The Lyme disease agent co-opts adiponectin receptor-mediated signaling in its arthropod vector
Figures
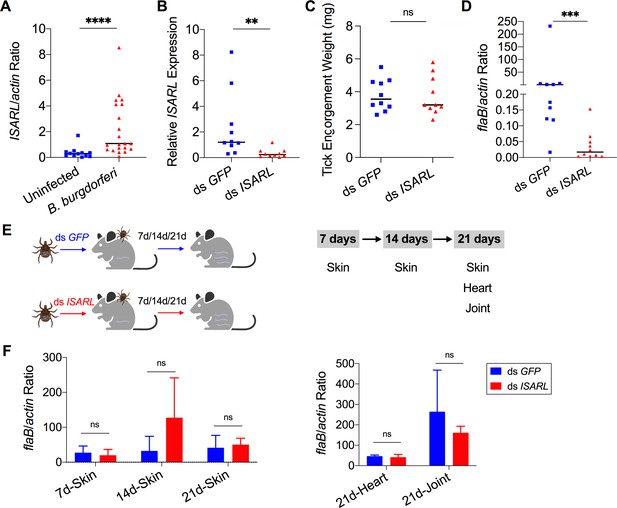
Silencing of ISARL significantly reduces the B. burgdorferi burden in nymphal tick guts.
(A) ISARL is significantly induced in nymphal tick guts after feeding on B. burgdorferi-infected mice. (B) qPCR assessment of ISARL transcript levels following RNAi silencing of ISARL after feeding on B. burgdorferi-infected mice. (C) Nymphal engorgement weights in ISARL-silenced and mock-injected nymphs. Each data point represents one engorged tick. (D) qPCR assessment of B. burgdorferi flaB levels in guts following RNAi silencing of ISARL after feeding on B. burgdorferi-infected mice. Each data point represents one nymph gut. Horizontal bars in the above figures represent the median. Statistical significance was assessed using a nonparametric Mann–Whitney test (ns, p>0.05; **p<0.01; ***p<0.001; ****p<0.0001). (E) Borrelia-infected nymphs microinjected with ds ISARL or ds GFP were fed on clean mice to assess transmission of the spirochete. The infection of Borrelia in murine skin 7, 14, and 21 days after infection, and in heart and joint tissues at 21 days was determined. (F) Murine skin 7, 14, and 21 days after infection, and in heart and joint tissues at 21 days was determined by qPCR of flaB and normalized to mouse actin. Data represent the means ± standard deviations from five biological replicates with two technical replicates.
-
Figure 1—source data 1
ISARL is involved in B. burgdorferi colonization in nymphal tick guts but has no effect on transmission.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig1-data1-v2.xlsx
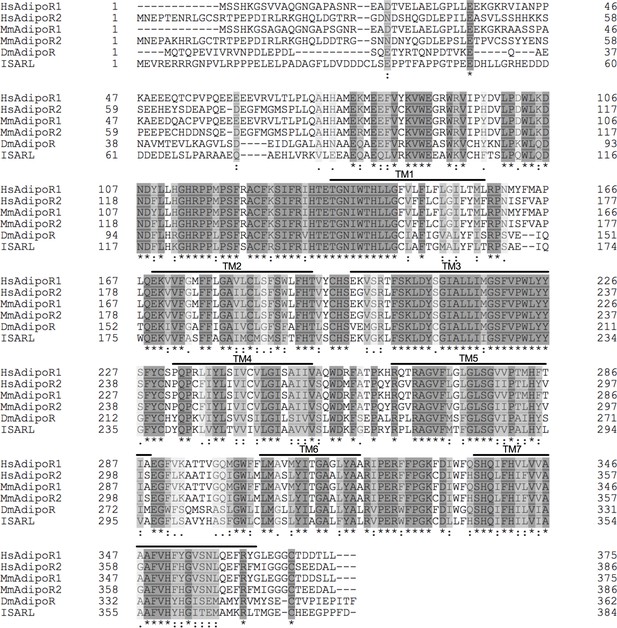
Protein sequence comparison of adiponectin receptors.
