Gross ways to live long: Parasitic worms as an anti-inflammaging therapy?
Abstract
Evolutionary medicine argues that disease can arise because modern conditions do not match those in which we evolved. For example, a decline in exposure to commensal microbes and gastrointestinal helminths in developed countries has been linked to increased prevalence of allergic and autoimmune inflammatory disorders (the hygiene hypothesis). Accordingly, probiotic therapies that restore ‘old friend’ microbes and helminths have been explored as Darwinian treatments for these disorders. A further possibility is that loss of old friend commensals also increases the sterile, aging-associated inflammation known as inflammaging, which contributes to a range of age-related diseases, including cardiovascular disease, dementia, and cancer. Interestingly, Crowe et al., 2020 recently reported that treatment with a secreted glycoprotein from a parasitic nematode can protect against murine aging by induction of anti-inflammatory mechanisms. Here, we explore the hypothesis that restorative helminth therapy would have anti-inflammaging effects. Could worm infections provide broad-spectrum protection against age-related disease?
Introduction
The aging process is the main cause of senescent multimorbidity, the co-occurrence of multiple chronic pathologies that includes the major diseases of late life. The geroscience approach views preventative intervention in the aging process as a means to simultaneously pre-empt the development of multiple age-related diseases (Austad, 2016). How does aging cause senescent multimorbidity? While there are clearly multiple contributory factors, one determinant whose importance is becoming increasingly clear is inflammaging, the state of systemic, low-grade inflammation that increases with age, independent of attack by infectious pathogens (Franceschi et al., 2000). Such inflammation is a contributory factor in diverse age-related pathologies, including cardiovascular disease, dementia, cancer, chronic obstructive pulmonary disease (COPD), osteoporosis, age-related macular degeneration (Xia et al., 2016), and perhaps even symptom severity during SARS-CoV-2 (COVID-19) infections (Akbar and Gilroy, 2020).
One cause of inflammaging is gut dysbiosis, an imbalance in the composition of the intestinal microbiome. Such imbalance can manifest as loss of immunomodulatory microbial species and is exacerbated by pro-dysbiotic aspects of the modern lifestyle, including antibiotic usage and the so-called Western diet (Buford, 2017). One interpretation of the cause of such dysbiotic effects utilizes the ‘old friends’ hypothesis (derived from the hygiene hypothesis [Strachan, 1989]). This argues that the human immune system evolved optimal function in a dirtier world, including the presence of various microbes and helminth parasites, whose removal leads to pathogenic immunological hyperactivity (Rook et al., 2004). Gut dysbiosis certainly does contribute to allergic and autoimmune inflammation prior to aging (Kamada et al., 2013). One corollary of the old friends hypothesis is that restoration of old friend species should attenuate such inflammatory hyperfunction and reduce disease. Indeed, fecal microbiota transfer from healthy donors has been suggested as a potential anti-inflammaging therapy (Franceschi and Campisi, 2014). While the possible role of the microbiome in inflammaging has engaged the imagination of biogerontologists, little consideration has been given to a possible similar role in aging of the macrobiome – in particular, helminth parasites, which include flukes, tapeworms, and nematodes.
Parasitic helminths have infected humans throughout their evolutionary history. As a consequence, helminths have become master manipulators of our immune system in order to dodge host attack. Meanwhile, we have evolved some levels of tolerance of their presence. The wisdom of sometimes putting up with helminths is illustrated by the pathologies that can result from overly aggressive anti-helminth responses, as in elephantiasis that can result from infection with the filarial nematode Wuchereria bancrofti (Figure 1a). Through such coevolution, normal human immune development and function is likely to have become dependent upon the presence of immunomodulatory helminths, as well as that of their microbial counterparts (Velasquez-Manoff, 2012). Indeed, like old friend microbes, reduced helminth infection in ultra-clean modern societies has been linked to increased rates of allergic and autoimmune inflammatory disease, while restorative helminth therapies can protect against these conditions (see next section). But does reduced helminth infection, like gut dysbiosis, also promote inflammaging in later life? And, consequently, could restorative therapy with helminths, given at low doses that do not cause illness, reduce inflammaging, and ameliorate the pathologies that it promotes (Figure 2a)?
