Tumor Biology: Cells under pressure
Packed like sardines, most cells in our body must operate in confined spaces. Neighboring cells, the extracellular fluid that surrounds cells, and the extracellular matrix that provides physical support to tissues, can all restrict the growth and motion of cells. This is exacerbated in tumors. As the cancer cells in the tumor multiply, they gradually push back the surrounding tissue, and in turn, experience varying degrees of compression or 'solid stress' (Nia et al., 2017). Increasing evidence suggests that the physical interactions between cancer cells and their microenvironment affect the signals that regulate their growth and spread.
Much of our knowledge about solid stress and its impact on tumor development stems from research on three-dimensional aggregates of cancer cells grown in the laboratory. When these aggregates – which are also known as multicellular tumor spheroids – multiply in a confined environment, such as a hydrogel, their growth is restricted (Helmlinger et al., 1997). These observations are somewhat intuitive, since cell division is an inherently physical process during which cells increase in volume and undergo striking morphological changes – both of which require space (Nam and Chaudhuri, 2018; Zlotek-Zlotkiewicz et al., 2015; Son et al., 2012).
It has been shown that restricting or reducing the volume of a cell through increased osmotic pressure – which draws water from the cell – stops them from multiplying (Nam et al., 2019; Delarue et al., 2014). Likewise, compression along one axis can suppress the growth of cells and even induce programmed cell death in tumor spheroids (Cheng et al., 2009). When applied transiently to single cells, it may also reverse the malignant phenotype, with the cells displaying behaviors of normal cells (Ricca et al., 2018).
However, full three-dimensional control of uniform compression of tumor spheroids has not been achieved to date. Now, in eLife, Giovanni Cappello, Pierre Recho and colleagues from the Université Grenoble Alpes, the Université de Lyon and the Collège de France – including Monika Dolega as first author – report a new way to study the impact of solid stress on tumor spheroids (Dolega et al., 2021).
Dolega et al. applied two strategies to compare the impact of solid stress on both single cells and tumor spheroids. To do so, they exerted osmotic pressure using osmolytes of varying sizes: small dextran molecules measuring less than 5 nm in diameter (following the standard approach), and large ones with a diameter of 15 nm or more (representing the new approach). The small dextran molecules were able to infiltrate the extracellular matrix of the spheroids, thereby applying osmotic pressure on the single cells within. However, the 15 nm dextran molecules were too big to enter the extracellular matrix: instead, the osmotic pressure acted on the entire spheroid, resulting in a global compression of the cells by the extracellular matrix (Figure 1).
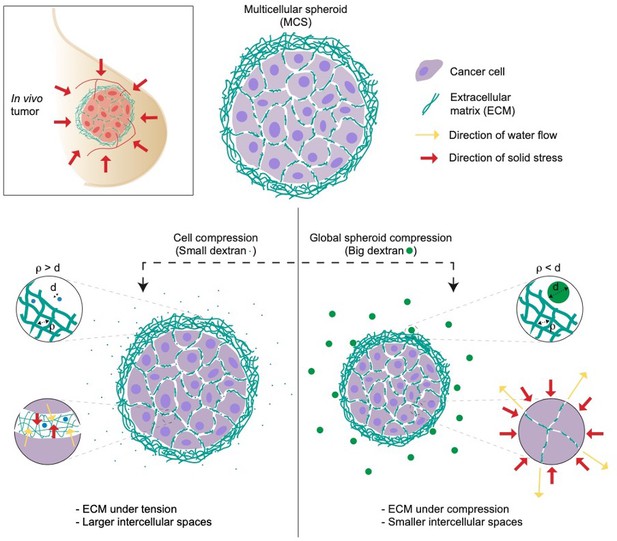
Studying the impact of solid stress on multicellular tumor spheroids.
Multicellular spheroids (MCS) can be used as a model of in vivo tumors due to their ability to mimic several features of solid tumors (top left). To test the effect of solid stress on tumor growth, Dolega et al. used dextran molecules of varying sizes to induce osmotic water flow (yellow arrows). They found that small dextran molecules (blue circles, left) can enter the pores of extracellular matrix (ECM, green strings), thereby applying osmotic pressure (red arrows) on individual cells. Large dextran molecules (green circles, right) cannot enter the extracellular matrix, so they exert osmotic pressure on the whole spheroid (red arrows), mimicking the solid stress in in vivo tumors. d = diameter of dextran molecules; ρ is the spacing between the fibers in the extracellular matrix.
