Science Forum: A Global lmmunological Observatory to meet a time of pandemics
Abstract
SARS-CoV-2 presents an unprecedented international challenge, but it will not be the last such threat. Here, we argue that the world needs to be much better prepared to rapidly detect, define and defeat future pandemics. We propose that a Global Immunological Observatory and associated developments in systems immunology, therapeutics and vaccine design should be at the heart of this enterprise.
Main text
Early in a pandemic, clinical surveillance and pathogen sequencing are essential first steps, bolstered by extensive testing to characterize and initiate control of the pandemic trajectory. But this tracking of infection provides only part of the picture (Figure 1A). Specifically, it cannot tell us the trajectory of susceptible individuals who, together with cases, determine the shape and size of the pandemic; the other major group, recovered (potentially immune) individuals, who drive the dynamics of herd immunity, is also not directly observable. Serological surveys can characterize these key hidden variables; however, validation and interpretation are difficult issues (especially for novel pathogens, as SARS-CoV-2 currently illustrates). Furthermore, extensive universal and frequent population sampling for serology is not part of the routine surveillance armory. A Global Immunological Observatory (GIO) would address all of these gaps (Metcalf et al., 2017; Metcalf et al., 2016).
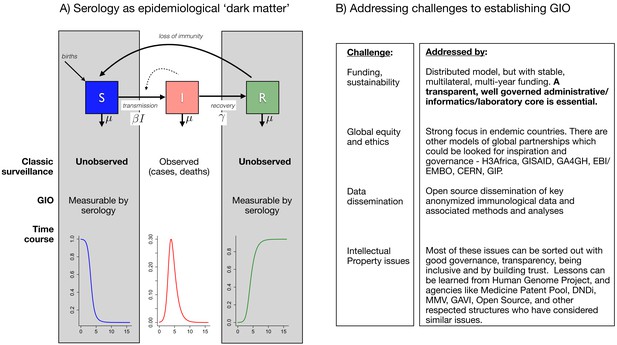
The goals of a Global Immunological Observatory, and the challenges involved in establishing such a body.
(A) The epidemiological process (at its simplest) can be captured as a set of flows from susceptibles (S) to infected individuals (I), which occurs at a rate defined by the numbers of infected individuals and the rate at which they encounter susceptible individuals (a function of human behavior) and then successfully transmit to them – these last two processes are here captured by the parameter . Infected individuals may then recover (entering the R class), and may or may not then become susceptible again. Typical surveillance only captures the I class: innovations around a Global Immunological Observatory (GIO) would provide a window onto the 'dark matter' of epidemiology (that is, the S and R classes). (B) Establishing a GIO will involve addressing challenges related to funding and sustainability, global equity and ethics, data dissemination, and intellectual property.
In theory, we have tremendous ability to deploy multiplex testing for immune responses to pathogens (using techniques ranging from classic ELISAs to phage display approaches) as well as pathogen presence (via genetic sequencing, antigen detection and so on). Further, these methods work with an increasing variety of accessible sample types (saliva, blood spots and so on) which are minimally invasive. Yet, despite decades of technical progress in measurement of both immune responses and pathogen presence (including SARS-CoV and MERS-CoV), which together reveal the core processes driving pathogen transmission (Figure 1A), the global health community was unable to identify and model local circulation of SARS-CoV-2 in a timely fashion in almost any setting.
How could we better deploy and refine tools for sophisticated pathogen surveillance to better meet likely future comparable threats (Metcalf et al., 2016)? Several problems have prevented this in the current pandemic. First, a shortage of testing capacity and a paucity of historical and contemporary samples to ground analyses combined to cripple our inferential capacity. More fundamentally, despite huge recent progress in immunology, the complexity of the immune system remains a barrier: a revolution in the infrastructure of immune surveillance and systems immunology to generate new understanding and resultant techniques is required. A number of innovations are in reach to step away from the status quo by building GIO, structured around three core sample types.
Routinely collected seasonal and international surveillance samples to define the baseline (such as clinical discard blood specimens from adults, or blood bank and plasma donor samples, representative random sampling and so on) and thus capture anomalies reflecting immune responses to emerging threats. Ideally, this would proceed in parallel with extensive pathogen detection and sequencing, see below.
Repeated samples from cohorts (ideally across the full age range, and including birth cohorts) to characterize the mechanisms underlying the ontogeny and time-course of immunity. This would be invaluable in the current crisis for teasing out immune correlates of protection.
