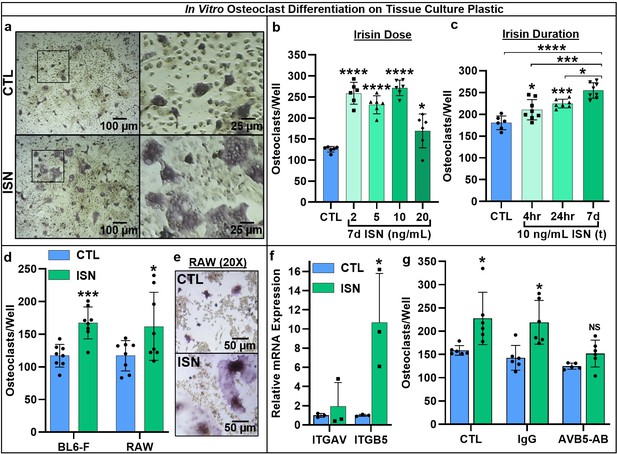
Representative 10× and 40× (boxed inset) images of TRAP-positive stained osteoclasts after 7-day differentiation with 10 ng/mL irisin (ISN) or untreated controls (CTL) (a).
Quantification of osteoclasts per well demonstrating enhanced osteoclastogenesis in response to continuous irisin across a physiologic range of 2–20 ng/mL (b, N = 6) and to treatment with 10 ng/mL irisin for only first 4 or 24 hr of culture compared to continuous treatment or CTL (c, N = 6–8). Quantification of osteoclasts per well confirming irisin stimulation of osteoclastogenesis with continuous 10 ng/mL treatment across primary murine gender with female BL6 mice, and with the macrophage cell line RAW 264.7 (d, N = 8), with representative images of differentiated RAW-derived osteoclasts (e). Expression of integrin receptor subunit αV (ITGAV) and β5 (ITGB5) in primary osteoclast cultures normalized to Hprt (f, N = 3), and quantification of osteoclast per well counts for CTL or continuous 10 ng/mL ISN in the presence of integrin αVβ5 neutralizing antibody (AVB5-AB), an IgG antibody control (IgG), or no antibody (CTL) (g, N = 5–6). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, not significant (NS) versus CTL within-group or as indicated.
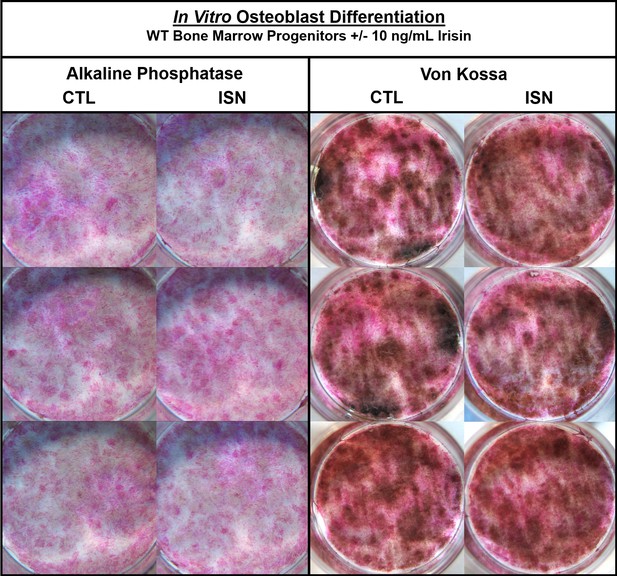
Representative images of in vitro osteoblast cultures with (ISN) or without (CTL) 10 ng/mL irisin during 18-day differentiation.
Alkaline phosphatase and von Kossa staining show no qualitative effect of irisin on differentiation or mineralization, respectively.