Multiple sequence alignment of Ixodes scapularis ISARL with the amino acid sequences of homologs identified in Homo sapiens (NP_001277482, HsAdipoR1; NP_001362293, HsAdipoR2), Mus musculus (NP_001292998, MmAdipoR1; NP_001342621, MmAdipoR2), and Drosophila melanogaster (NP_732759, DmAdipoR). Seven transmembrane (TM1–TM7) domain regions are marked by upper lines. (*) indicates positions that have a single, fully conserved residue (dark gray). (:) indicates conservation between groups of strongly similar properties (light gray). (.) indicates conservation between groups of weakly similar properties (white gray). The TM domains is based on the experimentally defined human adiponectin receptors.
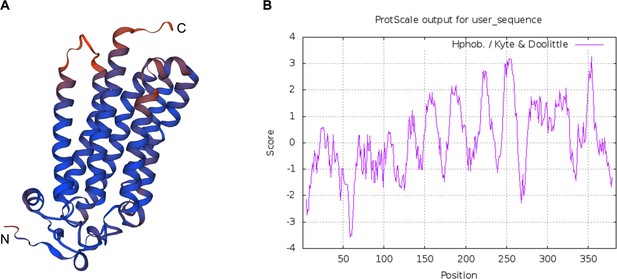
Predicted protein structure and hydrophobicity of ISARL.
Seven transmembrane (TM) domains were identified in the ISARL protein based on (A) protein structure prediction and (B) hydrophobicity analysis.
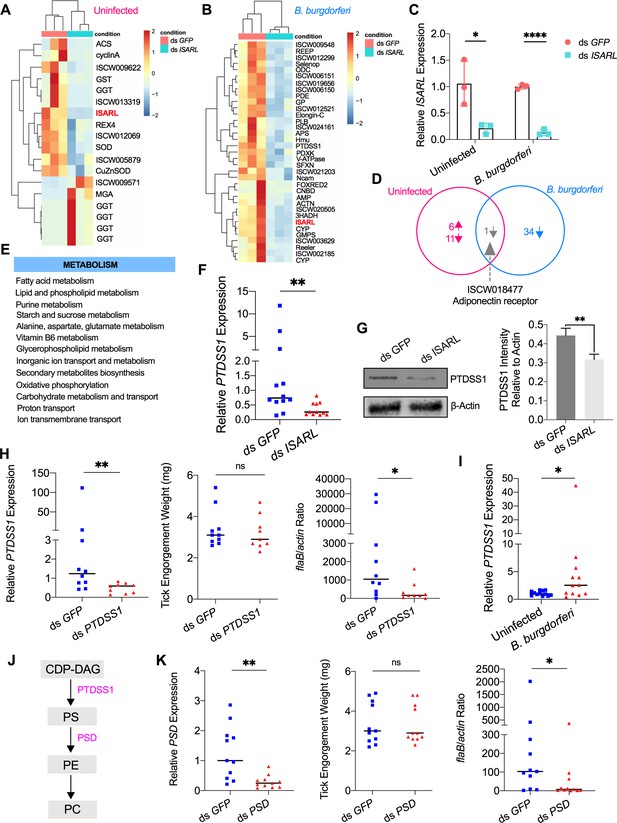
RNA-seq, qPCR validation, and RNAi-silencing assays revealed that phosphatidylserine synthase 1 (PTDSS1) is regulated by ISARL and is involved in B. burgdorferi colonization.
