T cell stiffness is enhanced upon formation of immunological synapse
Figures
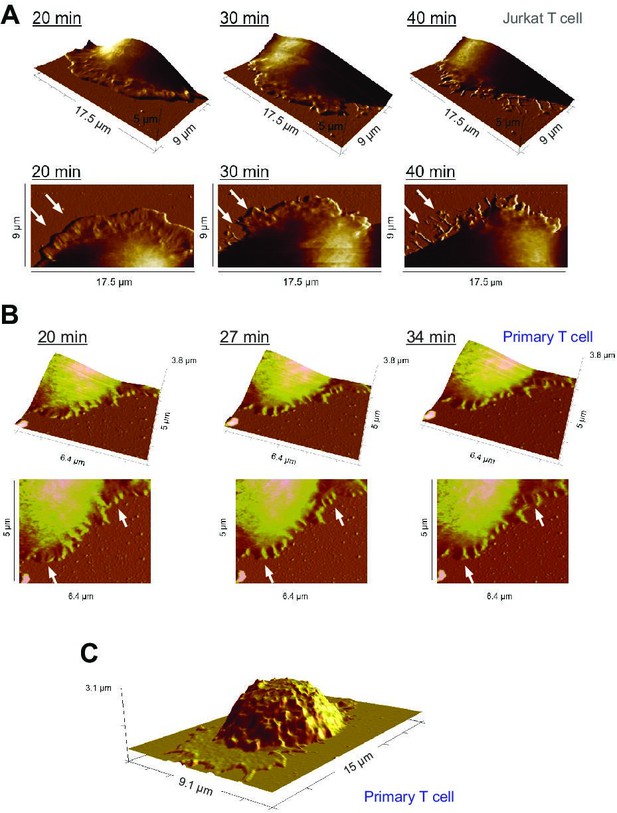
Representative time points of lamellipodial dynamics at the IS.
(A, B) Dynamic changes of the lamellipodium of a Jurkat T cell (A) or a human primary CD4+ T cell (B) during IS formation on a αLFA-1+αCD3+αCD28 antibody-coated coverslip. The height profile, examined by Peak Force QNM, is displayed (upper panel: 3D view, lower panel: top view). Exemplary dynamic parts are highlighted by arrowheads. (C) Height profile of a whole primary human CD4+ T cell during IS formation on a αLFA-1+αCD3+αCD28 antibody-coated coverslip. One representative cell from at least three independent experiments is shown.
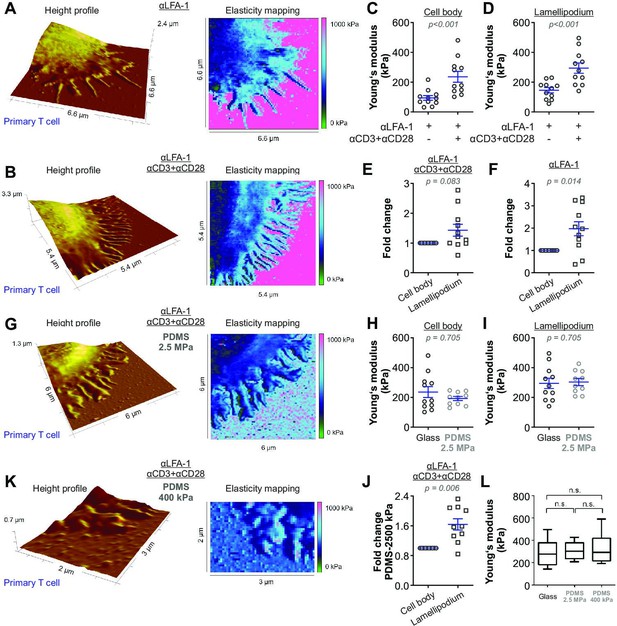
The stiffness of T cells increases upon activation.