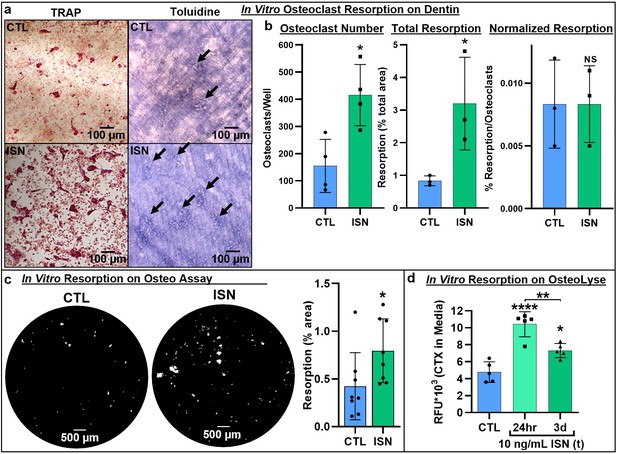
Representative images of TRAP-positive osteoclasts on dentin after 7-day osteoclast culture (left), and underlying resorption pits (arrows) stained with toluidine blue dye after decellularization via sonication, demonstrating increased resorption with irisin treatment (ISN) versus untreated controls (CTL) (a).
Quantification of osteoclast number, total resorption area, and resorption normalized to osteoclast number for dentin cultures (b, N = 3–4). Confirmation of irisin stimulation of resorption with Corning OsteoAssay resorbable calcium phosphate substrate, representative full-diameter images of 96-well plates with binary threshold to visualized resorption pits on von Kossa-stained substrate after 7-day osteoclast culture for ISN versus CTL, with quantification of total resorption by percent area (c, N = 8). Irisin stimulation of early-stage resorption with transient treatment for the first 24 hr alone, versus continuous ISN and CTL with quantification of resorption determined by ELISA of CTX release into media collected at day 3 of culture (d, N = 5). *p<0.05 versus CTL, **p<0.01, ****p<0.0001, not significant (NS) versus CTL within-group or as indicated.
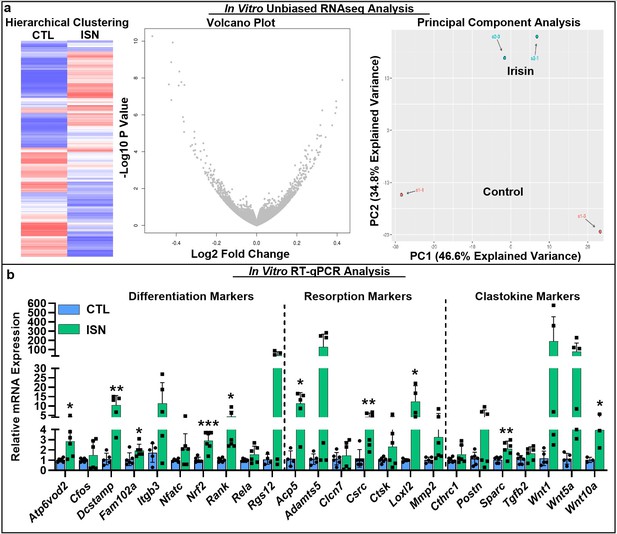
RNAseq analysis of differential gene expression pattern induced by continuous 10 ng/mL irisin treatment (ISN) compared to untreated controls (CTL) at day 7, as typified by representative sample hierarchical clustering, volcano plot, and principal component analysis (a, N = 2).
Relative mRNA expression quantified by RT-qPCR of markers for osteoclast differentiation, resorption, and clastokines in irisin-treated osteoclasts (ISN) compared to untreated controls (CTL), normalized to Hprt expression (b, N = 3–6). *p<0.05, **p<0.01, ***p<0.001 versus CTL within gene.