(A) Hierarchical clustering of differentially expressed genes was generated after feeding on clean mice. (B) Hierarchical clustering of differentially expressed genes was generated after feeding on B. burgdorferi-infected mice. Each column represents biological replicates. The gene names can be found in Supplementary file 1. The ISARL gene is highlighted with bold and red color. The expression levels were visualized, and the scale from least abundant to highest range is from –2.0 to 2.0. The phylogenetic relationships of differentially expressed genes are shown on the left tree. The top tree indicates the cluster relationship of the sequenced samples. (C) qPCR validation of ISARL knockdown in tick gut. Statistical significance was assessed using Student’s t test (*p<0.05; ****p<0.0001). (D) Venn diagram depicting unique and common differentially expressed genes between clean and B. burgdorferi-infected mice feeding. The up arrow indicates upregulation, and the down arrow indicates downregulation of differentially expressed genes. (E) Metabolism pathways inferred by GO and KEGG enrichment analyses of transcriptomes comparison between ds GFP and ds ISARL injection after feeding on B. burgdorferi-infected mice to repletion. (F) qPCR validation of PTDSS1 showed that PTDSS1 is positively regulated by ISARL. (G) Western blot of PTDSS1 protein showed that PTDSS1 is positively regulated by ISARL (**p<0.01). (H) qPCR assessment of PTDSS1 transcript level, nymphal engorgement weights, and B. burgdorferi flaB levels in guts following RNAi silencing of PTDSS1 after feeding on B. burgdorferi-infected mice. Each data point represents one nymph. (I) PTDSS1 is significantly induced in the nymphal tick gut after feeding on B. burgdorferi-infected mice. (J) PTDSS1 is involved in phospholipid pathway. Cytidine diphosphate diacylglycerol (CDP-DAG) is converted to phosphatidylserine (PS) by PTDSS1. PE, phosphatidylethanolamine; PC, phosphatidylcholine. (K) qPCR assessment of phosphatidylserine decarboxylase (PSD) transcript level, nymphal engorgement weights, and qPCR assessment of B. burgdorferi flaB levels in guts following RNAi silencing of PSD after feeding on B. burgdorferi-infected mice. Each data point represents one nymph. Horizontal bars in the above figures represent the median. Statistical significance was assessed using a nonparametric Mann–Whitney test (ns, p>0.05; *p<0.05; **p<0.01).
-
Figure 2—source data 1
Source data for PTDSS1 protein relative quantification.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-data1-v2.pdf
-
Figure 2—source data 2
Source data for PTDSS1 protein relative quantification.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-data2-v2.pdf
-
Figure 2—source data 3
PTDSS1 is regulated by ISARL and is involved in B. burgdorferi colonization.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-data3-v2.xlsx
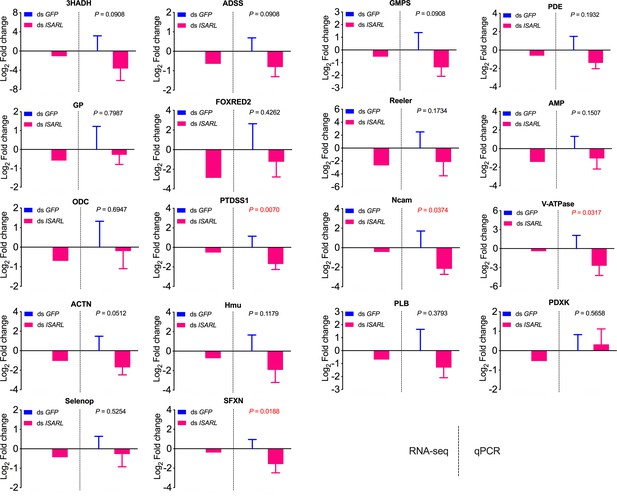
qPCR validation of 18 well-annotated and metabolism-related differentially expressed genes.
3HADH, 3-hydroxyacyl-CoA dehydrogenase, putative; ADSS, adenylosuccinate synthetase; GMPS, GMP synthase, putative; PDE, cAMP and cAMP-inhibited cGMP 3,5-cyclic phosphodiesterase; GP, glycogen phosphorylase; FOXRED2, FAD-dependent oxidoreductase domain-containing protein 2; Reeler, secreted protein with Reeler domain; AMP, AMP-dependent CoA ligase; ODC, oxodicarboxylate carrier protein; PTDSS1, phosphatidylserine synthase I; Ncam, N-CAM Ig domain-containing protein; V-ATPase, vacuolar H+-ATPase V1 sector, subunit G; ACTN, alpha-actinin, putative; Hmu, hemomucin, putative; PLB, phospholipase B-like; PDXK, pyridoxine kinase, putative; Selenop, selenoprotein P precursor; SFXN, sideroflexin 1, 2, 3, putative.