Height profiles and corresponding elasticity maps, Young’s moduli, and the respective fold changes of human primary CD4+ T cells. Either glass coverslips (A–F) or PDMS (G–K) were applied as the functionalized surfaces. (H–J, L) Young’s modulus of primary T cells on full antibody (αLFA-1+αCD3+αCD28)-functionalized glass (n = 11, the same dataset as in (D) full antibody), PDMS (2.5 MPa, n = 10), or PDMS (400 kPa, n = 5) substrates. The Mann-Whitney test (C), (D), (H), and (I), the Wilcoxon matched-pairs signed rank test (E), (F), and (J) or Mann-Whitney-U-test (L) was used for analyzing statistical significance. The results were presented as mean ± SEM, from 7 to 12 cells per condition as shown in the plots (LFA-1 vs full anybody-set) from six independent experiments for (A–F) (six donors), from 10 cells (four independent experiments/four donors) for PDMS condition (2.5 MPa) in (G–J), or from 5 cells (two independent experiments/two donors) for PDMS condition (400 kPa). Source data please refer to Figure 2—source data 1. For height profiles and elasticity maps of each value and condition, refer to Figure 2—figure supplements 3–6. For representative Force-Distance Curves, refer to Figure 2—figure supplement 7.
-
Figure 2—source data 1
Original values of stiffness shown in Figure 2.
- https://cdn.elifesciences.org/articles/66643/elife-66643-fig2-data1-v2.xlsx
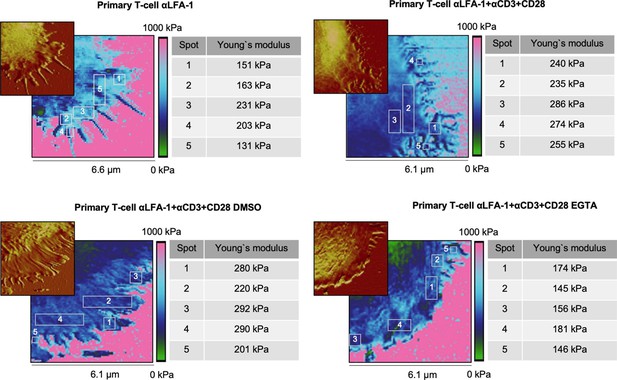
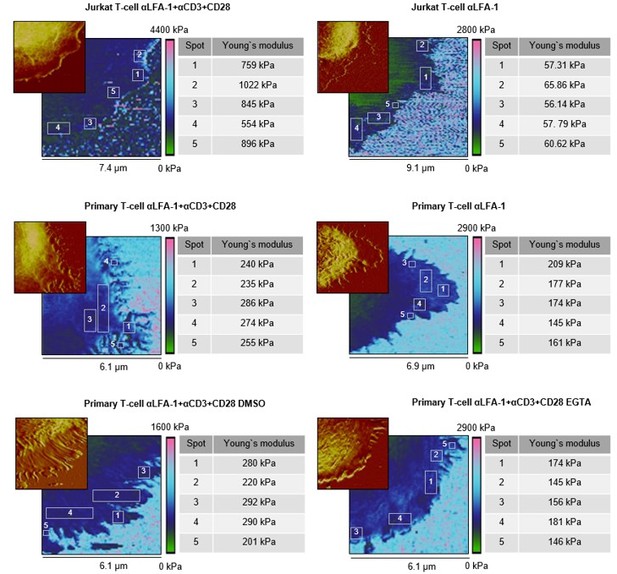
Local stiffness at lamellipodia is not influenced by positioning or topology.
Representative cells were taken from Figure 2. The Young’s moduli of five individual squares on the lamellipodium (at tips/edges, close to the cell body, and in between) of each cell was investigated. The observed areas are marked and numbered on the elasticity map. The corresponding height profiles are shown in the top left corner. Each individual Young’s modulus is given in the table on the right.
-
Figure 2—figure supplement 1—source data 1
Stiffness of T cells measured on funcationalized surfaces.
- https://cdn.elifesciences.org/articles/66643/elife-66643-fig2-figsupp1-data1-v2.xlsx
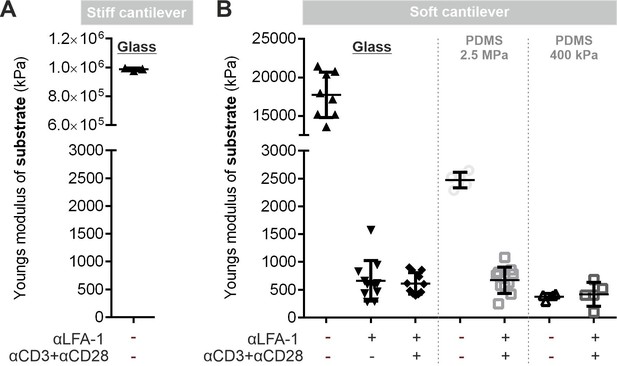
Stiffness of substrates.
