DNA-RNA hybrids at DSBs interfere with repair by homologous recombination
Figures
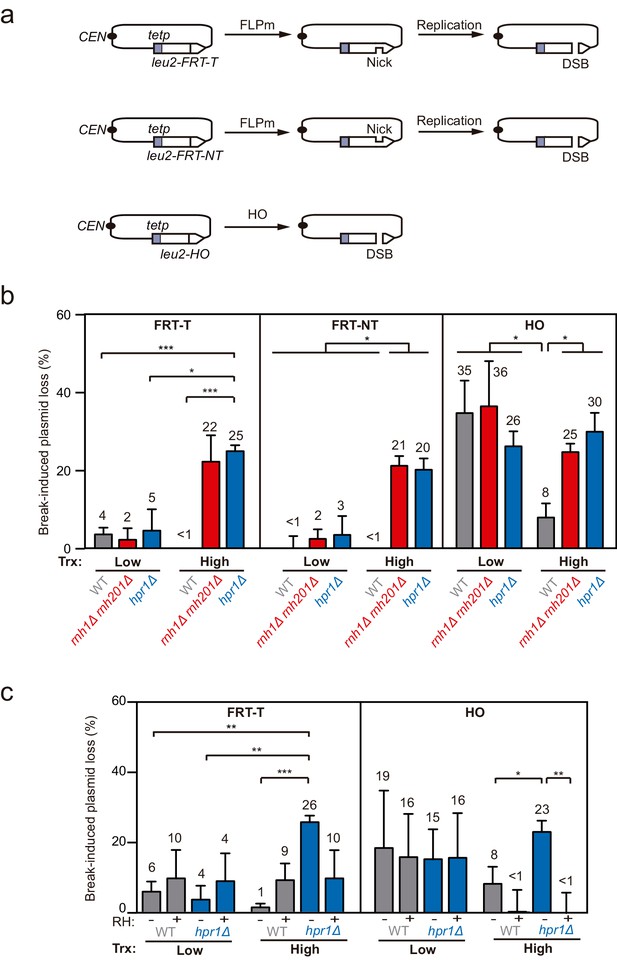
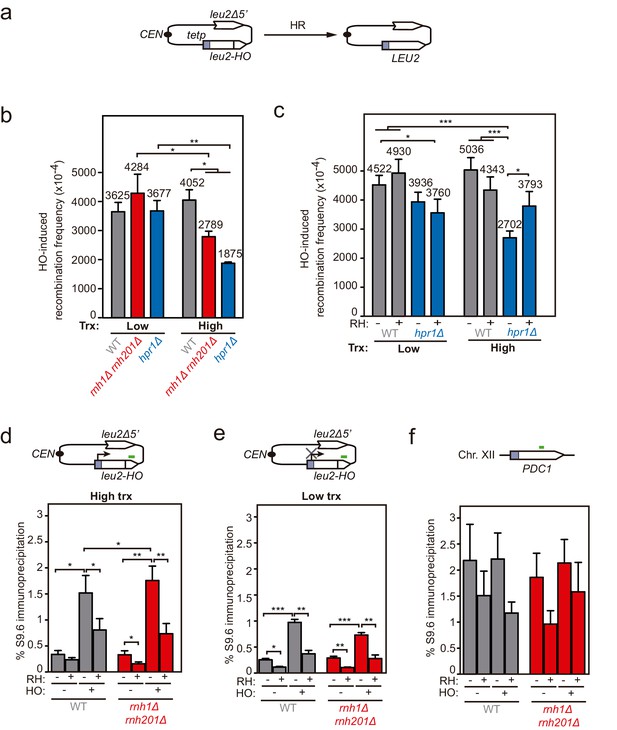
Loss of cleaved plasmids in DNA-RNA hybrid-accumulating mutants.
(a) Scheme of the pCM189-L2FRT-T (upper panel) or -NT (middle panel) and pCM189-L2HO (lower panel) plasmids. FLPm or HO endonuclease induction leads to either nicks that will be converted into DSBs by replication or to replication-independent DSBs, respectively. (b) Percentage of break-induced plasmid loss in wild type (WFLP and WS), rnh1Δ rnh201Δ (WFR1R2 and WSR1R2), and hpr1Δ (WFHPR1 and WSHPR1) strains transformed with pCM189-L2FRT-T (FRT-T), pCM189-L2FRT-NT (FRT-NT), or pCM189-L2FHO (HO) under low or high transcription and after either 24 hr of FLPm induction or 1 hr of HO induction (n≥3). (c) Percentage of break-induced plasmid loss in wild type (WFLP and WS) and hpr1Δ (WFHPR1 and WSHPR1) strains transformed with pCM189-L2FRT-T (FRT-T) or pCM189-L2FHO (HO) and either pRS314 (RH−) or pRS314-GALRNH1 (RH+) under low or high transcription and after either 24 hr of FLPm induction or 1 hr of HO induction (n≥4). Mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted in (b, c) panels. *p≤0.05; **p≤0.01; ***p≤0.001 (unpaired Student’s t-test). See also Figure 1—figure supplement 1. Data underlying this figure are provided as Figure 1—source data 1. Trx, transcription.