To anticipate zoonotic threats, a multi-species extension of GIO replicating these surveillance streams in key reservoir species (notably bats), and associated at-risk occupations, is an important extension (Daszak et al., 2020).
Such samples are a necessary condition for GIO, however, they will not, in themselves, be sufficient – a series of technological developments are also required: to define the core endemic pathogen imprint on the individual and thus population level immune function, necessary to enable identification of departures from it. Traditionally, ELISAs are the foundation of public health immunological surveillance, although they largely remain limited in throughput, both in the numbers of specimens tested and numbers of pathogen-specific antibodies detected. Advances in highly multiplexed, comprehensive serological evaluations of known and potential pathogen exposure (e.g., microarray chips, VirScan [Xu et al., 2015; Khan et al., 2020]) are increasingly available. Simultaneous epitope and T and B cell repertoire identification such as T-scan (Kula et al., 2019), coupled with direct pathogen detection will provide a much more exhaustive picture than currently available of pathogen exposure and immune response.
Critically, deployment of the observatory would have allowed us to rapidly achieve a preliminary understanding of the dynamics of immunity – even a few weeks gained would have been beneficial in the current crisis. For example, with GIO operational, ongoing evaluation of clinical discard or blood donor specimens (e.g., using VirScan) would have enabled fast detection of serological shifts away from baseline. In particular, we would have readily detected excess cross-reactive antibodies for seasonal coronaviruses, SARS, MERS or any of an additional number of animal coronaviruses already present in the VirScan library. The value of routine measurements of such a broad diversity of antibodies (e.g. VirScan detects antibodies to hundreds of thousands of potential pathogen epitopes with only a microliter of blood) is high sensitivity to perturbations in the antibody repertoires including from zoonotic pathogens not known to infect humans. Through GIO, routine evaluation of international longitudinal sample sets would have been available to guide our understanding of attack-rate in vulnerable populations and in children, sero-prevalence, acquisition of sero-positivity and how they map to immunity, potentially vital weeks earlier. Further, even before the outbreak, many of the fundamental immunological uncertainties revealed by the current pandemic could have been addressed in 'peacetime': mass sampling coupled with technical refinements in measurements, and immuno-epidemiological modeling would have shed important light on the potential for and magnitude of cross-reactivity to existing coronaviruses, the role of cellular vs. humoral immunity, the duration of immunity, and the potential for immune pathogenesis from autoimmunity to antibody dependent enhancement.
Beyond immune-based detection, ideally GIO would exist next to a similar Global Pathogen Observatory (GPO) for a parallel effort surveilling and sequencing pathogens themselves, potentially informed by the immunological context GIO reveals. In particular, GPO could build on seminal previous efforts in pathogen discovery (eg, PREDICT, the Human Virome Project), rooted in the disease ecosystem perspective of OneHealth (Daszak et al., 2020; Carroll et al., 2018).
From detection to control
Of course, we must not simply observe a pandemic as it evolves. We must act in parallel and we must translate our knowledge into directly reducing morbidity and mortality in all vulnerable populations. GIO and GPO provide a unique lens for achieving this. The necessary technologies fundamentally already exist. The time from release of the SARS-CoV-2 sequence to a needle in an arm in the first Phase one vaccine trial was only 65 days (https://www.clinicaltrials.gov/ct2/show/NCT04283461). This is a remarkable achievement made possible through the recent development of easily manufactured, implementable and deployable vaccine platforms, such as mRNA or DNA constructs.
Contemporaneously, permissive animal challenge models should be established to test the vaccine and also to aid in the identification of neutralizing monoclonal antibodies which could be used in short term prevention and as therapeutic agents. This endeavor should go hand in hand with identification of neutralizing monoclonal antibodies in people who have recovered from the infection as well as with detailed analysis of their T cell responses. Finally, and this all flows logically from the initial serological signal and identification of the pathogen’s sequence and in vitro production of its proteins, the establishment of assays to measure the breadth, affinity, receptor binding competition analyses and neutralizing capacity of specific antibodies would be a critical part of this process. Importantly, all these activities can and should be done in parallel. Indeed, with 'peacetime' sampling of reservoir species by GIO and GPO, a library of mRNA/DNA candidates could be generated preemptively. More broadly, GIO could provide considerable insights into other immune-related diseases: from cancer to autoimmune disorders.