(A) Stiffness of uncoated glass coverslips measured by Peak Force Tapping mode in air, utilizing ScanAsyst Air cantilevers (Bruker) with a spring constant of 0.8 N/m. (B) Stiffness of uncoated/functionalized substrates measured by the Peak Force Tapping mode in fluid, utilizing MLCT cantilever type B cantilever (Bruker) with a spring constant of 0.06–0.1 N/m. Further details please see Materials and methods. Source data please refer to Figure 2—figure supplement 2—source data 1.
-
Figure 2—figure supplement 2—source data 1
Source data of stiffness of uncoated and coated substrates.
- https://cdn.elifesciences.org/articles/66643/elife-66643-fig2-figsupp2-data1-v2.xlsx
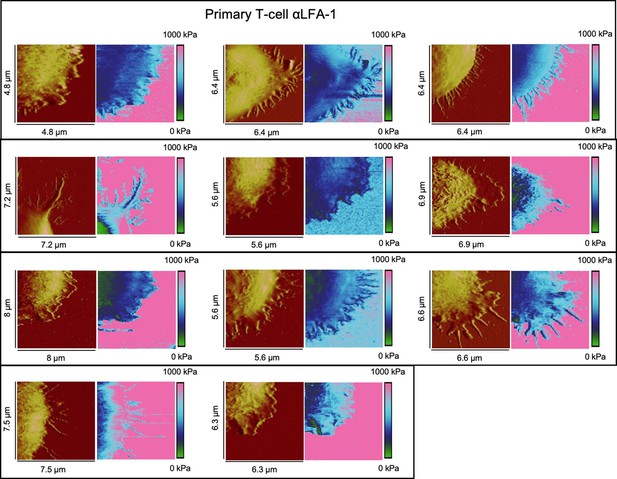
Height profiles and elasticity maps (Young's modulus) of primary T-cells on αLAF-1-functionalized glass.
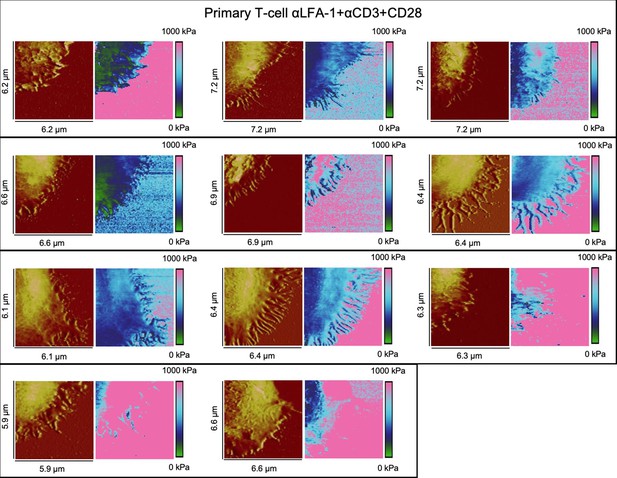
Height profiles and elasticity maps (Young`s modulus) of primary T-cells on full antibody (αLFA-1+αCD3+αCD28)-functionalized glass.