-
Figure 2—figure supplement 1—source data 1
Source data for qPCR validation.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-figsupp1-data1-v2.xlsx
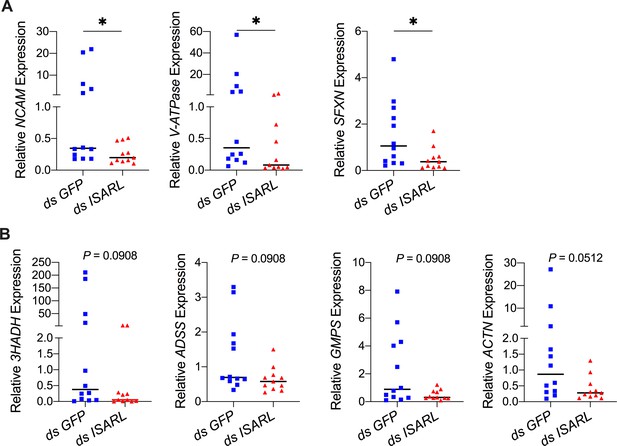
QPCR validation of differentially expressed genes from the RNA-seq dataset.
(A) N-CAM Ig domain-containing protein (NCAM), vacuolar H+-ATPase V1 sector, subunit G (V-ATPase), and sideroflexin 1,2,3, putative (SFXN) were significantly downregulated following RNAi silencing of ISARL after feeding on B. burgdorferi-infected mice. (B) 3-Hydroxyacyl-CoA dehydrogenase, putative (3HADH), adenylosuccinate synthetase (ADSS), GMP synthase, putative (GMPS), and alpha-actinin, putative (ACTN) were downregulated following RNAi silencing of ISARL after feeding on B. burgdorferi-infected mice (p-values are close to significant of 0.05). Each data point represents one nymph gut. Horizontal bars in the above figures represent the median. Statistical significance was assessed using a nonparametric Mann–Whitney test (*p<0.05).
-
Figure 2—figure supplement 2—source data 1
Source data for qPCR validation.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-figsupp2-data1-v2.xlsx
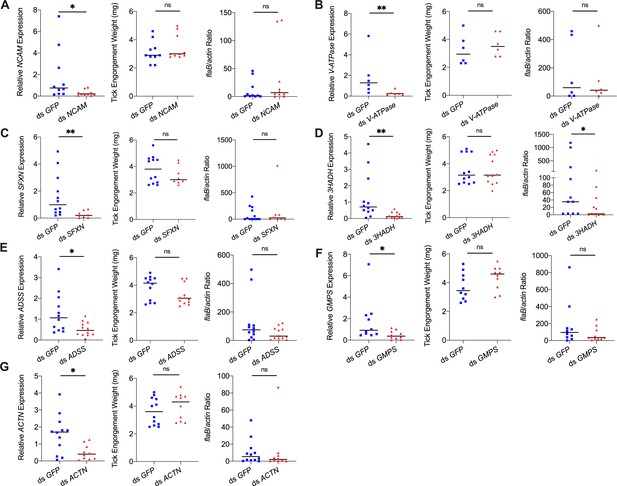
Silencing of differentially expressed genes and effects on B. burgdorferi acquisition.
Silencing of (A) NCAM, (B) V-ATPase, (C) SFXN, (E) ADSS, (F) GMPS, and (G) ACTN has no effect on B. burgdorferi acquisition. Silencing of (D) 3HADH decreased the B. burgdorferi burden in tick gut. 3HADH is involved in fatty acid metabolic processes, suggesting that tick fatty acid metabolism may also influence acquisition of B. burgdorferi. 3HADH is not significantly regulated by ISARL, it was not considered further in this study. Each data point represents one nymph gut. Horizontal bars in the above figures represent the median. Statistical significance was assessed using a nonparametric Mann–Whitney test (ns, p>0.05; *p<0.05; **p<0.01).