We argue that a Global Immunological Observatory is essential for understanding and combating future pandemics. The GIO/GPO infrastructure would bootstrap our ability to engage with a series of fundamental questions associated with the other key processes in pandemics, such as the transmission process – encompassing the biophysics of pathogen spread between individuals, as well as the role of patterns of social mixing. To make full use of immunological and pathogen observatories, cross fertilization between many fields will be required: spanning systems immunology, virology and physical sciences, through to public health and social science. Major progress in computational biology and disease modeling would also be enabled by these rich and varied datasets. In particular, a key gap in pandemic and endemic disease understanding is the cross-scale phylodynamic interaction between viral evolution and immune kinetics in individuals and nonlinear epidemic dynamics at population and global scales (Grenfell et al., 2004). Establishment of GIO/GPO would generate a step change in knowledge at this key interface.
Many lessons will emerge from this pandemic, but we need to learn even more before the next inevitable outbreaks occur. GIO and a larger pathogen enterprise will require significant annual investments, but this will be trivial compared to the costs of future pandemics. Establishing GIO/GPO would require solving a range of important practical issues, from sustainable funding, governance, global equity, intellectual property and relationships with industry, but there are existing models and clear paths to addressing all of these challenges (Figure 1B). Lessons for infrastructure, funding and governance could also be learned from the virological and immune phenotypes collected to inform strain selection for seasonal influenza vaccination coordinated by the WHO (Morris et al., 2018). A variety of distributed models are possible for GIO/GPO, but an essential condition is the transparent and effective governance of administrative, informatics and laboratory cores.
Another field where understanding of spatio-temporal dynamics is crucial to addressing a deeply applied problem is weather and climate science. The skill of weather forecasts was dramatically improved by deployment of buoys measuring Sea Surface Temperature across the oceans of the world (http://www.argo.ucsd.edu/About_Argo.html). Similarly, in the context of global health, we have the means to deploy measurements crucial both to pandemic preparedness, and endemic disease control, using the delicate and responsive sensor that is all of our immune systems (and the immune systems of all the reservoir species). And as this pandemic has repeatedly shown, early warning and rapid response can make dramatic differences that translate directly to immensely favorable outcomes. Susceptibility and immunity have been the 'dark matter' of epidemic dynamics; GIO could reveal them, to the considerable benefit of global health.
Data availability
No data is involved in this manuscript.
References
-
Predictive modeling of influenza shows the promise of applied evolutionary biologyTrends in Microbiology 26:102–118.https://doi.org/10.1016/j.tim.2017.09.004
Decision letter
-
Clifford J RosenSenior and Reviewing Editor; Maine Medical Center Research Institute, United States
-
Kinna ThakararReviewer; Maine Medical Center, United States
-
Michael WhiteReviewer; Institut Pasteur, France
In the interests of transparency, eLife publishes the most substantive revision requests and the accompanying author responses.
Thank you for submitting your article, "A Global Immunological Observatory to meet a time of pandemics" to eLife. Your article has been reviewed by two reviewers [reports below] and I am pleased to tell you that it will be accepted for publication as a Feature Article once it has been revised in response to the comments from the referees and a number of editorial points.
To help expedite the revision process I have extracted the points from the reviews that you need to address – please see points 1-5 below.
Points to address from the reviewers
The key question is not should a GIO & GPO be implemented (they should), but, why has progress not been made on implementing such structures since they were originally proposed. What was not discussed were the financial, governance and equity barriers that have prevented the establishment of a GIO.
1) "Finance. Biobanking at scale is expensive - who pays? How is this sustained in the long term? Where are all the -80C freezers? And who is maintaining them?"
- From the Features Editor: Please consider adding a few sentences about these questions, or at least mention that these questions will need to be answered if the GIO project is to move ahead.
2) "Governance. To meet the ambitious vision of the authors, the 'global' part of GIO is especially important. Who owns the samples? Who determines access? How does transfer occur across international borders?"
- From the Features Editor: Again, please consider adding a few sentences about these questions, or at least mention that these questions will need to be answered.
3) "Equity. Most important of all in my opinion. There is a big gap between the low and middle income countries (LMIC) where many emergent pathogens originate, and high income countries with elite institutions and laboratories possessing advanced technologies (e.g. VirScan). Global observatories require global participation, and the perspective of LMIC researchers and public health personnel is missing."