-
Figure 1—source data 1
Loss of cleaved plasmids in DNA-RNA hybrid-accumulating mutants.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig1-data1-v2.xlsx
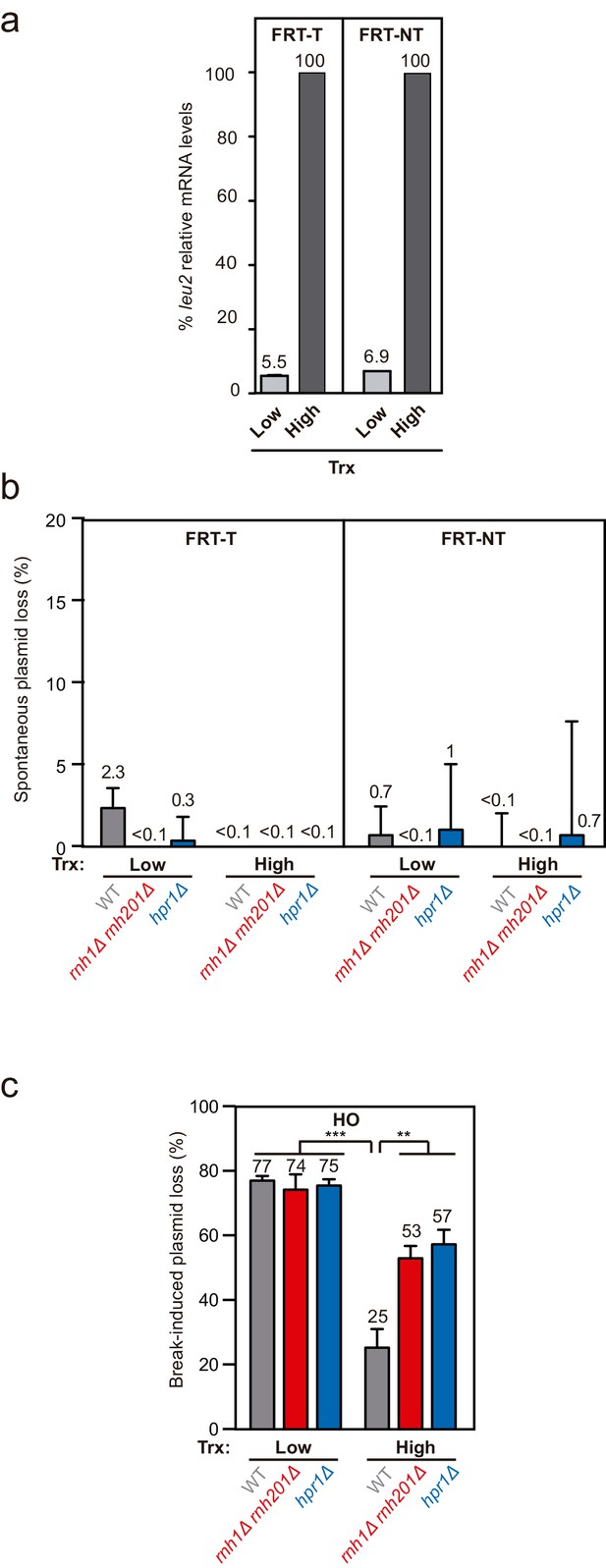
leu2 expression levels in the TINV-FRT system, spontaneous plasmid loss and break-induced plasmid loss after 3h.
Related to Figure 1. (a) Relative RNA levels at the leu2 allele carrying the FRT-T or FRT-NT site in wild type (WFLP) strain transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low or high transcription (n=3). (b) Percentage of spontaneous plasmid loss in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2), and hpr1Δ (WFHPR1) strains transformed with pCM189-L2FRT-T (FRT-T) or pCM189-L2FRT-NT (FRT-NT) under low or high transcription and after either 24 hr of FLPm induction (n=3). In both, all panels, mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted. Data underlying this figure are provided as Figure 1—figure supplement 1—source data 1. (c) Percentage of break-induced plasmid loss in wild type (WS), rnh1Δ rnh201Δ (WSR1R2), and hpr1Δ (WSHPR1) strains transformed with pCM189-L2FHO (HO) under low or high transcription and after 3 hr of HO induction (n=4). Mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted in (b, c) panels. **p≤0.01; ***p≤0.001 (unpaired Student’s t-test). Data underlying this figure are provided as Figure 1—figure supplement 1—source data 1. Trx, transcription.