-
Figure 2—figure supplement 3—source data 1
Source data for RNAi of differentially expressed genes and effects on B. burgdorferi acquisition.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig2-figsupp3-data1-v2.xlsx
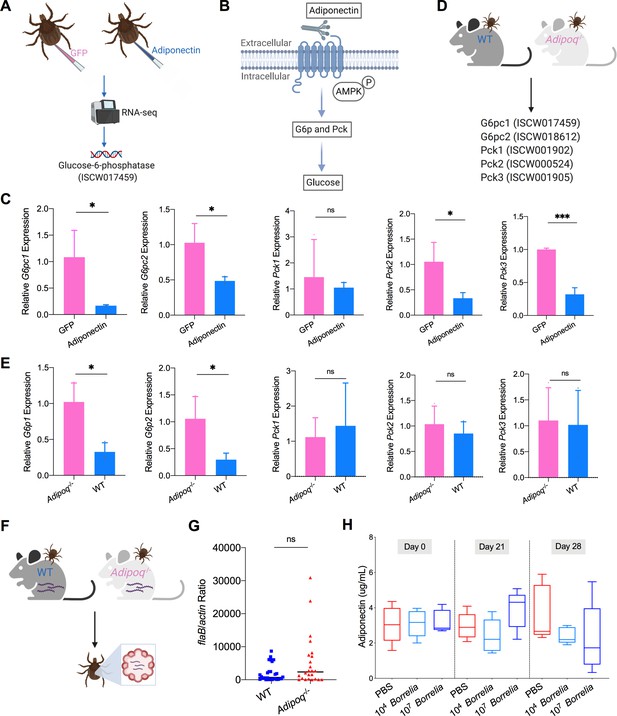
Mammalian adiponectin regulates tick glucose metabolism.
(A) RNA-seq of injection of recombinant mouse adiponectin and GFP (control) proteins. One classic downstream gene of mammalian adiponectin receptor signaling, glucose-6-phosphatase (G6p), was significantly downregulated in the presence of mammalian adiponectin. (B) Interaction of mammal adiponectin and adiponectin receptor suppresses G6p and phosphoenolpyruvate carboxykinase (Pck) expression through an AMP-activated protein kinase (AMPK)-dependent mechanism, which further inhibits glycogenolysis and gluconeogenesis. (C) Injection of recombinant mouse adiponectin significantly downregulates the expression of G6pc1, G6pc2, Pck2, and Pck3 in the tick gut. (D) Feed ticks on C57BL/6J WT and Adipoq-/- mice and then evaluate the expression of G6pc1, G6pc2, Pck1, Pck2, and Pck3. (E) After feeding on WT and Adipoq-/- mice, G6pc1 and G6pc2 showed significant downregulation profile in the presence of adiponectin, while Pck genes did not exhibit marked downregulation. (F) Ticks were fed on B. burgdorferi-infected WT and Adipoq-/- mice, and then B. burgdorferi flaB levels in guts were assessed. (G) qPCR assessment of B. burgdorferi burden after feeding on B. burgdorferi-infected WT and Adipoq-/- mice. No significant difference of B. burgdorferi burden in tick gut was observed between feeding on WT and Adipoq-/- mice. (H) Adiponectin concentration in mice sera following 21 and 28 days after injection of B. burgdorferi at the density of 104 and 107 cells/mL, respectively. Statistical significance was assessed using a nonparametric Mann–Whitney test (ns, p>0.05; *p<0.05; **p<0.01; ***p<0.001).