- From the Features Editor: Please add a paragraph about equity barriers.
4) "The proposed GIO would also build on existing infrastructure (GPO, Global Virome project); if space allows, it may be helpful to further expand on this part."
- From the Features Editor: Please add a few sentences about how GIO might build on existing infrastructure.
5) "I really like the analogy of the deployment of buoys for measuring Sea Surface Temperature. Such buoys produce data which is easily shared and reproduced. A GIO would produce serum of finite quantity which must be stored somewhere, and can only be used by a select few.
- From the Features Editor: Please add a few sentences about this issue (unless it is already covered by your answers to the points above).
Reports from the reviewers
Reviewer 1
I enjoyed reading your paper, "A Global Immunological Observatory to meet a time of pandemics." In addition to clinical surveillance and pathogen sequencing, you highlight the following points to managing future pandemics: 1) collection of core samples 2) pairing advanced serologic sampling with technical refinements in measurements and immuno-epidemiological modeling 3) building on serologic sampling to test vaccines and neutralizing monoclonal antibodies.
Core samples would collected through seasonal and international surveillance , repeated sampling through cohorts, and these surveillance streams would be collected in key reservoir species as well. Importantly, the inclusion of longitudinal cohorts internationally would help us better understand the attack-rates of pandemics in vulnerable populations. COVID-19 has exacerbated longstanding inequities in health, and it is an important point to include vulnerable populations in the proposed serologic sampling.
The authors assert that a Global Immunological Observatory (GIO) essential for understanding and combating future pandemics. There are multiple strengths to this article; it is well-written, and it clearly outlines the need for immunological observatory. I have no major concerns.
The proposed GIO would also build on existing infrastructure (GPO, Global Virome project); if space allows, it may be helpful to further expand on this part.
Reviewer #2
With this reviewer, Mina and Metcalf are preaching to the choir. I am in full agreement that it would have been incredibly beneficial to have had an established Global Immunological Observatory (GIO) to respond to the ongoing SARS-CoV-2 pandemic. Indeed, the benefit of such a structure was just as clear four years ago when a subset of the authors proposed essentially the same thing under the name of a World Serum Bank in a Viewpoint in The Lancet (a piece I very much admired). This article revisits the same territory as before but highlights the important benefits that would have been possible had a GIO been implemented. Admittedly, this is shutting the stable door after the horse has bolted. Indeed, this point was made in the original Viewpoint with respect to the 2009 influenza pandemic. Despite this, this point is more valid than ever and deserves to be heard again, and widely discussed in a forum such as eLife.
Since this is an opinion piece, I'll brazenly take the opportunity to throw in my 2 cents. As these are personal opinions related to the topic, and not criticisms of the authors' valid points, they can be ignored at the discretion of the editor and authors.
The key question is not should a GIO & GPO be implemented (they should), but, why has progress not been made on implementing such structures since they were originally proposed. What was not discussed were the financial, governance and equity barriers that have prevented the establishment of a GIO.
Finance. Biobanking at scale is expensive - who pays? How is this sustained in the long term? Where are all the -80C freezers? And who is maintaining them?
Governance. To meet the ambitious vision of the authors, the 'global' part of GIO is especially important. Who owns the samples? Who determines access? How does transfer occur across international borders?
Equity. Most important of all in my opinion. There is a big gap between the low and middle income countries (LMIC) where many emergent pathogens originate, and high income countries with elite institutions and laboratories possessing advanced technologies (e.g. VirScan). Global observatories require global participation, and the perspective of LMIC researchers and public health personnel is missing.
I really like the analogy of the deployment of buoys for measuring Sea Surface Temperature. Such buoys produce data which is easily shared and reproduced. A GIO would produce serum of finite quantity which must be stored somewhere, and can only be used by a select few.
https://doi.org/10.7554/eLife.58989.sa1Author response
[We repeat the reviewers’ points here in italic, and include our replies point by point, as well as a description of the changes made, in Roman.]
Points to address from the reviewers
The key question is not should a GIO & GPO be implemented (they should), but, why has progress not been made on implementing such structures since they were originally proposed. What was not discussed were the financial, governance and equity barriers that have prevented the establishment of a GIO.