-
Figure 1—figure supplement 1—source data 1
leu2expression levels in the TINV-FRT system, spontaneous plasmid loss and break-induced plasmid loss after 3h.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig1-figsupp1-data1-v2.xlsx
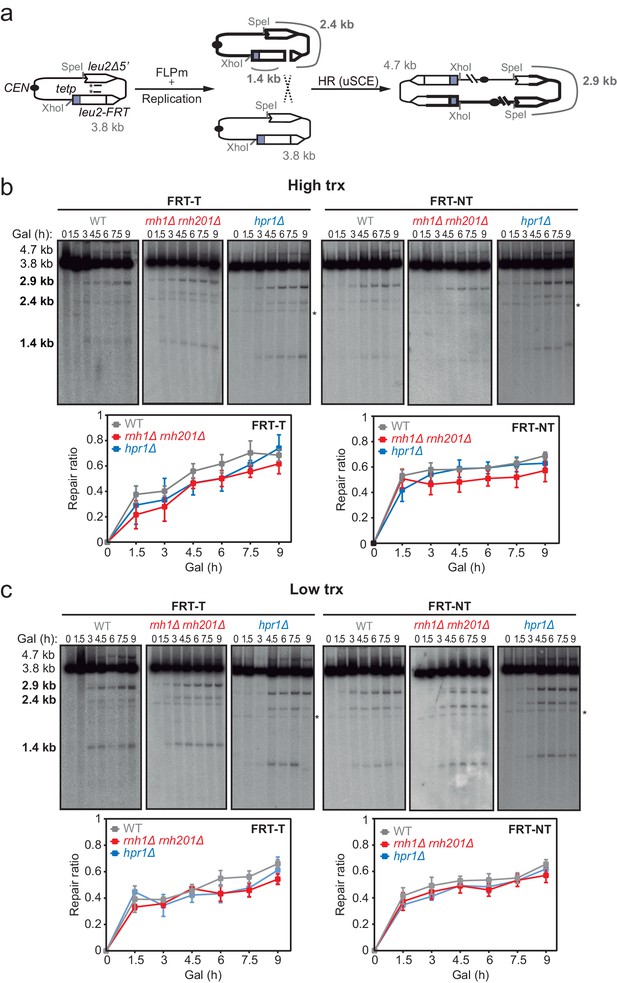
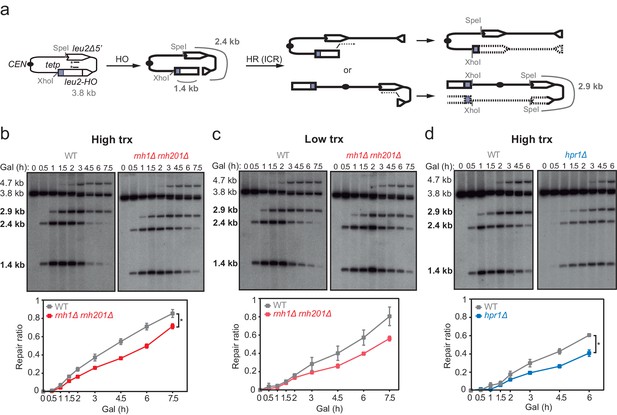
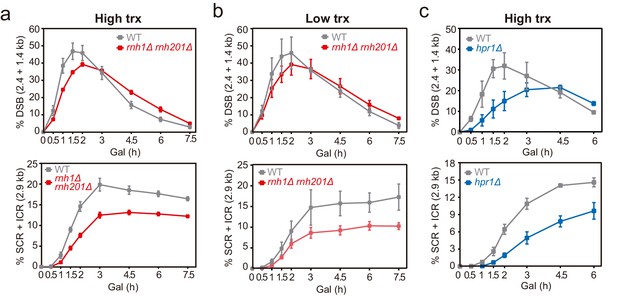
Effect of rnh1Δ rnh201Δ and hpr1Δ in the repair of replication-born DSBs with the sister chromatid.
(a) Schemes of the TINV-FRT system, in which FLPm induction of nicks leads to replication-born DSBs in one of the sister chromatids so that the intact sister chromatid can be used as a template for repair. The repair intermediate resulting from sister chromatid recombination (SCR) involving an exchange between unequal repeats in the two sister chromatids (unequal sister chromatid exchange, uSCE) is depicted. Fragments generated after XhoI-SpeI digestion are indicated with their corresponding sizes in kb and were detected by Southern blot hybridization with a LEU2 probe, depicted as a line with an asterisk. Note that the 2.9-kb band can also appear as a consequence of a break-induced replication event with the sister chromatid (sister chromatid BIR) or within the same chromatid (intrachromatid recombination, ICR), but both reactions are known to occur at a minor and irrelevant frequency. (b) Representative Southern blots and quantified repair ratios from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under high transcription (n≥3). (c) Representative Southern blots and quantified repair ratios from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low transcription (n≥3). In (b, c), the 3.8-kb band corresponds to the intact plasmid, the 2.9-kb band to the repair intermediate, and 1.4 and 2.4-kb bands to the DSBs. Asterisks beside Southern blots indicate non-specific hybridization. Mean and SEM are plotted in (b, c) panels. In all cases, p>0.1 (two-way ANOVA test). See also Figure 2—figure supplements 1 and 2. Data underlying this figure are provided as Figure 2—source data 1. DSB, double-strand break; HR, homologous recombination; uSCE, unequal sister chromatid exchange; trx, transcription.
-
Figure 2—source data 1
Effect ofrnh1Δ rnh201Δandhpr1Δin the repair of replication-born DSBs with the sister chromatid.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig2-data1-v2.xlsx
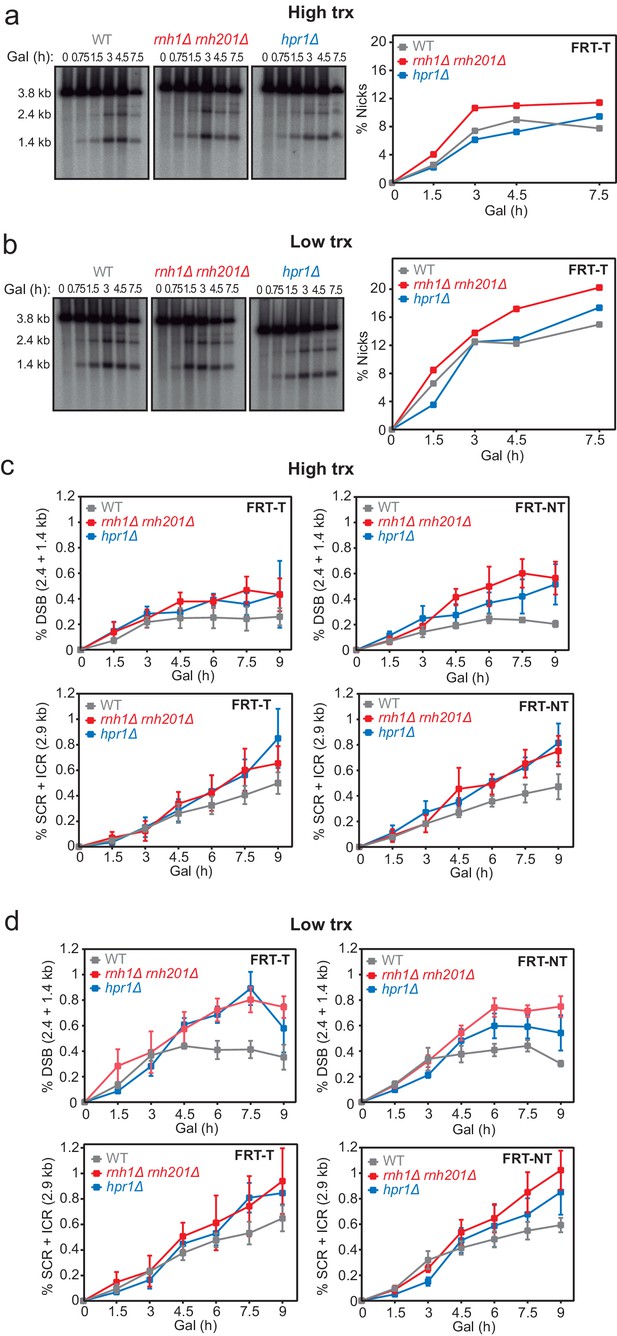
Analysis of FLPm-induced breaks and repair intermediates in rnh1Δ rnh201Δ and hpr1Δ.