-
Figure 3—source data 1
Mammalian adiponectin regulates tick glucose metabolism but has no effect on B. burgdorferi colonization.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig3-data1-v2.xlsx
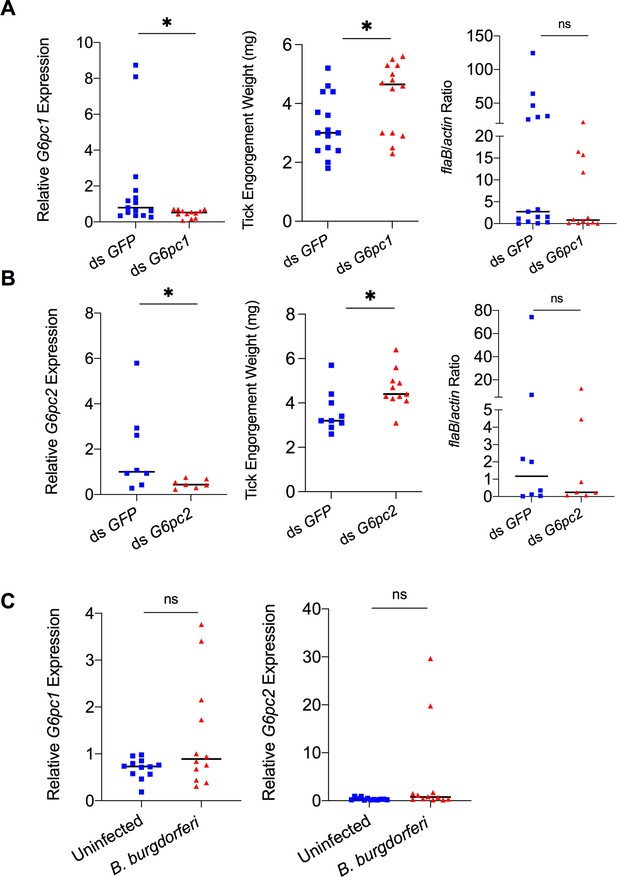
Mammalian adiponectin and tick glucose metabolism changes have no effect on B. burgdorferi acquisition.
(A) qPCR assessment of G6pc1 transcript level, nymphal engorgement weights, and qPCR assessment of B. burgdorferi flaB levels in guts following RNAi silencing of G6pc1 after feeding on B. burgdorferi-infected mice. (B) qPCR assessment of G6pc2 transcript level, nymphal engorgement weights, and qPCR assessment of B. burgdorferi flaB levels in guts following RNAi silencing of G6pc2 after feeding on B. burgdorferi-infected mice. (C) qPCR assessment of G6pc1 and G6pc2 transcript level in nymphal tick gut after feeding on clean and B. burgdorferi-infected mice (ns, p>0.05; *p<0.05).
-
Figure 3—figure supplement 1—source data 1
Source data for effects of tick glucose metabolism on B. burgdorferi acquisition.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig3-figsupp1-data1-v2.xlsx
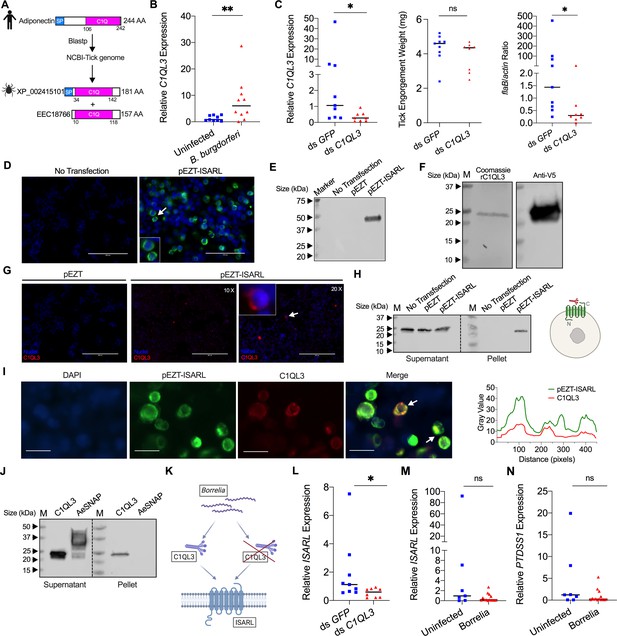
Tick complement C1q-like protein 3 (C1QL3) is involved in ISARL signaling pathways.