Many thanks for these suggestions - we have altered the manuscript along the lines suggested, as detailed below. The principle change is the extension of Figure 1 to include a second panel that details some of the issues raised; but we have also added some specific points to the text.
1) "Finance. Biobanking at scale is expensive - who pays? How is this sustained in the long term? Where are all the -80C freezers? And who is maintaining them?"
- From the Features Editor: Please consider adding a few sentences about these questions, or at least mention that these questions will need to be answered if the GIO project is to move ahead.
In the second panel to the figure (that lists challenges to establishing GIO/GPO and approaches to meeting them), we also emphasize the importance of sustainable funding from multiple sources. However, we don’t think it is helpful to be specific about such sources at this point and in this particular manuscript, so we have not expanded further on this.
2) "Governance. To meet the ambitious vision of the authors, the 'global' part of GIO is especially important. Who owns the samples? Who determines access? How does transfer occur across international borders?"
- From the Features Editor: Again, please consider adding a few sentences about these questions, or at least mention that these questions will need to be answered.
Both the mixed funding sources (described above) and the rich collaboration across the research community that we envisage will result in complex ownership of samples. The emergent data (rather than the samples) encompass much of the value and are much more straightforward to share equitably and publicly. We therefore focus on dissemination of anonymized data (rather than samples) for GIO/GPO. We now make this point in Figure 1B.
3) "Equity. Most important of all in my opinion. There is a big gap between the low and middle income countries (LMIC) where many emergent pathogens originate, and high income countries with elite institutions and laboratories possessing advanced technologies (e.g. VirScan). Global observatories require global participation, and the perspective of LMIC researchers and public health personnel is missing."
- From the Features Editor: Please add a paragraph about equity barriers.
We point to this in the second panel of Figure 1B, where we note specific models that could be used; and also in the text, where we state that: “Establishing GIO/GPO would require solving a range of important practical issues, from sustainable funding, governance, global equity, intellectual property and relationships with industry, but there are existing models and clear paths to addressing all of these challenges.”
4) "The proposed GIO would also build on existing infrastructure (GPO, Global Virome project); if space allows, it may be helpful to further expand on this part."
- From the Features Editor: Please add a few sentences about how GIO might build on existing infrastructure.
We have included a paragraph towards the end of the text, where we state: “Many lessons will emerge from this pandemic- but we need to learn even more before the next inevitable outbreaks occur. GIO and a larger pathogen enterprise will be significant annual investments - but trivial compared to the costs of future pandemics. Establishing GIO/GPO would require solving a range of important practical issues, from sustainable funding, governance, global equity, intellectual property and relationships with industry, but there are existing models and clear paths to addressing all of these challenges (Figure 1B). Lessons for infrastructure, funding and governance could also be learned from the virological and immune phenotypes collected to inform strain selection for seasonal influenza vaccination coordinated by the WHO (Morris et al. 2018). A variety of distributed models are possible for GIO/GPO, but an essential condition is the transparent and effective governance of administrative, informatics and laboratory cores.”
5) "I really like the analogy of the deployment of buoys for measuring Sea Surface Temperature. Such buoys produce data which is easily shared and reproduced. A GIO would produce serum of finite quantity which must be stored somewhere, and can only be used by a select few.
- From the Features Editor: Please add a few sentences about this issue (unless it is already covered by your answers to the points above).
This is an excellent point. To address this to some extent, we now focus on public and equitable sharing of the data, rather than the samples, as sharing these is more clearly straightforwardly actionable.
Reports from the reviewers
Reviewer 1
I enjoyed reading your paper, "A Global Immunological Observatory to meet a time of pandemics." In addition to clinical surveillance and pathogen sequencing, you highlight the following points to managing future pandemics: 1) collection of core samples 2) pairing advanced serologic sampling with technical refinements in measurements and immuno-epidemiological modeling 3) building on serologic sampling to test vaccines and neutralizing monoclonal antibodies.
Core samples would collected through seasonal and international surveillance , repeated sampling through cohorts, and these surveillance streams would be collected in key reservoir species as well. Importantly, the inclusion of longitudinal cohorts internationally would help us better understand the attack-rates of pandemics in vulnerable populations. COVID-19 has exacerbated longstanding inequities in health, and it is an important point to include vulnerable populations in the proposed serologic sampling.
The authors assert that a Global Immunological Observatory (GIO) essential for understanding and combating future pandemics. There are multiple strengths to this article; it is well-written, and it clearly outlines the need for immunological observatory. I have no major concerns.