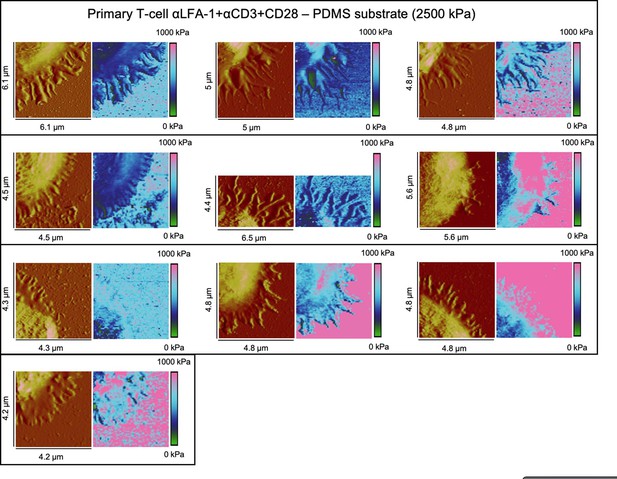
Height profiles and elasticity maps (Young`s modulus) of primary T-cells on full antibody (αLFA-1+αCD3+αCD28)-functionalized PDMS substrate with 2.5 MPa.
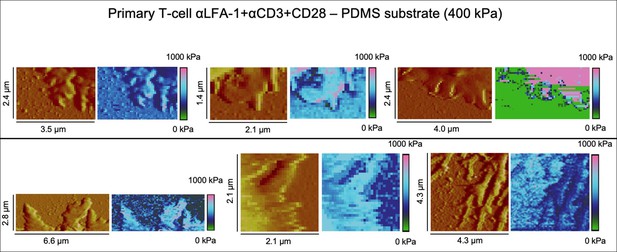
Height profiles and elasticity maps (Young`s modulus) of primary T-cells on full antibody (αLFA-1+αCD3+αCD28)-functionalized PDMS substrate with 400 kPa.
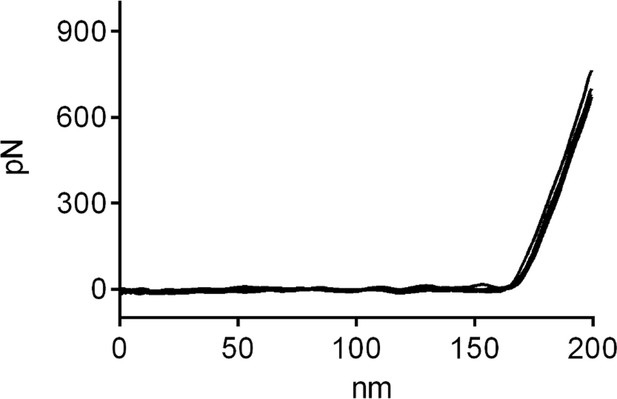
Exemplary Force-Distance Curves during Elasticity mapping of primary T cells on full antibody (αLFA-1+αCD3+αCD28)-functionalized glass.
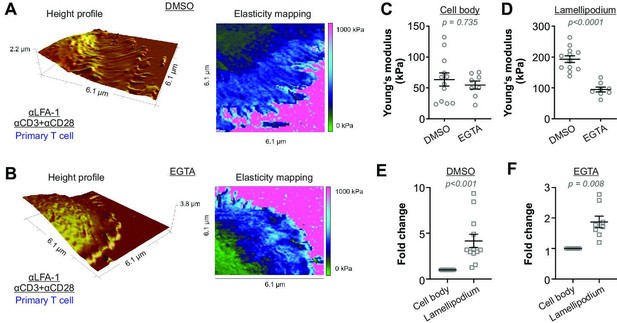
Activation-induced T cell stiffening is regulated by intracellular calcium.
Primary human CD4+ T cells were treated with either EGTA-AM or DMSO at room temperature for 30 min. Height profiles and corresponding elasticity maps (A, B), Young’s moduli (C, D), and the respective fold changes (E, F) are shown. The Mann-Whitney test (C, D) or the Wilcoxon matched-pairs signed rank test (E, F) was used for statistical significance. Results were presented as mean ± SEM, from 11 cells for each condition (LFA-1 vs full anybody-set) from four independent experiments (four donors). Source data please refer to Figure 3—source data 1. For height profiles and elasticity maps of each value and condition, refer to Figure 3—figure supplements 1 and 2.
-
Figure 3—source data 1
Original values of stiffness shown in Figure 3.