By keeping the magnitude of pressure the same, Dolega et al. discovered that global compression decreased the volume of the tumor spheroids significantly more than the osmotic compression of single cells, with cells within the spheroid growing and migrating more slowly. Comparable results were also observed in single cells encapsulated in an extracellular matrix-like hydrogel. There, global compression reduced the growth and migration of the cells while osmotic compression did not. These findings suggest that solid stress in the form of global compression does indeed regulate the growth and spread of a tumor.
Dolega et al. have developed a robust approach to probe the impact of solid stress on cells in three-dimensional microenvironments, and the different outcomes observed for osmotic compression of single cells and global compression of tumor spheroids draws attention to the possibility that the extracellular matrix can act as a pressure sensor that might regulate cell behavior. It remains unclear if cells sense solid stress through the same pathways that are implicated in osmotic compression, including stretch-activated ion channels, or through alternate pathways, perhaps involving the cytoskeleton and cell-matrix adhesions (Nam et al., 2019). Probing these mechanisms will help advance our understanding of how solid stress regulates cell behavior in a wide range of contexts, from embryonic development to cancer.
References
-
Compressive stress inhibits proliferation in tumor spheroids through a volume limitationBiophysical Journal 107:1821–1828.https://doi.org/10.1016/j.bpj.2014.08.031
-
Solid stress inhibits the growth of multicellular tumor spheroidsNature Biotechnology 15:778–783.https://doi.org/10.1038/nbt0897-778
-
Solid stress and elastic energy as measures of tumour mechanopathologyNature Biomedical Engineering 1:0004.https://doi.org/10.1038/s41551-016-0004
-
Optical volume and mass measurements show that mammalian cells swell during mitosisJournal of Cell Biology 211:765–774.https://doi.org/10.1083/jcb.201505056
Article and author information
Author details
Publication history
Copyright
© 2021, Indana and Chaudhuri
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,897
- views
-
- 283
- downloads
-
- 5
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Physics of Living Systems
In social behavior research, the focus often remains on animal dyads, limiting the understanding of complex interactions. Recent trends favor naturalistic setups, offering unique insights into intricate social behaviors. Social behavior stems from chance, individual preferences, and group dynamics, necessitating high-resolution quantitative measurements and statistical modeling. This study leverages the Eco-HAB system, an automated experimental setup that employs radiofrequency identification tracking to observe naturally formed mouse cohorts in a controlled yet naturalistic setting, and uses statistical inference models to decipher rules governing the collective dynamics of groups of 10–15 individuals. Applying maximum entropy models on the coarse-grained co-localization patterns of mice unveils social rules in mouse hordes, quantifying sociability through pairwise interactions within groups, the impact of individual versus social preferences, and the effects of considering interaction structures among three animals instead of two. Reproducing co-localization patterns of individual mice reveals stability over time, with the statistics of the inferred interaction strength capturing social structure. By separating interactions from individual preferences, the study demonstrates that altering neuronal plasticity in the prelimbic cortex – the brain structure crucial for sociability – does not eliminate signatures of social interactions, but makes the transmission of social information between mice more challenging. The study demonstrates how the joint probability distribution of the mice positions can be used to quantify sociability.
-
- Physics of Living Systems
Chromosome structure is complex, and many aspects of chromosome organization are still not understood. Measuring the stiffness of chromosomes offers valuable insight into their structural properties. In this study, we analyzed the stiffness of chromosomes from metaphase I (MI) and metaphase II (MII) oocytes. Our results revealed a tenfold increase in stiffness (Young’s modulus) of MI chromosomes compared to somatic chromosomes. Furthermore, the stiffness of MII chromosomes was found to be lower than that of MI chromosomes. We examined the role of meiosis-specific cohesin complexes in regulating chromosome stiffness. Surprisingly, the stiffness of chromosomes from three meiosis-specific cohesin mutants did not significantly differ from that of wild-type chromosomes, indicating that these cohesins may not be primary determinants of chromosome stiffness. Additionally, our findings revealed an age-related increase of chromosome stiffness for MI oocytes. Since aging is associated with elevated levels of DNA damage, we investigated the impact of etoposide-induced DNA damage on chromosome stiffness and found that it led to a reduction in stiffness in MI oocytes. Overall, our study underscores the dynamic and cyclical nature of chromosome stiffness, modulated by both the cell cycle and age-related factors.