The proposed GIO would also build on existing infrastructure (GPO, Global Virome project); if space allows, it may be helpful to further expand on this part.
Great suggestion - as mentioned above, we now point to some of these prior examples explicitly, stating: “In particular, GPO could build on seminal previous efforts in pathogen discovery (eg, PREDICT, the Human Virome Project), rooted in the disease ecosystem perspective rooted in OneHealth (Daszak, Olival, and Li 2020; Carroll et al. 2018).” And also: “Lessons for infrastructure, funding and governance could also be learned from the virological and immune phenotypes collected to inform strain selection for seasonal influenza vaccination coordinated by the WHO (Morris et al. 2018). A variety of distributed models are possible for GIO/GPO, but an essential condition is the transparent and effective governance of administrative, informatics and laboratory cores.
Reviewer #2:
With this reviewer, Mina and Metcalf are preaching to the choir. I am in full agreement that it would have been incredibly beneficial to have had an established Global Immunological Observatory (GIO) to respond to the ongoing SARS-CoV-2 pandemic. Indeed, the benefit of such a structure was just as clear four years ago when a subset of the authors proposed essentially the same thing under the name of a World Serum Bank in a Viewpoint in The Lancet (a piece I very much admired). This article revisits the same territory as before but highlights the important benefits that would have been possible had a GIO been implemented. Admittedly, this is shutting the stable door after the horse has bolted. Indeed, this point was made in the original Viewpoint with respect to the 2009 influenza pandemic. Despite this, this point is more valid than ever and deserves to be heard again, and widely discussed in a forum such as eLife.
Many thanks!
Since this is an opinion piece, I'll brazenly take the opportunity to throw in my 2 cents. As these are personal opinions related to the topic, and not criticisms of the authors' valid points, they can be ignored at the discretion of the editor and authors.
The key question is not should a GIO & GPO be implemented (they should), but, why has progress not been made on implementing such structures since they were originally proposed. What was not discussed were the financial, governance and equity barriers that have prevented the establishment of a GIO.
We take this point, and have tried to address it in the current version, specifically expanding on the practicalities in both Figure 1B, and in the text (as described above). However, we have stopped short of outlining the full scope of how this might work in detail –this would be a much longer set of documents, and is perhaps beyond the scope of what is achievable here. In terms of why so little progress towards GIO has been made so far, we agree whole-heartedly with your sentiments, but think that it is important to look forward rather than back at this point.
Finance. Biobanking at scale is expensive - who pays? How is this sustained in the long term? Where are all the -80C freezers? And who is maintaining them?
We now mention this in Figure 1B, and further outline our thinking above. Although we agree that sustainable funding will be critical, we don’t think it is helpful to be specific about the funding such sources at this point and in this particular manuscript, so we have not expanded further on this. We state that: “Establishing GIO/GPO would require solving a range of important practical issues, from sustainable funding, governance, global equity, intellectual property and relationships with industry, but there are existing models and clear paths to addressing all of these challenges (Figure 1B).”
Governance. To meet the ambitious vision of the authors, the 'global' part of GIO is especially important. Who owns the samples? Who determines access? How does transfer occur across international borders?
We strongly agree with this point, and try and reinforce it in both the text (stating that: “Establishing GIO/GPO would require solving a range of important practical issues, from sustainable funding, governance, global equity, intellectual property and relationships with industry, but there are existing models and clear paths to addressing all of these challenges (Figure 1B).”) and in Figure 1B.
Equity. Most important of all in my opinion. There is a big gap between the low and middle income countries (LMIC) where many emergent pathogens originate, and high income countries with elite institutions and laboratories possessing advanced technologies (e.g. VirScan). Global observatories require global participation, and the perspective of LMIC researchers and public health personnel is missing.
We strongly agree with this point, as articulated above.
I really like the analogy of the deployment of buoys for measuring Sea Surface Temperature. Such buoys produce data which is easily shared and reproduced. A GIO would produce serum of finite quantity which must be stored somewhere, and can only be used by a select few.
This is a great point: clearly, the data from GIO/GPO could never be quite as timely as that emanating from the buoy network; the samples, in particular, are not easily shared. Accordingly, in this version, we focus on provision of anonymized data, which should be feasible in a timely fashion assuming requisite technical and informatic advances.
https://doi.org/10.7554/eLife.58989.sa2Article and author information
Author details
Funding
The authors declare that there was no funding for this work.