- https://cdn.elifesciences.org/articles/66643/elife-66643-fig3-data1-v2.xlsx
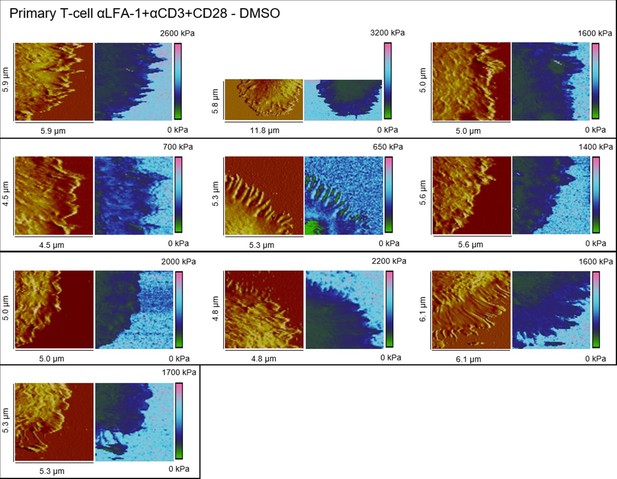
Height profiles and elasticity maps (Young`s modulus) of DMSO-treated primary T cells on full antibody (αLFA-1+αCD3+CD28)-functionalized glass.
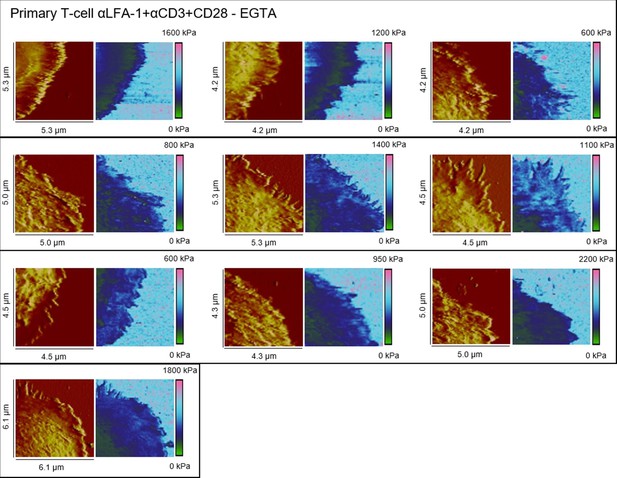
Height profiles and elasticity maps (Young`s modulus) of EGTA-treated primary T-cells on full antibody (αLFA-1+αCD3+CD28)-functionalized glass.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | Jurkat E6.1 cell line | ATCC | ATCC Cat# TIB-152, RRID:CVCL_0367 | |
| Biological sample (Homo sapiens) | Primary human CD4+ T cells | Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors provided by Institute of Clinical Hemostaseology and Transfusion Medicine. Faculty of Medicine. University of Saarland.PMID:24599783 | Negatively isolated from PBMCs using CD4+ T Cell Isolation Kit human (Miltenyl). | |
| Commercial assay or kit | CD4+ T Cell Isolation Kit human | Miltenyi | Cat# 130-096-533 | |
| Commercial assay or kit | Sylgard 184 Silicone Elastomer Kit | Dow Europe GmbH | Material Number 1317318 | |
| Peptide, recombinant protein | Polyornithine | Sigma-Aldrich(Merck) | MDL number MFCD00286305 | |
| Chemical compound, drug | EGTA/AM | Calbiochem (Merck) | Cat# 324,628 | |
| Antibody | anti-LFA-1 (ITGAL) antibody(Mouse monoclonal) | Antibodies-online | Cat# ABIN135680, RRID:AB_10773722 | Diluted to 9 µg/ml in 20 μl PBS |
| Antibody | mouse anti-human CD28 antibody(Mouse monoclonal) | BD Pharmingen | Cat# 555725, RRID:AB_396068 | Diluted to 90 µg/ml in 20 μl PBS |
| Antibody | mouse anti-human CD3 antibody(Mouse monoclonal) | Diaclone | Cat# 854.010.000, RRID:AB_1155287 | Diluted to 30 µg/ml in 20 μl PBS |
| Software, algorithm | GraphPad Prism | GraphPad | RRID:SCR_002798 | |
| Software, algorithm | Research NanoScope 9.1 | Bruker Corp. | R3.119071 | |
| Software, algorithm | NanoScope Analysis 1.80 | Bruker Corp. | R2.132257 |