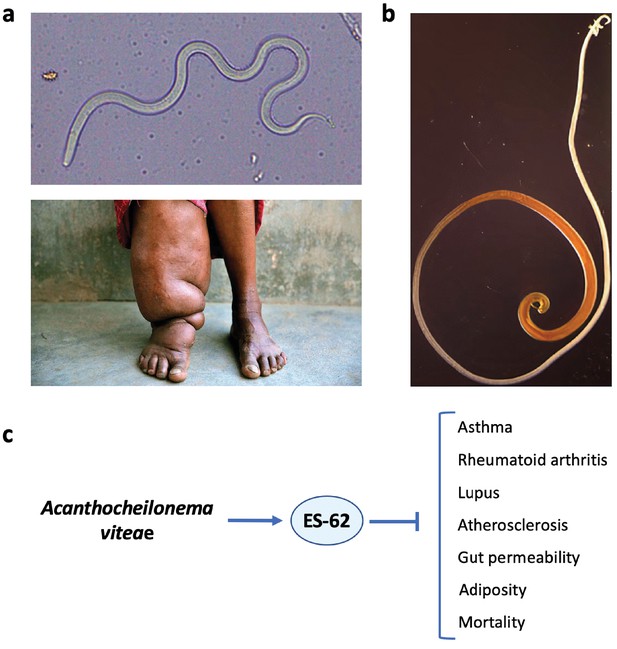
Helminth therapy to prevent Darwinian diseases of inflammatory hyperactivity.
(a) Resistance is futile: the filarial nematode Wuchereria bancrofti (top) that causes elephantiasis in individuals with a hyperactive response to the parasite (bottom). (b) The whipworm Trichuris suis, a candidate for use in helminth therapy. (c) The rodent filarial worm Acanthocheilonema viteae secretes a glycoprotein, ES-62, which is protective in murine models of asthma, rheumatoid arthritis, lupus, and atherosclerosis. In addition, in a mouse model of high-calorie diet-accelerated aging, ES-62 administration prevented age-associated increases in gut permeability and adiposity, and increased longevity (Crowe et al., 2020).
© 2015, Pandey et al. Figure 1a (top) is reproduced from Pandey et al., 2015. Published under a CC BY NC 4.0 license.
© 2019, Cheena Kapoor. Figure 1a (bottom) reproduced with permission from the copyright holder, Cheena Kapoor. This image is not covered by the CC-BY 4.0 licence and further reproduction of this panel would require permission from the copyright holder.
© 2013, Friederike Ebner. Figure 1b was reproduced with permission from the copyright holder, Friederike Ebner, Institute of Immunology (Freie Universität Berlin). This image is not covered by the CC-BY 4.0 licence and further reproduction of this panel would require permission from the copyright holder.
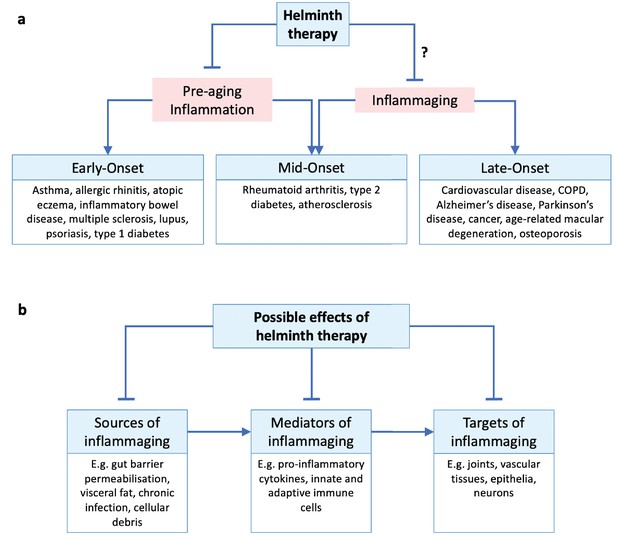
Helminth therapy and inflammaging.
(a) Inflammatory disorders throughout the life course may be classed as early-, mid-, and late-onset disorders. Pre-aging inflammation (e.g. allergic and autoimmune inflammation) contributes to early- and some mid-onset disorders (all of which may persist into later life), while inflammaging appears later in life and contributes to late- and some mid-onset disorders. Helminth therapy has been demonstrated to protect against early- and mid-onset disorders by controlling pre-aging inflammation, but whether it can also protect against mid- and late-onset disorders through anti-inflammaging mechanisms remains uncertain. We note that MS (age of onset 20–50 years) and lupus (15–45 years) are sometimes described as inflammaging related (Sanai et al., 2016; Musella et al., 2018; Tsai et al., 2019). (b) Hypothetical scheme of helminth-mediated attenuation of inflammaging acting at different pathophysiological stages. ‘Sources of inflammaging’ are the mechanisms by which new inflammation is introduced; ‘mediators of inflammaging’ are the components of existing inflammation itself; ‘targets of inflammaging’ are the tissues damaged by the inflammation. Helminth therapy could in principle target the inflammatory source and/or mediator and/or the damaged tissues.