Related to Figure 2. (a) Representative Southern blot and quantified nicks from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) under high transcription (n=3). (b) Representative Southern blot and quantified nicks from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) under low transcription (n=3). (c) Quantification of the 2.4 and 1.4-kb DSB and 2.9-kb SCR+ICR bands from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under high transcription (n≥3). (d) Quantification of the 2.4 and 1.4-kb DSB and 2.9-kb SCR+ICR bands from time-course experiments performed at the indicated times after FLPm induction in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low transcription (n≥3). In (a, c), fragments generated after XhoI-SpeI digestion are indicated with their corresponding sizes in kb and were detected by Southern blot hybridization with a LEU2 probe, depicted as a line with an asterisk. Mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted in (b, d) panels, whereas the mean is plotted in (a, c). Data underlying this figure are provided as Figure 2—figure supplement 1—source data 1. DSB, double-strand break; ICR, intrachromatid recombination; SCR, sister chromatid recombination; trx, transcription.
-
Figure 2—figure supplement 1—source data 1
Analysis of FLPm-induced breaks and repair intermediates inrnh1Δ rnh201Δandhpr1Δ.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig2-figsupp1-data1-v2.xlsx
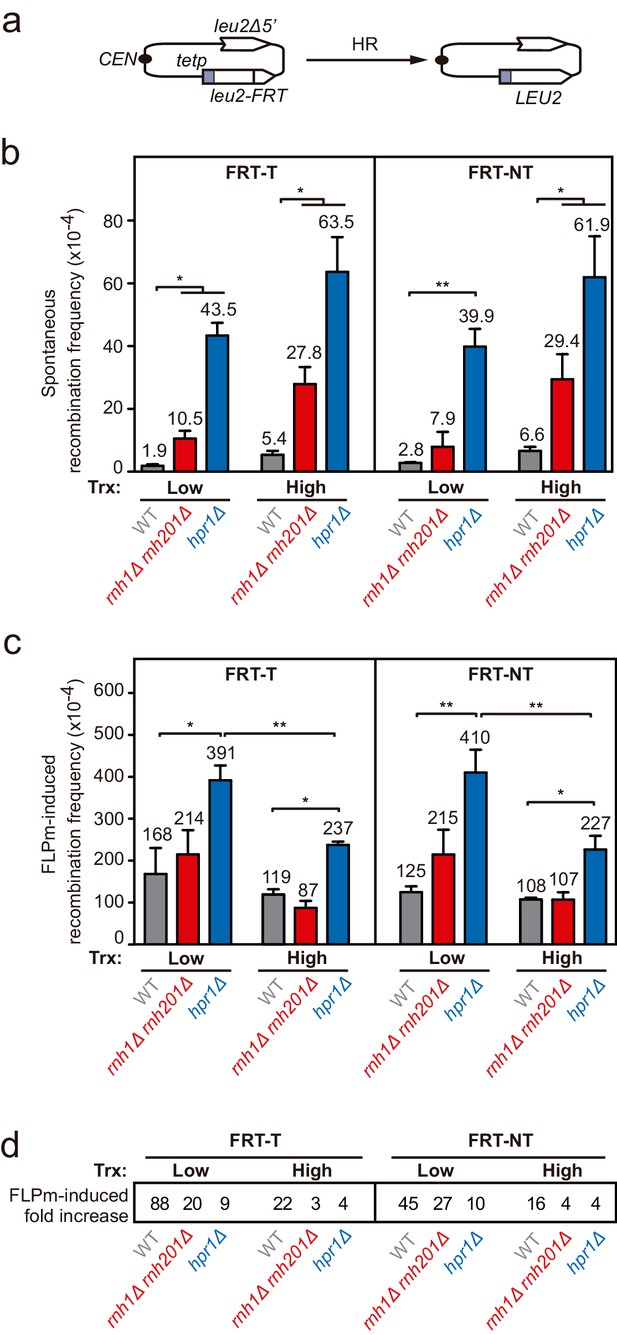
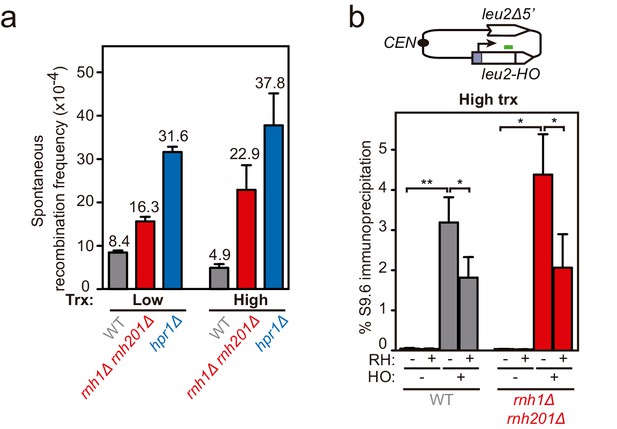
Frequency of spontaneous and FLPm-induced recombination in rnh1Δ rnh201Δ and hpr1Δ.