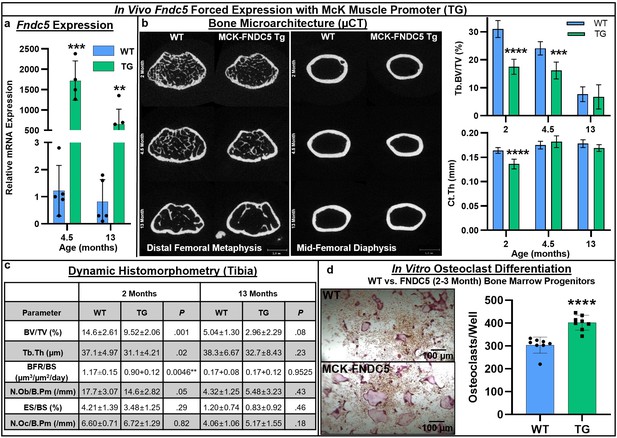
Skeletal phenotype and osteoclastogenic potential of Fndc5 forced expression with McK muscle promoter mice (MCK-FNDC5, TG) compared to wild type C57BL/6J controls (WT).
Whole-bone gene expression from tibia shows increased Fndc5 expression out to 13 months (a, N = 3–5). Representative transverse slice images of trabecular bone at the midpoint of the distal femoral metaphysis (left) and cortical bone at the mid-diaphysis (right) demonstrates reduced cortical bone area in TG versus WT, especially at early ages, with trabecular BV/TV significantly lower at 2 and 4.5 months, and cortical thickness significantly lower at 2 months (b, N = 6). Tibial histomorphometry at 2 and 13 months demonstrated reduction of bone volume fraction at both time points, with slight increase in osteoclast numbers in tibia at 13 months (c, N = 6). In vitro MCSF/RANKL-induced osteoclast differentiation from bone marrow progenitors at 2–3 months of age yielded higher osteoclast numbers in TG versus WT (d, N = 8). ***p<0.001, ****p<0.0001 versus WT.
Representative images of in vitro osteoblast cultures from wild type (WT) or Fndc5-transgenic bone marrow progenitors after 18-day differentiation.
Crystal violet and alkaline phosphatase staining for differentiation and von Kossa staining for mineralization show no qualitative difference between genotypes.
Tables
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus male/female) |
C57BL/6J | Jackson Laboratories | IMSR Cat# JAX:000664, RRID:IMSR_JAX:000664 | Wild type mouse line |
| Cell line (Mus musculus) |
RAW 264.7 | ATCC | TIB-71 | Cryopreserved monocyte cell line |
| Antibody | Anti-Integrin aVb5 (Mouse monoclonal) | Abcam | ab78289, RRID:AB_1566022 | Neutralizing antibody (1:1000) |
| Peptide, recombinant protein | Irisin | Lake Pharma Kim et al., 2018 | N/A (custom) | 10 his-tag recombinant from HEK 293 cells |
| Peptide, recombinant protein | RANKL, recombinant human | PeproTech | 310–01 | Osteoclast growth factor |
| Peptide, recombinant protein | M-CSF, recombinant murine | PeproTech | 315–02 | Osteoclast growth factor |
| Commercial assay or kit | Acid Phosphatase Kit | Sigma-Aldrich | 387A | TRAP assay |
| Commercial assay or kit | Corning Osteo Assay | VWR | 89184–614 | Resorption assay |
| Commercial assay or kit | OsteoLyse Assay Kit | Lonza | PA-1500 | Resorption assay |
| Software, algorithm | Prism 8 | Graphpad | RRID:SCR_002798 | Statistics/graphing software |
| Software, algorithm | FIJI | NIH | RRID:SCR_002285 | Image analysis software |
Additional files
-
Source data 1
Source data tables for figures.
- https://cdn.elifesciences.org/articles/58172/elife-58172-data1-v3.docx
-
Supplementary file 1
Primer Sequence List. Primer sequences used for quantitative RT-PCR on RNA isolated from irisin treated and control primary osteoclast cultures.
- https://cdn.elifesciences.org/articles/58172/elife-58172-supp1-v3.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/58172/elife-58172-transrepform-v3.pdf