Using a mouse model, Crowe et al., 2020 recently provided the first tantalizing evidence that helminth therapy can protect against aging through anti-inflammatory mechanisms. In the light of their study, we have taken a conceptual research approach (surveying and repurposing published findings) (Blagosklonny and Pardee, 2002) to investigate the plausibility of anti-inflammaging helminth therapy. We will begin by describing the idea of helminth therapy in more detail.
Helminth therapy as a treatment for putative old friend loss disorders
A variety of inflammatory disorders that afflict people prior to aging have been linked to a loss of old friend helminths. These include asthma, atopic eczema, inflammatory bowel disease, multiple sclerosis (MS), rheumatoid arthritis, and type 1 diabetes (Maizels, 2020). For example, a study from Argentina found that accidental acquisition of helminth infections not only caused a reduction in inflammatory cytokines, but also alleviated disease symptoms in MS patients, and that clearance of parasites reversed these effects (Correale and Farez, 2007; Correale and Farez, 2011). In a similar vein, studies from Uganda found that hookworm infections in pregnant women confer protection against infantile atopic eczema, protection that is abrogated by anthelmintic treatment during pregnancy (Mpairwe et al., 2011; Mpairwe et al., 2014).
More directly, a number of studies have documented beneficial effects of deliberate helminth infections, beginning with J.E. Turton's 1976 report in The Lancet, which describes how maintaining an infection with the intestinal hookworm Necator americanus alleviated his allergies (Turton, 1976). Helminth therapy (including treatment with individual parasite antigens) has also been explored extensively in rodents. For example, to date, 20 studies of the autoimmune encephalomyelitis mouse model of MS have reported helminth-induced reductions in disease severity (Charabati et al., 2020).
Unsurprisingly, the use of live helminths to treat old friend disorders remains controversial, and giving oneself worms seems unwholesome, to say the least. More seriously, there are obvious safety worries: live helminth therapy might cause a resurgence of harmful infections in de-wormed countries or induce harmful side effects. However, these concerns may be assuaged by controlling dose size and using helminths with different definitive hosts, such as the pig whipworm Trichuris suis which does not reproduce in humans (Figure 1b). However, although some initial clinical trials of T. suis ova therapy to treat patients with inflammatory bowel disease yielded promising results (Elliott and Weinstock, 2017), follow-up trials have not thus far (Huang et al., 2018). A possibility here is that only true old friends (i.e. anthropophilic helminths) will provide therapeutic benefits; notably, there is evidence that treatment with N. americanus hookworms is beneficial against celiac disease, an autoimmune disease affecting the bowel (Croese et al., 2015).
Another strategy is to identify the mechanisms by which helminths manipulate host immunity and then apply those therapeutically. For example, administration of the glycoprotein ES-62 secreted by the rodent filarial nematode Acanthocheilonema viteae can recapitulate many of the immunomodulatory changes resulting from filarial infections and is protective in several pathological contexts (Figure 1c). Similarly, a variety of helminth-derived proteins are protective in rodent models of MS (Dixit et al., 2017). Such approaches seem likely to yield safer and more precisely targeted therapeutics and are certainly more palatable. However, long-lasting, low-level helminth infections might still represent cheaper and more efficient forms of therapy, providing the full panoply of immunomodulatory agents and in a form that is easy to administer; c.f. ES-62 that was given in weekly injections (Crowe et al., 2020).
Taken together, helminth therapy currently shows promise as a means to treat inflammatory conditions such as allergy and autoimmunity (Figure 2a). But could it also protect against inflammaging? Could maintaining half a dozen hookworms in your bowel help slow aging?
Helminth therapy to counter inflammaging?
Inflammaging can be detected as a sterile, persistent elevation of pro-inflammatory molecules in the blood, including cytokines such as IL-6 and TNF-α and acute phase proteins such as C-reactive protein (CRP). Interestingly, reduced levels of circulating pro-inflammatory cytokines and CRP have been seen in people infected by strongylid and filarial helminths in a number of studies (Aravindhan et al., 2010; George et al., 2014; Rajamanickam et al., 2019; Rajamanickam et al., 2020) (though not all [Aravindhan et al., 2012]), and experimental helminth infection similarly reduces pro-inflammatory cytokines in human serum (Gaze et al., 2012). Furthermore, expulsion of helminths by anthelmintic medication induces elevated pro-inflammatory cytokine responsiveness in human blood (Bourke et al., 2013; Wammes et al., 2016). Thus, it is possible that helminth infections could attenuate the systemic inflammation that is inflammaging. But what about actual benefits in terms of improved health outcomes, such as reduced pathology and increased lifespan?