(A) Blastp of the tick genome with the human adiponectin C1Q domain in NCBI generated two homologs and were annotated as complement C1q-like protein 3 (C1QL3) (GenBank number: XP_002415101) and conserved hypothetical protein (GenBank number: EEC18766), respectively. These are identical proteins except that C1QL3 has a signal peptide sequence. (B) C1QL3 is significantly induced in replete nymphal tick guts after feeding on B. burgdorferi-infected mice. (C) qPCR assessment of C1QL3 transcript levels, nymphal engorgement weights, and B. burgdorferi flaB levels in guts following RNAi silencing of C1QL3 after feeding on B. burgdorferi-infected mice. (D) Human HEK293T cells were transfected with HA-tagged ISARL-expressing vector (pEZT-ISARL-HA). 40 hr post transfection, the cells were examined. The results showed that ISARL can be successfully expressed on the HEK293T cell membrane. The white arrow indicates examples of membrane expression. (E) Western blot confirmed ISARL expression in the HEK293T cells. (F) Generation of tick C1QL3 protein with His/V5-tag in a Drosophila expression system. Recombinant protein was assessed by SDS-PAGE gel and western blot. (G) C1QL3 is bound on the membrane of ISARL-expressed HEK293T cells. 10× and 20× are the microscope magnifications. The white arrow indicates one example of binding. (H) Binding of C1QL3 to ISARL as analyzed by a pull-down assay. HRP V5-tag monoclonal antibody was used to detect protein. C1QL3 was only detected in ISARL-expressed cells pellet. (I) Co-immunolocalization of ISARL (green) and C1QL3 (red). The specific signal of C1QL3 protein was observed on the surface of some of ISARL-expressed cells, and no signal was shown on nonsuccessfully expressed cells membrane. The white arrows indicate examples of binding. Bar: 20 μm. The plot profile of co-localization was conducted by ImageJ software. (J) Binding of C1QL3 to tick ISE6 cells as analyzed by a pull-down assay. The Aedes aegypti synaptosomal-associated protein (AeSNAP) was used as control. HRP V5-tag monoclonal antibody was used to detect protein. (K) Analysis of how silencing of C1QL3 influences ISARL expression after feeding on B. burgdorferi-infected mice. (L) qPCR assessment showed that ISARL transcript levels following RNAi silencing of C1QL3 were significantly lower than in control ds GFP-injected tick guts after feeding on B. burgdorferi-infected mice. (M) qPCR assessment showed that a blood meal containing B. burgdorferi did not significantly increase expression of ISARL in the nymphal tick guts as compared to feeding on clean mice after RNAi silencing of C1QL3. (N) qPCR assessment showed that a blood meal containing B. burgdorferi did not significantly increase expression of PTDSS1 in the nymphal tick guts as compared to feeding on clean mice after RNAi silencing of C1QL3. Each data point represents one nymph. Horizontal bars in the above figures represent the median. Statistical significance was assessed using a nonparametric Mann–Whitney test (ns, p>0.05; *p<0.05; **p<0.01).
-
Figure 4—source data 1
Source data for ISARL expression.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data1-v2.pdf
-
Figure 4—source data 2
Source data for C1QL3 protein purification.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data2-v2.pdf
-
Figure 4—source data 3
Source data for C1QL3 protein purification.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data3-v2.pdf
-
Figure 4—source data 4
Source data for binding of C1QL3 to ISARL.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data4-v2.pdf
-
Figure 4—source data 5
Source data for binding of C1QL3 to tick ISE6 cells.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data5-v2.pdf
-
Figure 4—source data 6
C1QL3 is involved in the ISARL signaling pathway and modulates B. burgdorferi colonization.