Related to Figure 2. (a) Schemes of the TINV-FRT recombination systems. (b) Frequency of spontaneous recombination in wild type (WFLP), rnh1Δ rnh201Δ (WFLPR1R2) and hpr1Δ (WFLPHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low or high transcription (n=3). (c) Frequency of FLPm-induced recombination in wild type (WFLP), rnh1Δ rnh201Δ (WFLPR1R2) and hpr1Δ (WFLPHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low or high transcription (n=4). (d) Fold increase of FLPm-induced recombination versus spontaneous recombination, as obtained by the ratio between the data in (b, c). Mean and SEM are plotted in (b, c) panels. *p≤0.05; **p≤0.01 (unpaired Student’s t-test). Data underlying this figure are provided as Figure 2—figure supplement 2—source data 1.
-
Figure 2—figure supplement 2—source data 1
Frequency of spontaneous and FLPm-induced recombination inrnh1Δ rnh201Δandhpr1Δ.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig2-figsupp2-data1-v2.xlsx
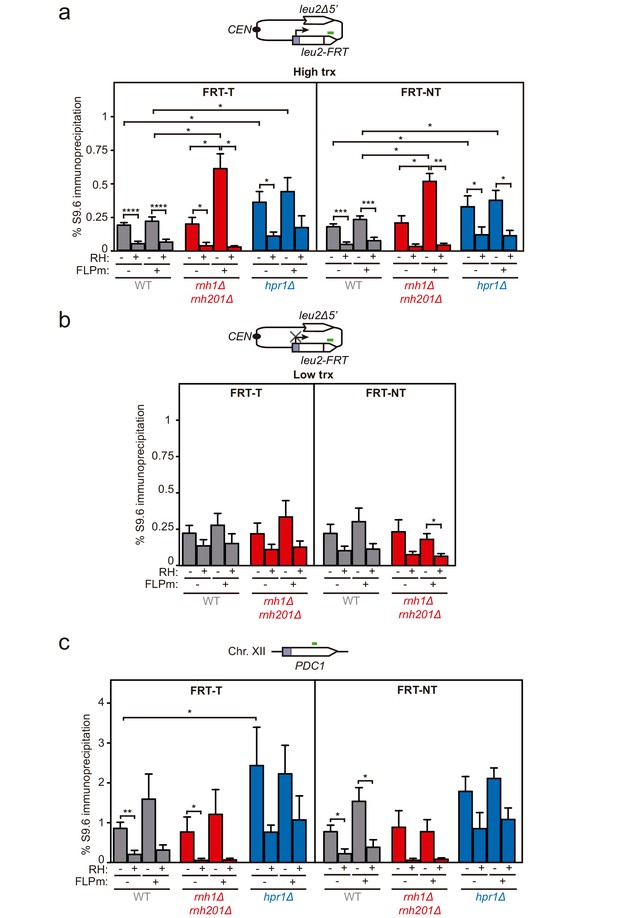
DNA-RNA hybrid accumulation at replication-born DSBs.
(a) DRIP with the S9.6 antibody in the leu2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n≥3). (b) DRIP with the S9.6 antibody in the leu2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in wild type (WFLP) and rnh1Δ rnh201Δ (WFR1R2) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under low transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=4). (c) DRIP with the S9.6 antibody in the PDC1 gene as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n≥3). Mean and SEM are plotted in all panels. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001 (unpaired Student’s t-test). See also Figure 3—figure supplement 1. Data underlying this figure are provided as Figure 3—source data 1. DRIP, DNA-RNA immunoprecipitation; trx, transcription.
-
Figure 3—source data 1
DNA-RNA hybrid accumulation at replication-born DSBs.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig3-data1-v2.xlsx
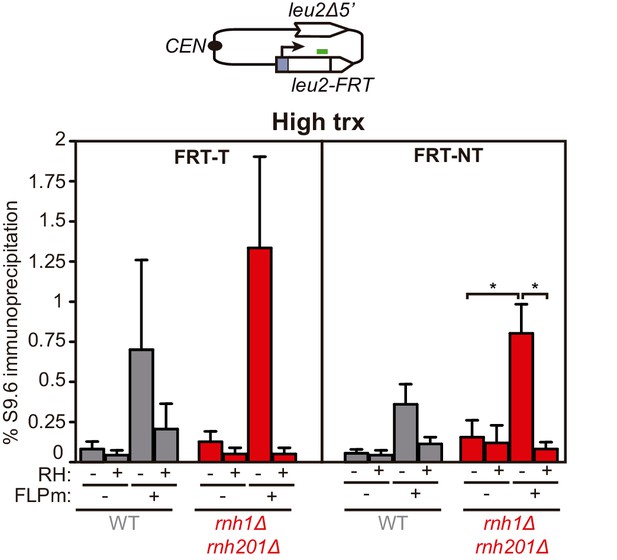
DNA-RNA hybrid accumulation upstream of the FRT site.