ES-62 is a 62 kDa glycoprotein found in the excretory secretory products of the filarial nematode parasite A. viteae with anti-inflammatory properties. In the first study of its kind, Crowe et al., 2020 reported that weekly administration of ES-62 improved late-life health and increased lifespan (+12%, median lifespan) in a C57BL/6J mouse model of high-calorie diet-accelerated aging. This suggests that the anti-inflammatory capacities of helminth therapy can exert protective effects even in later life.
Administration of ES-62 prevented the age-related decline in gut barrier integrity seen in high-calorie diet-accelerated aging (Crowe et al., 2020). Of interest in the present context is that permeabilization of the gut lining can contribute to inflammaging, by allowing foreign antigens to leak into the blood and induce systemic immune activation (Thevaranjan et al., 2017; Ferrucci and Fabbri, 2018). But how could helminth therapy maintain gut barrier integrity? One way may be through the prevention of gut dysbiosis, a known contributor to inflammaging. Indeed, ES-62 prevented and, to some extent, even reversed age-related decreases in Bacteroidetes:Firmicutes ratio and increases in Proteobacteria in the gut (Crowe et al., 2020), both of which are associated with inflammaging (Fransen et al., 2017). Another possible mode of action is helminth-induced production of IL-22, an essential cytokine for maintaining gut barrier integrity (Sonnenberg et al., 2011). Interestingly, the intestinal murine roundworm Heligmosomoides polygyrus can even induce fetal-like reversion in intestinal cells to effect tissue repair (Nusse et al., 2018). Thus, it is plausible that the extended lifespan was due to suppression of intestinal changes that contribute to inflammaging.
Administration of ES-62 also prevented adipocyte hypertrophy in gonadal visceral fat (Crowe et al., 2020). This, again, is notable given that adiposity is a major source of inflammaging. Adipose tissue, particularly visceral fat, is highly infiltrated by immune cells, such as monocytes, B and T lymphocytes, mast cells, and neutrophils, and is also a major site of senescent cell accumulation (Tchkonia et al., 2010; Mraz and Haluzik, 2014). Together, these adipocytes, immune cells, and senescent cells secrete a potent and destructive cocktail of pro-inflammatory molecules, including TNF-α, IL-6, IFN-γ, and leptin, which enter systemic circulation and contribute to the inflammaging phenotype. At the same time, anti-inflammatory mechanisms are reduced in adipose tissues, as shown by reduced numbers of eosinophils and of adiponectin secretion. In line with this, ES-62 administration reduced the decline in eosinophil number in visceral fat (Crowe et al., 2020).
Thus, one potential mode of action of helminth therapy is suppression of adiposity. How could it achieve this? Nippostrongylus brasiliensis, a gastrointestinal roundworm of rodents, reduces adiposity apparently by reducing intestinal glucose absorption through altered STAT6 signaling (Moyat et al., 2019). Whether humans show similar responses is unclear. One study of humans reported an inverse correlation between previous schistosome infection and central obesity, though adjustment for socio-economic status was not performed (Shen et al., 2015). More generally, given the relationship between adiposity and inflammaging, helminth therapy might be of particular benefit to people who are overweight or obese.
The findings by Crowe et al., 2020 provide new support for the possibility that helminth therapy could protect against inflammaging. It would be interesting to know how ES-62 affects systemic markers of inflammation during inflammaging, which were not measured in the study, and also to examine effects on specific diseases linked to inflammaging. A number of earlier studies suggest that helminths can protect against such diseases, including rheumatoid arthritis (Ferrucci and Fabbri, 2018; Rea et al., 2018), type 2 diabetes (de Candia et al., 2019; Zuo et al., 2019), atherosclerosis (Zhuang and Lyga, 2014), and cancer (Leonardi et al., 2018; Zuo et al., 2019; Figure 2a). We will consider the evidence for each of these in turn.