- https://cdn.elifesciences.org/articles/72568/elife-72568-fig4-data6-v2.xlsx
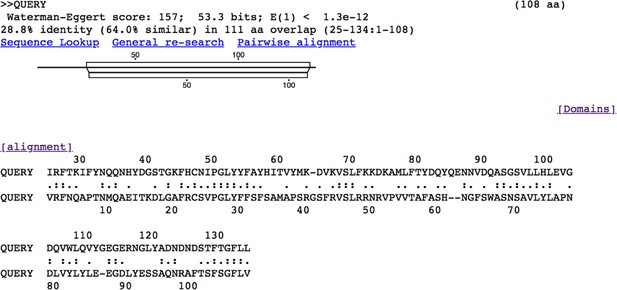
Alignment of human adiponectin C1Q domain and C1QL3 C1Q domain.
Tick C1QL3 C1Q domain has 64.0% similarity and 28.8% identity with human adiponectin C1Q domain. The alignment was conducted in LALIGN/PLALIGN (https://fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi?rm=lalign&pgm=lal).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
| Biological sample (Mus musculus) | C3H/HeJ | Jackson Laboratory | Stock #: 000659;RRID:IMSR_JAX:000659 | |
| Biological sample (M. musculus) | WT C57BL/6J | Jackson Laboratory | Stock #: 000664; RRID:IMSR_JAX:000664 | |
| Biological sample (M. musculus) | Adipo-/- C57BL/6J | Jackson Laboratory | Stock #: 008195; RRID:IMSR_JAX:008195 | |
| Biological sample (Borrelia burgdorferi) | Borrelia burgdorferi strain N40 | Dr. Erol Fikrig Laboratory | ||
| Biological sample (Ixodes scapularis) | Black-legged tick | Oklahoma State University | ||
| Cell line (Homo sapiens) | Human embryonic kidney HEK293T | ATCC | #CRL-3216; RRID:CVCL_0063 | |
| Cell line (I. scapularis) | Tick ISE6 | ATCC | #CRL-11974; RRID:CVCL_Z170 | |
| Antibody | Anti-HA (rabbit monoclonal) | Cell Signaling Technology | #C29F4; RRID:AB_10693385 | IF (1:100) |
| Antibody | Anti-V5 (mouse monoclonal) | Invitrogen | #R960-25; RRID:AB_2556564 | IF (1:100) |
| Antibody | Goat anti-rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen | #A-11034; RRID:AB_2576217 | IF (1:100) |
| Antibody | Goat anti-mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 | Invitrogen | #A-21422; RRID:AB_141822 | IF (1:100) |
| Antibody | HRP Anti-His tag antibody (chicken polyclonal) | Abcam | #ab3553; RRID:AB_303900 | WB (1:10,000) |
| Antibody | HRP V5-tag (mouse monoclonal) | Invitrogen | #R961-25; RRID:AB_2556565 | WB (1:1000) |
| Peptide, recombinant protein | Mouse adiponectin | MilliporeSigma | #SRP3297 | |
| Peptide, recombinant protein | Aequorea victoria green fluorescent protein (GFP) | SinoBiological | #13105-S07E | |
| Commercial assay or kit | Mouse adiponectin/Acrp30 Quantikine ELISA Kit | R&D Systems | #MRP300;RRID:AB_2832917 | |
| Software, algorithm | Prism | GraphPad | RRID:SCR_002798 |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/72568/elife-72568-transrepform1-v2.docx
-
Supplementary file 1
Supplementary files in this study.
(a) Summary of differently expressed genes of comparison between ds GFP and ds ISARL injection after 96 hr feeding on clean mice. (b) Summary of differently expressed genes of comparison between ds GFP and ds ISARL injection after 96 hr feeding on B. burgdorferi-infected mice. (c) Summary of differently expressed genes of comparison between recombinant GFP and adiponectin proteins injection after 8 hr. (d) The primers used in this study.
- https://cdn.elifesciences.org/articles/72568/elife-72568-supp1-v2.docx