Related to Figure 3. DRIP with the S9.6 antibody in the leu2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in wild type (WFLP), rnh1Δ rnh201Δ (WFR1R2) and hpr1Δ (WFHPR1) strains transformed with pTINV-FRT-T (FRT-T) or pTINV-FRT-NT (FRT-NT) under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=4). Means and SEM are plotted in all panels. *p≤0.05 (unpaired Student’s t-test). Data underlying this figure are provided as Figure 3—figure supplement 1—source data 1. DRIP, DNA-RNA immunoprecipitation; trx, transcription.
-
Figure 3—figure supplement 1—source data 1
DNA-RNA hybrid accumulation upstream of theFRTsite.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig3-figsupp1-data1-v2.xlsx
Effect of rnh1Δ rnh201Δ and hpr1Δ in the repair of endonuclease-induced DSBs.
(a) Schemes of the TINV-FHO system, in which HO induction leads to replication-independent DSBs which when occurring by break-induced replication (BIR) from one of the DSB ends invading the truncated repeat located in the same chromatid (intrachromatid recombination, ICR) leads to the depicted repair intermediate. Other recombination reactions (such as uSCE depicted in Figure 2) are also possible. Fragments generated after XhoI-SpeI digestion are indicated with their corresponding sizes in kb and were detected by Southern blot hybridization with a LEU2 probe, depicted as a line with an asterisk. (b) Representative Southern blots and quantified repair ratios from time-course experiments performed at the indicated times after HO induction in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under high transcription (n=4). (c) Representative Southern blots and quantified repair ratios from during time-course experiments performed at the indicated times after HO induction in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under low transcription (n=3). (d) Representative Southern blots and quantified repair ratios from time-course experiments performed at the indicated times after HO induction in wild type (WS) and hpr1Δ (WSHPR1) strains transformed with pTINV-FHO under high transcription (n=3). In (b–d), the 3.8-kb band corresponds to the intact plasmid, 2.9-kb band to the repair intermediates, and 1.4 and 2.4-kb bands to the DSBs. The 4.7-kb band corresponds to a repair intermediate that has not been used for the quantification analysis. Mean and SEM are plotted in (b–d) panels. *p≤0.05 (two-way ANOVA test). See also Figure 4—figure supplement 1. Data underlying this figure are provided as Figure 4—source data 1. DSB, double-strand break; HR, homologous recombination; ICR, intrachromatid recombination; trx, transcription.
-
Figure 4—source data 1
Effect ofrnh1Δ rnh201Δandhpr1Δin the repair of endonuclease-induced DSBs.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig4-data1-v2.xlsx
Analysis of HO-induced DSBs and repair intermediates in rnh1Δ rnh201Δ and hpr1Δ.
Related to Figure 4. (a) Quantification of the 2.4 and 1.4-kb DSB and 2.9-kb SCR+ICR bands from during time-course experiments performed at the indicated times after HO induction in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under high transcription (n=4). (b) Quantification of the 2.4 and 1.4-kb DSB and 2.9-kb SCR+ICR bands from during time-course experiments performed at the indicated times after HO induction in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under low transcription (n=3). (c) Quantification of the 2.4 and 1.4-kb DSB and 2.9-kb SCR+ICR bands from during time-course experiments performed at the indicated times after HO induction in wild type (WS) and hpr1Δ (WSHPR1) strains transformed with pTINV-FHO under high transcription (n=3). Mean and SEM are plotted in all panels. Data underlying this figure are provided as Figure 4—figure supplement 1—source data 1. DSB, double-strand break; ICR, intrachromatid recombination; SCR, sister chromatid recombination.
-
Figure 4—figure supplement 1—source data 1
Analysis of HO-induced DSBs and repair intermediates inrnh1Δ rnh201Δandhpr1Δ.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig4-figsupp1-data1-v2.xlsx
Genetic analysis of the repair and DNA-RNA hybrid accumulation at endonuclease-induced DSBs.
(a) Scheme of the pCM189-L2FHO recombination system. (b) Frequency of HO-induced recombination in wild type (WS), rnh1Δ rnh201Δ (WSR1R2) and hpr1Δ (WSHPR1) strains transformed with pTINV-FHO under low or high transcription (n≥3). (c) Frequency of HO-induced recombination in wild type (WS) and hpr1Δ (WSHPR1) strains transformed with either pRS314 (RH−) or pRS314-GALRNH1 (RH+) and pTINV-FHO under low or high transcription (n=9). (d) DRIP with the S9.6 antibody in the leu2-HO allele as depicted on top and in either spontaneous conditions (HO−) or after HO induction (HO+) in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=5). (e) DRIP with the S9.6 antibody in the leu2-HO allele as depicted on top and in either spontaneous conditions (HO−) or after HO induction (HO+) in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under low transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=4). (f) DRIP with the S9.6 antibody in the PDC1 gene as depicted on top and in either spontaneous conditions (HO−) or after HO induction (HO+) in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under high transcription and either untreated (RH−) or after in vitro RNase H treatment (RH+) (n=5). Mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted in (b–f) panels. *p≤0.05; **p≤0.01; ***p≤0.001 (unpaired Student’s t-test in (b) panel and paired Student’s t-test in (c–f) panels). See also Figure 5—figure supplement 1. Data underlying this figure are provided as Figure 5—source data 1. DRIP, DNA-RNA immunoprecipitation; HR, homologous recombination; trx, transcription.
-
Figure 5—source data 1
Genetic analysis of the repair and DNA-RNA hybrid accumulation at endonuclease-induced DSBs.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig5-data1-v2.xlsx
Frequency of recombination and hybrid accumulation upstream of the HO site.
