Structural basis for the absence of low-energy chlorophylls in a photosystem I trimer from Gloeobacter violaceus
Abstract
Photosystem I (PSI) is a multi-subunit pigment-protein complex that functions in light-harvesting and photochemical charge-separation reactions, followed by reduction of NADP to NADPH required for CO2 fixation in photosynthetic organisms. PSI from different photosynthetic organisms has a variety of chlorophylls (Chls), some of which are at lower-energy levels than its reaction center P700, a special pair of Chls, and are called low-energy Chls. However, the sites of low-energy Chls are still under debate. Here, we solved a 2.04-Å resolution structure of a PSI trimer by cryo-electron microscopy from a primordial cyanobacterium Gloeobacter violaceus PCC 7421, which has no low-energy Chls. The structure shows the absence of some subunits commonly found in other cyanobacteria, confirming the primordial nature of this cyanobacterium. Comparison with the known structures of PSI from other cyanobacteria and eukaryotic organisms reveals that one dimeric and one trimeric Chls are lacking in the Gloeobacter PSI. The dimeric and trimeric Chls are named Low1 and Low2, respectively. Low2 is missing in some cyanobacterial and eukaryotic PSIs, whereas Low1 is absent only in Gloeobacter. These findings provide insights into not only the identity of low-energy Chls in PSI, but also the evolutionary changes of low-energy Chls in oxyphototrophs.
Editor's evaluation
This work presents a high-resolution structure of a photosystem I complex of the primordial cyanobacterium Gloeobacter violaceus. It relates the structural differences with oher cyanobacteria to differences in the optical properties.
https://doi.org/10.7554/eLife.73990.sa0Introduction
Oxygenic photosynthetic reactions convert light energy into chemical energy and produce molecular oxygen, thereby maintaining the aerobic life on the earth (Blankenship, 2021). The light-induced photosynthetic reactions occur in two multisubunit pigment–protein complexes, photosystem I and photosystem II (PSI and PSII, respectively). PSI functions in light-harvesting, charge-separation, and electron-transfer reactions, leading to the reduction of NADP to NADPH required for CO2 fixation (Blankenship, 2021; Brettel and Leibl, 2001; Nelson and Yocum, 2006). PSI contains many cofactors, including chlorophylls (Chls), carotenoids (Cars), quinones, and iron–sulfur clusters, which serve in the photochemical reactions (Nelson and Yocum, 2006). These cofactors are well conserved in PSI cores among oxyphototrophs, although the oligomerization states and subunit compositions of PSI differ significantly among different species of organisms (Fromme et al., 2001; Suga and Shen, 2020; Hippler and Nelson, 2021).
Among photosynthetic organisms, cyanobacteria are a large group of prokaryotes, and their PSI cores are present in either trimeric (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021) or tetrameric (Kato et al., 2019; Zheng et al., 2019; Chen et al., 2020; Semchonok et al., 2022) forms. Cyanobacterial PSI complexes also possess unique Chl molecules at energy levels lower than the level of P700, a special pair Chl that performs charge separation in each PSI-monomer unit (Brettel and Leibl, 2001). The low-energy Chls are historically called red Chls, which differ in their energy levels among different species of cyanobacteria (Gobets and van Grondelle, 2001; Schlodder et al., 2005; Karapetyan et al., 2006; Schlodder et al., 2007). The main functions of low-energy Chls in PSI are either uphill energy transfer from low-energy Chls to other Chls (Gobets and van Grondelle, 2001) or excitation-energy quenching upon P700 oxidation (Shubin et al., 1995; Shibata et al., 2010; Schlodder et al., 2011). From these observations, the low-energy Chls are thought to regulate energy balance for energy transfer and energy quenching (Gobets and van Grondelle, 2001; Schlodder et al., 2005; Karapetyan et al., 2006; Schlodder et al., 2007). Despite numerous structural and functional analyses of the cyanobacterial PSI, the locations of low-energy Chls are still under debate both experimentally and theoretically (Gobets and van Grondelle, 2001; Schlodder et al., 2005; Karapetyan et al., 2006; Schlodder et al., 2007; Byrdin et al., 2002; Adolphs et al., 2010). This is because it is very difficult to identify specific low-energy Chls from ~95 Chl molecules at different energy levels in a PSI monomer.
The sites of low-energy Chls in PSI have been suggested by spectroscopic techniques (Gobets and van Grondelle, 2001; Schlodder et al., 2005; Karapetyan et al., 2006; Schlodder et al., 2007). Absorption spectroscopy showed various peaks and shoulders around/over 700 nm, reflecting a complexity and difficulty of the identification of low-energy Chls in PSI. In contrast, fluorescence spectroscopy is a convenient method to observe low-energy Chls in PSI. It is known that under the liquid-nitrogen condition, two types of prominent fluorescence peaks from low-energy Chls were mainly found. These two fluorescence peaks appear at around 723 and 730 nm in PSI trimers isolated from the representative cyanobacteria, Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis) (van der Lee et al., 1993; Mimuro et al., 2010; Turconi et al., 1996) and Thermosynechococcus elongatus (Pålsson et al., 1996), respectively. The 723 and/or 730 nm fluorescence peaks are conserved in most cyanobacteria, although their band widths and peak positions differ slightly depending on the species of cyanobacteria as well as the experimental conditions employed to measure. The fluorescence feature of low-energy Chls is also conserved in the tetrameric PSI cores of cyanobacteria (Kato et al., 2019; Nagao et al., 2020b). However, it is unclear regarding the locations of these low-energy forms, because the PSI core contains about 95 Chls, and it is difficult to identify specific Chls from the bulk of the PSI Chls.