A prediction of the ‘anti-inflammaging helminth hypothesis’ is that in areas endemic for helminth infection, there should be lower rates of inflammaging-related disease, and there is some evidence for this. For example, in a region of Eastern India endemic for lymphatic filariasis infection, it was found that not one of 207 rheumatoid arthritis patients tested positive for circulating filarial nematode antigens; by contrast, 40% of 222 healthy controls were antigen positive (Panda et al., 2013). This is consistent with a protective role of helminth infection against rheumatoid arthritis. Indeed, experimental helminth infection or treatment with helminth antigens can protect against pathology in the collagen-induced arthritis (CIA) and MRL/lpr mouse models of rheumatoid arthritis (Smallwood et al., 2017; Langdon et al., 2019). Notably, ES-62-mediated protection against CIA was accompanied by reduced serum levels of the pro-inflammatory cytokine IL-17 (Pineda et al., 2012), consistent with protection against systemic inflammation.
Broadly similar findings have been reported for type 2 diabetes and atherosclerosis. Several (but not all [Mendonça et al., 2006]) epidemiological studies have reported an inverse relationship between the incidence of helminth infection and type 2 diabetes (Aravindhan et al., 2010; Chen et al., 2013; Hays et al., 2015), and also serum levels of the pro-inflammatory cytokines IL-6 and GM-CSF (Aravindhan et al., 2010); these studies controlled for age and body mass index (and/or income). And again, experimental helminth infections protected mice from type 2 diabetes-associated states such as hyperglycemia and insulin resistance (Wu et al., 2011; Morimoto et al., 2016; Pace et al., 2018), while anthelmintic treatment elevated blood glucagon and insulin resistance, and several circulating pro-inflammatory cytokines (Tahapary et al., 2017; Rajamanickam et al., 2019; Rajamanickam et al., 2020). Regarding atherosclerosis, the burden of Opisthorchis felineus (cat liver fluke) in cadavers was found to be negatively correlated with the severity of aortic atherosclerosis (Magen et al., 2013). In atherosclerotic mouse models, infection with the blood fluke Schistosoma mansoni reduced atherosclerotic lesions by 50% (Doenhoff et al., 2002); similarly, administration of Schistosoma egg antigens reduced atherosclerotic plaque size (Zhang et al., 2012; Wolfs et al., 2014), and ES-62 reduced atherosclerotic lesion area by 60% in the gld.apoE−/− mouse model of lupus-accelerated atherosclerosis (Aprahamian et al., 2015).
There is also some very limited evidence that helminth therapy might promote resistance to some forms of cancer. The tapeworm Taenia crassiceps and its antigens have been found to attenuate and prevent colon tumorigenesis in mice through anti-inflammatory mechanisms (León-Cabrera et al., 2014; Callejas et al., 2019). However, in the same mouse model, the nematode H. polygyrus was found to promote tumorigenesis (Pastille et al., 2017). Moreover, certain parasitic helminths are well known to increase cancer rates, for example the trematode parasite Schistosoma haematobium causes bladder cancer (Vennervald and Polman, 2009).
There is also some evidence linking helminth loss to other inflammaging-linked conditions. This includes COPD, given that some helminths are known to suppress lung inflammation and to reduce levels of the pro-inflammatory cytokine IL-33, a driver of COPD pathology (Osbourn et al., 2017). It should be noted however that other helminths such as N. brasiliensis can promote COPD pathology (Marsland et al., 2008). Meanwhile, IL-33 also promotes age-related macular degeneration (Xi et al., 2016), another late-life inflammaging-linked disease. Anecdotal evidence from home-users of live helminth therapy suggests that old friend helminths may also provide protection against Parkinson’s disease and depression, although whether the latter includes geriatric depression is not known (Cheng et al., 2015). Both Parkinson’s disease and geriatric depression are considered to be inflammaging-linked conditions (Teixeira et al., 2012; Calabrese et al., 2018).
Perspectives
There is clear evidence that an absence of helminth infection leads to increased incidence of inflammatory disorders, including some inflammaging-linked conditions, such as rheumatoid arthritis. Furthermore, experimental and clinical work has demonstrated the protective benefits of restorative helminth therapy against some inflammaging-linked diseases. Whether this reflects the ability of helminth therapy to reduce inflammaging remains uncertain, but this possibility clearly warrants further investigation.