Related to Figure 5. (a) Frequency of spontaneous recombination in wild type (WS), rnh1Δ rnh201Δ (WSR1R2) and hpr1Δ (WSHPR1) strains transformed with pTINV-FHO under low or high transcription (n≥2). (b) DRIP with the S9.6 antibody in the leu2-HO allele as depicted on top and in either spontaneous conditions (HO−) or after HO induction (HO+) in wild type (WS) and rnh1Δ rnh201Δ (WSR1R2) strains transformed with pTINV-FHO under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=5). Mean and SEM are plotted in all panels. *p≤0.05; **p≤0.01 (paired Student’s t-test). Data underlying this figure are provided as Figure 5—figure supplement 1—source data 1. DRIP, DNA-RNA immunoprecipitation; trx, transcription.
-
Figure 5—figure supplement 1—source data 1
Frequency of recombination and hybrid accumulation upstream of theHOsite.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig5-figsupp1-data1-v2.xlsx
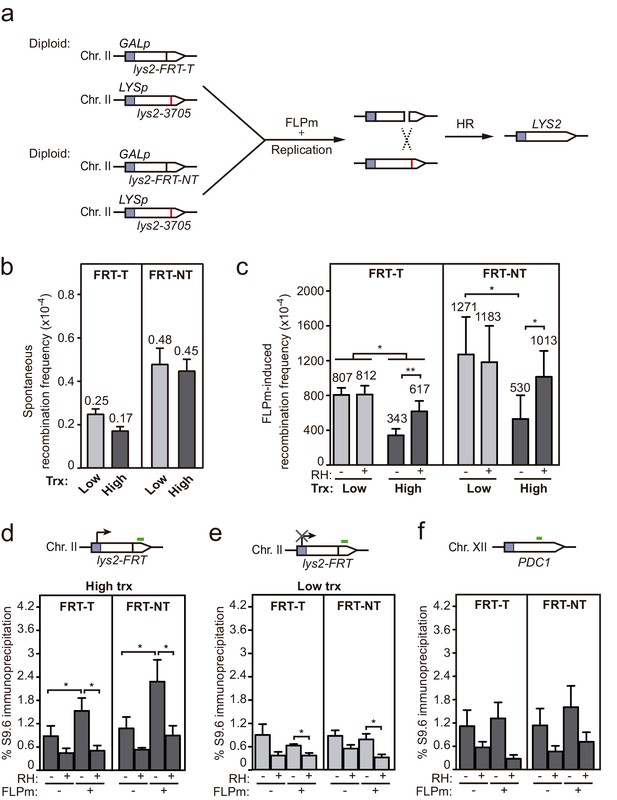
Interference of DNA-RNA hybrids with DSB repair in a chromosome.
(a) Scheme of the diploid chromosome-based FLPm recombination systems (DGL-FRT-T and NT), in which FLPm induction of nicks leads to replication-born DSBs that when repaired with the homologous chromosome would lead to the restoration of the LYS2 gene. (b) Frequency of spontaneous recombination in DGLFT and DGFLNT strains carrying the FRT-T and NT constructs respectively and transformed with pCM190 under low or high transcription (n=3). (c) Frequency of FLPm-induced recombination DGLFT and DGFLNT strains carrying the FRT-T and NT constructs respectively and transformed with pCM190-FLP and either pRS313 (RH−) or pRS313-GALRNH1 (RH+) under low or high transcription (n=5). (d) DRIP with the S9.6 antibody in the lys2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in GLFT and GFLNT strains carrying the FRT-T and NT constructs respectively, transformed with pCM190-FLP under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=4). (e) DRIP with the S9.6 antibody in the lys2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in GLFT and GFLNT strains carrying the FRT-T and NT constructs, respectively, transformed with pCM190-FLP under low transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=4). (f) DRIP with the S9.6 antibody in the PDC1 gene as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in GLFT and GFLNT strains carrying the FRT-T and NT constructs, respectively, transformed with pCM190-FLP under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=3). Mean and SEM of independent experiments consisting in the median value of six independent colonies each are plotted in (b–f) panels. *p≤0.05; **p≤0.01 (paired Student’s t-test). See also Figure 6—figure supplement 1. Data underlying this figure are provided as Figure 6—source data 1. DRIP, DNA-RNA immunoprecipitation; DSB, double-strand break; HR, homologous recombination; trx, transcription.
-
Figure 6—source data 1
Interference of DNA-RNA hybrids with DSB repair in a chromosome.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig6-data1-v2.xlsx
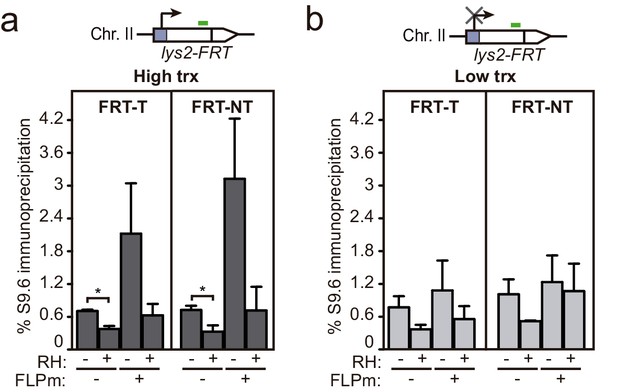
DNA-RNA hybrids accumulation upstream the FRT site.