Publication history
- Received:
- Accepted:
- Accepted Manuscript published:
- Version of Record published:
Copyright
This is an open-access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Metrics
-
- 5,054
- views
-
- 544
- downloads
-
- 65
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
- Microbiology and Infectious Disease
Background:
In many settings, a large fraction of the population has both been vaccinated against and infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hence, quantifying the protection provided by post-infection vaccination has become critical for policy. We aimed to estimate the protective effect against SARS-CoV-2 reinfection of an additional vaccine dose after an initial Omicron variant infection.
Methods:
We report a retrospective, population-based cohort study performed in Shanghai, China, using electronic databases with information on SARS-CoV-2 infections and vaccination history. We compared reinfection incidence by post-infection vaccination status in individuals initially infected during the April–May 2022 Omicron variant surge in Shanghai and who had been vaccinated before that period. Cox models were fit to estimate adjusted hazard ratios (aHRs).
Results:
275,896 individuals were diagnosed with real-time polymerase chain reaction-confirmed SARS-CoV-2 infection in April–May 2022; 199,312/275,896 were included in analyses on the effect of a post-infection vaccine dose. Post-infection vaccination provided protection against reinfection (aHR 0.82; 95% confidence interval 0.79–0.85). For patients who had received one, two, or three vaccine doses before their first infection, hazard ratios for the post-infection vaccination effect were 0.84 (0.76–0.93), 0.87 (0.83–0.90), and 0.96 (0.74–1.23), respectively. Post-infection vaccination within 30 and 90 days before the second Omicron wave provided different degrees of protection (in aHR): 0.51 (0.44–0.58) and 0.67 (0.61–0.74), respectively. Moreover, for all vaccine types, but to different extents, a post-infection dose given to individuals who were fully vaccinated before first infection was protective.
Conclusions:
In previously vaccinated and infected individuals, an additional vaccine dose provided protection against Omicron variant reinfection. These observations will inform future policy decisions on COVID-19 vaccination in China and other countries.
Funding:
This study was funded the Key Discipline Program of Pudong New Area Health System (PWZxk2022-25), the Development and Application of Intelligent Epidemic Surveillance and AI Analysis System (21002411400), the Shanghai Public Health System Construction (GWVI-11.2-XD08), the Shanghai Health Commission Key Disciplines (GWVI-11.1-02), the Shanghai Health Commission Clinical Research Program (20214Y0020), the Shanghai Natural Science Foundation (22ZR1414600), and the Shanghai Young Health Talents Program (2022YQ076).
-
- Epidemiology and Global Health
Background:
The role of circulating metabolites on child development is understudied. We investigated associations between children’s serum metabolome and early childhood development (ECD).
Methods:
Untargeted metabolomics was performed on serum samples of 5004 children aged 6–59 months, a subset of participants from the Brazilian National Survey on Child Nutrition (ENANI-2019). ECD was assessed using the Survey of Well-being of Young Children’s milestones questionnaire. The graded response model was used to estimate developmental age. Developmental quotient (DQ) was calculated as the developmental age divided by chronological age. Partial least square regression selected metabolites with a variable importance projection ≥1. The interaction between significant metabolites and the child’s age was tested.
Results:
Twenty-eight top-ranked metabolites were included in linear regression models adjusted for the child’s nutritional status, diet quality, and infant age. Cresol sulfate (β=–0.07; adjusted-p <0.001), hippuric acid (β=–0.06; adjusted-p <0.001), phenylacetylglutamine (β=–0.06; adjusted-p <0.001), and trimethylamine-N-oxide (β=–0.05; adjusted-p=0.002) showed inverse associations with DQ. We observed opposite directions in the association of DQ for creatinine (for children aged –1 SD: β=–0.05; pP=0.01;+1 SD: β=0.05; p=0.02) and methylhistidine (–1 SD: β = - 0.04; p=0.04;+1 SD: β=0.04; p=0.03).
Conclusions:
Serum biomarkers, including dietary and microbial-derived metabolites involved in the gut-brain axis, may potentially be used to track children at risk for developmental delays.
Funding:
Supported by the Brazilian Ministry of Health and the Brazilian National Research Council.