Given the role of inflammaging in numerous diseases of aging, further studies examining the effect of helminth therapy on multimorbidity would be especially useful. For example, it would be helpful to extend ongoing helminth therapy trials aimed at individual diseases to look at a broader range of conditions. The anti-inflammaging potential of helminth therapy could also be assessed by monitoring pro-inflammatory cytokine levels following administration of helminth therapy in patients with detectable inflammaging. Where live helminth therapy is used, infections can be easily terminated by anthelmintics should the need arise; conveniently, this would also provide additional data on the therapy’s efficacy (i.e. does inflammaging then worsen?). Regarding ES-62 in particular, it would be worthwhile to test its effects on aging in mice maintained on a normal diet rather than on a life-shortening high-calorie diet.
The possibility of anti-aging helminth therapy raises various questions. How do responses to such therapy change with age? Would higher or lower parasite (or antigen) levels be needed to take account of immunosenescence? To what extent do risks of helminth therapy increase in old age? What are optimal ages to apply such therapy to reduce inflammaging? Would helminth therapy act only in a preventative fashion (typical of anti-aging treatments), delaying the genesis or worsening of inflammaging-linked pathologies, or could it reverse existing disease symptoms? At least for murine rheumatoid arthritis, there is some evidence that helminth therapy could be both prophylactic and therapeutic (McInnes et al., 2003; Rzepecka et al., 2015). Thus, it is possible that helminth therapy could, to some extent, reverse symptoms of aging as well as decelerate them. Would responses to helminth therapy differ between the sexes? Here it is notable that ES-62 extended lifespan in male, but not female obese mice (Crowe et al., 2020). A further possibility is that helminths might inhibit tissue aging in their hosts in order to protect their local niche; notably, the pro-aging mTOR pathway was found to be inhibited in human dendritic cells by Brugia malayi microfilariae (Narasimhan et al., 2016).
Effective application of helminth therapy would also require a clear understanding of its specific anti-inflammaging mechanisms. Theoretically, helminths could counter inflammaging in several ways. For example, they could inhibit sources of inflammaging by preventing gut barrier permeabilization and obesity, neutralize existing inflammaging by increasing the proportion of anti-inflammatory to pro-inflammatory cytokines, or repair inflammaging-afflicted tissue damage, for example through promotion of IL-22 secretion (Figure 2b).
Conclusions
It goes without saying that improvements in hygiene and elimination of helminth parasites have been of incalculable benefit to humanity. But a cost coupled with this benefit is abnormalities of immune function, specifically inflammatory hyperfunction. Available evidence suggests that restorative helminth therapies are effective against not only allergic and autoimmune inflammatory disorders, but also age-associated inflammation in later life, at least to some extent. Should this be confirmed, helminth therapy could provide protection against the wide spectrum of age-related diseases promoted by inflammaging. In the wake of successes during the last century in eliminating the evils of helminth infections, the time now seems propitious to explore further their possible benefits, particularly for our aging population – strange though this may sound.
References
-
The immunomodulatory parasitic worm product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse modelInternational Journal for Parasitology 45:203–207.https://doi.org/10.1016/j.ijpara.2014.12.006
-
Effect of filarial infection on serum inflammatory and atherogenic biomarkers in coronary artery disease (CURES-121)The American Journal of Tropical Medicine and Hygiene 86:828–833.https://doi.org/10.4269/ajtmh.2012.11-0773
-
BookThe geroscience hypothesis: Is it possible to change the rate of aging?In: Sierra F, Kohanski R, editors. Advances in Geroscience. Cham: Springer. pp. 1–36.https://doi.org/10.1007/978-3-319-23246-1_1
-
Aging and Parkinson's Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesisFree Radical Biology and Medicine 115:80–91.https://doi.org/10.1016/j.freeradbiomed.2017.10.379
-
Helminth-derived molecules inhibit colitis-associated Colon cancer development through NF-κB and STAT3 regulationInternational Journal of Cancer 145:3126–3139.https://doi.org/10.1002/ijc.32626
-
A critical analysis of helminth immunotherapy in multiple sclerosisMultiple Sclerosis Journal 26:1448–1458.https://doi.org/10.1177/1352458519899040
-
Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural chinaThe Journal of Clinical Endocrinology & Metabolism 98:E283–E287.https://doi.org/10.1210/jc.2012-2517
-
Overcoming evolutionary mismatch by Self-Treatment with helminths: current practices and experienceJournal of Evolutionary Medicine 3:1–22.https://doi.org/10.4303/jem/235910
-
Association between parasite infection and immune responses in multiple sclerosisAnnals of Neurology 61:97–108.https://doi.org/10.1002/ana.21067