Related to Figure 6. (a) DRIP with the S9.6 antibody in the lys2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in GLFT and GFLNT strains carrying the FRT-T and NT constructs, respectively, transformed with pCM190-FLP under high transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+). (b) DRIP with the S9.6 antibody in the lys2-FRT alleles as depicted on top and in either spontaneous conditions (FLPm−) or after FLPm induction (FLPm+) in GLFT and GFLNT strains carrying the FRT-T and NT constructs, respectively, transformed with pCM190-FLP under low transcription and either non-treated (RH−) or after in vitro RNase H treatment (RH+) (n=3). Mean and SEM are plotted. *p≤0.05 (unpaired Student’s t-test). Data underlying this figure are provided as Figure 6—figure supplement 1—source data 1. DRIP, DNA-RNA immunoprecipitation; trx, transcription.
-
Figure 6—figure supplement 1—source data 1
DNA-RNA hybrids accumulation upstream theFRTsite.
- https://cdn.elifesciences.org/articles/69881/elife-69881-fig6-figsupp1-data1-v2.xlsx
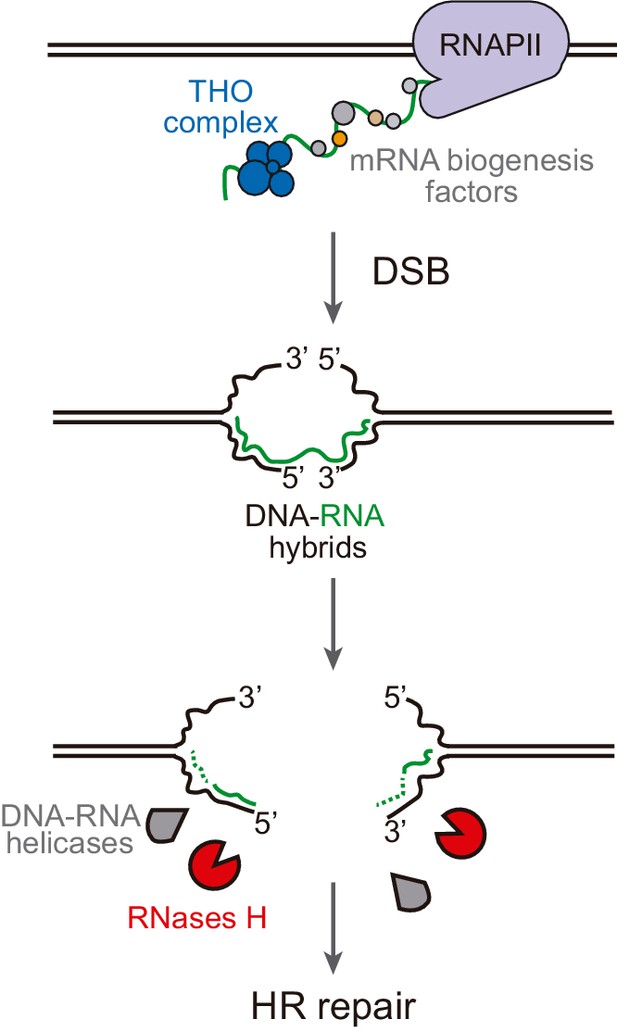
Model of DNA-RNA hybrid formation upon breakage of transcribed DNA.
During transcription, nascent RNA is coated by mRNA biogenesis factors, such as the THO complex, that prevent RNA from hybridizing with its complementary DNA. In their absence, DNA-RNA hybrids can remain even when the DNA is broken. Moreover, double-strand break (DSB) induction leads to incidental DNA-RNA hybridization at both sides of the break site. Such hybrids need to be removed by RNase H enzymes or helicases to allow further repair by homologous recombination (HR).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent Saccharomyces cerevisiae | W303 background strains with different gene deletions | Various | (See Materials and methods section) | |
| Recombinant DNA reagent | Yeast expression plasmids and recombination systems | Various | (See Materials and methods section) | |
| Sequence-based reagent | Primers for DRIP, RT-PCR and probe amplification | Condalab | (See Materials and methods section) | |
| Antibody | S9.6 anti DNA:RNA hybrids (mouse monoclonal) | ATCC Hybridoma cell line | Cat # HB-8730, RRID:CVCL_G144 | (1 mg/ml) |
| Commercial assay kit | Macherey-Nagel DNA purification | Macherey-Nagel | Cat # 740588.250 | |
| Commercial assay kit | Qiagen’s RNeasy | Qiagen | Cat # 75162 | |
| Commercial assay kit | Reverse transcription kit | Qiagen | Cat # 205311 | |
| Peptide, recombinant protein | Zymolyase 20T | US Biological | Z1001 | (15 mg/ml) |
| Chemical compound, drug | Doxycyclin hyclate | Sigma-Aldrich | D9891 | (5 mg/ml) |
| Peptide, recombinant protein | Proteinase K (PCR grade) | Roche | Cat # 03508811103 | |
| Peptide, recombinant protein | Rnase A | Roche | Cat # 10154105103 | |
| Peptide, recombinant protein | Rnase III | Thermo Fisher Scientific | Cat # AM2290 | |
| Peptide, recombinant protein | Spermidine | Sigma-Aldrich | Cat # S2626 | |
| Peptide, recombinant protein | Spermine | Sigma-Aldrich | Cat # S3256 | |
| Other | iTaq Universal SYBR Green | Bio-Rad | Cat # 1725120 | |
| Software, algorithm | GraphPad Prism V8.4.2 | GraphPad Software, La Jolla, CA, USA | RRID:SCR_002798 |
Additional files
-
Supplementary file 1
Strains used in this study.
- https://cdn.elifesciences.org/articles/69881/elife-69881-supp1-v2.docx
-
Supplementary file 2
Primers used in this study.
- https://cdn.elifesciences.org/articles/69881/elife-69881-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/69881/elife-69881-transrepform-v2.pdf