-
The impact of parasite infections on the course of multiple sclerosisJournal of Neuroimmunology 233:6–11.https://doi.org/10.1016/j.jneuroim.2011.01.002
-
Experimental hookworm infection and gluten microchallenge promote tolerance in celiac diseaseJournal of Allergy and Clinical Immunology 135:508–516.https://doi.org/10.1016/j.jaci.2014.07.022
-
Type 2 diabetes: how much of an autoimmune disease?Frontiers in Endocrinology 10:451.https://doi.org/10.3389/fendo.2019.00451
-
Novel therapeutics for multiple sclerosis designed by parasitic wormsInternational Journal of Molecular Sciences 18:2141.https://doi.org/10.3390/ijms18102141
-
Nematodes and human therapeutic trials for inflammatory diseaseParasite Immunology 39:e12407.https://doi.org/10.1111/pim.12407
-
Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailtyNature Reviews Cardiology 15:505–522.https://doi.org/10.1038/s41569-018-0064-2
-
Inflamm-aging. an evolutionary perspective on immunosenescenceAnnals of the New York Academy of Sciences 908:244–254.https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
-
Chronic inflammation (Inflammaging) and its potential contribution to Age-Associated diseasesThe Journals of Gerontology Series A: Biological Sciences and Medical Sciences 69:S4–S9.https://doi.org/10.1093/gerona/glu057
-
Coincident helminth infection modulates systemic inflammation and immune activation in active pulmonary tuberculosisPLOS Neglected Tropical Diseases 8:e3289.https://doi.org/10.1371/journal.pntd.0003289
-
Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? evidence from australian aboriginal adultsDiabetes Research and Clinical Practice 107:355–361.https://doi.org/10.1016/j.diabres.2015.01.012
-
Role of the gut Microbiota in immunity and inflammatory diseaseNature Reviews Immunology 13:321–335.https://doi.org/10.1038/nri3430
-
Helminth-based therapies for rheumatoid arthritis: a systematic review and meta-analysisInternational Immunopharmacology 66:366–372.https://doi.org/10.1016/j.intimp.2018.11.034
-
Extraintestinal helminth infection reduces the development of colitis-associated tumorigenesisInternational Journal of Biological Sciences 10:948–956.https://doi.org/10.7150/ijbs.9033
-
Ageing: from inflammation to CancerImmunity & Ageing 15:.https://doi.org/10.1186/s12979-017-0112-5
-
Chronic Opisthorchis felineus infection attenuates atherosclerosis--an autopsy studyInternational Journal for Parasitology 43:819–824.https://doi.org/10.1016/j.ijpara.2013.04.008
-
Regulation of type 2 diabetes by helminth-induced Th2 immune responseJournal of Veterinary Medical Science 78:1855–1864.https://doi.org/10.1292/jvms.16-0183
-
The interplay of type 2 immunity, helminth infection and the Microbiota in regulating metabolismClinical & Translational Immunology 8:e01089.https://doi.org/10.1002/cti2.1089
-
Maternal hookworm modifies risk factors for childhood eczema: results from a birth cohort in UgandaPediatric Allergy and Immunology 25:481–488.https://doi.org/10.1111/pai.12251
-
The role of adipose tissue immune cells in obesity and low-grade inflammationJournal of Endocrinology 222:R113–R127.https://doi.org/10.1530/JOE-14-0283
-
Interplay between age and neuroinflammation in multiple sclerosis: effects on motor and cognitive functionsFrontiers in Aging Neuroscience 10:238.https://doi.org/10.3389/fnagi.2018.00238
-
Microfilariae of Brugia malayi inhibit the mTOR pathway and induce autophagy in human dendritic cellsInfection and Immunity 84:2463–2472.https://doi.org/10.1128/IAI.00174-16
-
Metabolic consequences of concomitant Strongyloides stercoralis infection in patients with type 2 diabetes mellitusClinical Infectious Diseases 69:697–704.https://doi.org/10.1093/cid/ciy935
-
Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesisPLOS Neglected Tropical Diseases 14:e0008101.https://doi.org/10.1371/journal.pntd.0008101
-
Age and Age-Related diseases: role of inflammation triggers and cytokinesFrontiers in Immunology 9:586.https://doi.org/10.3389/fimmu.2018.00586
-
Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disordersSpringer Seminars in Immunopathology 25:237–255.https://doi.org/10.1007/s00281-003-0148-9
-
Aging and multiple sclerosisMultiple Sclerosis Journal 22:717–725.https://doi.org/10.1177/1352458516634871
-
Helminth immunomodulation in autoimmune diseaseFrontiers in Immunology 8:453.https://doi.org/10.3389/fimmu.2017.00453
-
Effect of anthelmintic treatment on insulin resistance: a Cluster-Randomized, Placebo-Controlled trial in IndonesiaClinical Infectious Diseases 65:764–771.https://doi.org/10.1093/cid/cix416
-
Biomarkers in mood disorders among the elderly: can they contribute to diagnosis and prognosis?Current Translational Geriatrics and Experimental Gerontology Reports 1:111–120.https://doi.org/10.1007/s13670-012-0010-9
-
Molecular and cellular bases of immunosenescence, inflammation, and cardiovascular complications mimicking “Inflammaging” in Patients with Systemic Lupus ErythematosusInternational Journal of Molecular Sciences 20:3878.https://doi.org/10.3390/ijms20163878
-
BookAn Epidemic of Absence: A New Way of Understanding Allergies and Autoimmune DiseasesScribner.
-
Helminths and malignancyParasite Immunology 31:686–696.https://doi.org/10.1111/j.1365-3024.2009.01163.x
-
IL-33 amplifies an innate immune response in the degenerating retinaJournal of Experimental Medicine 213:189–207.https://doi.org/10.1084/jem.20150894
-
An update on Inflamm-Aging: mechanisms, prevention, and treatmentJournal of Immunology Research 2016:1–12.https://doi.org/10.1155/2016/8426874
-
Anti-atherogenic effect and its mechanisms of soluble egg antigen of schistosomia japonicum in ApoE-/- miceZhongguo Xue Xi Chong Bing Fang Zhi Za Zhi = Chinese Journal of Schistosomiasis Control 24:654–658.
-
Inflammaging in skin and other tissues - the roles of complement system and macrophageInflammation & Allergy Drug Targets 13:153–161.https://doi.org/10.2174/1871528113666140522112003
-
Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatmentsInternational Journal of Molecular Sciences 20:4472.https://doi.org/10.3390/ijms20184472
Article and author information
Author details
Funding
Wellcome Trust (098565/Z/12/Z)
- David Gems
Wellcome Trust (215574/Z/19/Z)
- David Gems
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We would like to thank J Crowe, JM Leeb, FE Lumb, and M Blaxter for useful discussion. Figure 1a image (top, Wuchereria bancrofti) was reproduced from Pandey et al., 2015. We thank Cheena Kapoor for permission to reproduce the Figure 1a image of elephantiasis, and Friederike Ebner (Institute for Immunology, Freie Universität Berlin) for the Figure 1b image of Trichuris suis. This work was supported by a Wellcome Trust Strategic Award (098565/Z/12/Z) and a Wellcome Trust Investigator Award (215574/Z/19/Z) to D Gems.
Copyright
© 2021, Zhang and Gems
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 12,420
- views
-
- 703
- downloads
-
- 12
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
Molecules released by parasites may have helped to reduce inflammation in the past.
-
- Cell Biology
- Immunology and Inflammation
The endothelial blood-brain barrier (BBB) strictly controls immune cell trafficking into the central nervous system (CNS). In neuroinflammatory diseases such as multiple sclerosis, this tight control is, however, disturbed, leading to immune cell infiltration into the CNS. The development of in vitro models of the BBB combined with microfluidic devices has advanced our understanding of the cellular and molecular mechanisms mediating the multistep T-cell extravasation across the BBB. A major bottleneck of these in vitro studies is the absence of a robust and automated pipeline suitable for analyzing and quantifying the sequential interaction steps of different immune cell subsets with the BBB under physiological flow in vitro. Here, we present the under-flow migration tracker (UFMTrack) framework for studying immune cell interactions with endothelial monolayers under physiological flow. We then showcase a pipeline built based on it to study the entire multistep extravasation cascade of immune cells across brain microvascular endothelial cells under physiological flow in vitro. UFMTrack achieves 90% track reconstruction efficiency and allows for scaling due to the reduction of the analysis cost and by eliminating experimenter bias. This allowed for an in-depth analysis of all behavioral regimes involved in the multistep immune cell extravasation cascade. The study summarizes how UFMTrack can be employed to delineate the interactions of CD4+ and CD8+ T cells with the BBB under physiological flow. We also demonstrate its applicability to the other BBB models, showcasing broader applicability of the developed framework to a range of immune cell-endothelial monolayer interaction studies. The UFMTrack framework along with the generated datasets is publicly available in the corresponding repositories.