Gloeobacter violaceus PCC 7421 (hereafter referred to as Gloeobacter) is a unique cyanobacterium that has no thylakoid membranes, and its photosynthesis takes place in the cytoplasmic membranes similarly as that seen in anoxygenic photosynthetic bacteria (Guglielmi et al., 1981). Molecular phylogenetic analyses showed that Gloeobacter branched off from the main cyanobacterial tree at an early stage of evolution (Nelissen et al., 1995). Therefore, Gloeobacter is considered as an evolutionary primordial cyanobacterium. Unlike other cyanobacteria, the Gloeobacter PSI did not exhibit characteristic fluorescence peaks at around 723 or 730 nm in fluorescence-emission spectra both in vivo (Koenig and Schmidt, 1995) and in vitro (Mimuro et al., 2010). These observations lead to an attractive notion that Chls absent in the structure of the Gloeobacter PSI are plausible candidates for Chls fluorescing at around 723 and 730 nm observed in other cyanobacteria.
To reveal the locations of characteristic low-energy Chls in PSI from Synechocystis and thermophilic cyanobacteria as well as the possible structural differences among PSI of Gloeobacter and other cyanobacteria, we solved the structure of the PSI trimer isolated from Gloeobacter using cryo-electron microscopy (cryo-EM) at a resolution of 2.04 Å. The structure obtained showed the absence of some subunits commonly found in other cyanobacteria, confirming the primordial nature of this cyanobacterium. By comparing the Gloeobacter PSI structure with the structures of Synechocystis and thermophilic cyanobacteria, we propose that one Chl dimer and one trimer, which are absent in the Gloeobacter PSI, are responsible for the characteristic fluorescence peaks at around 723 and 730 nm observed in other cyanobacteria.
Results and discussion
Overall structure of the PSI trimer
The PSI trimers were purified from Gloeobacter, and its biochemical characterization was summarized in Appendix 1—figure 1, which showed that the sample is very pure and suitable for structural analysis. Cryo-EM images of the PSI trimer were obtained by a JEOL CRYO ARM 300 electron microscope operated at 300 kV. After processing of the images with RELION (Appendix 1—figure 2 and Appendix 1—table 1), the final cryo-EM map with a C3 symmetry enforced was determined at a resolution of 2.04 Å, based on the ‘gold standard’ Fourier shell correlation (FSC) = 0.143 criterion (Figure 1A and Appendix 1—figure 2). This resolution is the highest for the structure of PSI cores ever determined by X-ray crystallography and single-particle cryo-EM so far (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). This was realized by imaging with a cold-field emission electron beam that produces superior high-resolution signals (Hamaguchi et al., 2019). The atomic model of PSI was built based on the 2.04 Å map (Figure 1B and Appendix 1—table 2), and most of the cofactors and amino acid residues were precisely assigned to this high-resolution map.
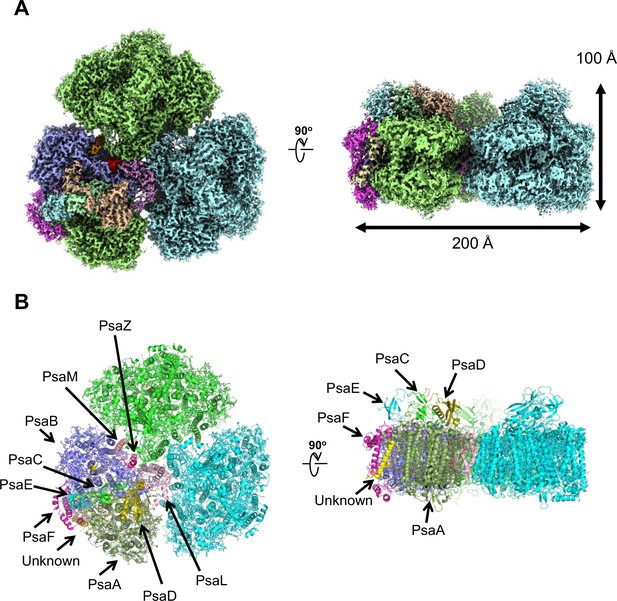
Overall structure of the Gloeobacter photosystem I (PSI) trimer.
(A) Cryo-electron microscopy (cryo-EM) density of the PSI trimer at 2.04 Å resolution. (B) Structural model of the PSI trimer. Views are from the top of the cytosolic side (left) and side of the membrane (right) for both panels (A and B).
The components of the electron-transfer chain are assigned unambiguously, which consists of a special pair Chls P700, accessory Chl Acc, primary electron acceptor A0, secondary electron acceptor A1 (menaquinone-4), and three iron–sulfur clusters FX/FA/FB (Figure 2A). The densities for the Mg atoms in the Chl molecules are clearly visualized at their centers (Figure 2B–E). In addition, the characteristic structures of Chl a′ and Chl a that constitute the pair of P700 are distinguished clearly in the high-resolution map (red arrows in Figure 2B, C, respectively). The densities of individual heavy atoms in the iron–sulfur clusters are also clearly separated, allowing their precise assignment possible (Figure 2G–I). On the other hand, menaquinone-4 is assigned here (Figure 2F) because it has been detected in the Gloeobacter PSI core by HPLC (Mimuro et al., 2005), although it is difficult to distinguish between menaquinone-4 and phylloquinone at the present resolution of the cryo-EM map due to the same length of their prenyl chain. The electron-transfer chain is arranged in two branches approximately symmetrically, and no significant differences between the branches are found. Thus, it cannot be determined if the electrons are transferred through both branches or proceed preferentially through one branch based on the structure obtained.
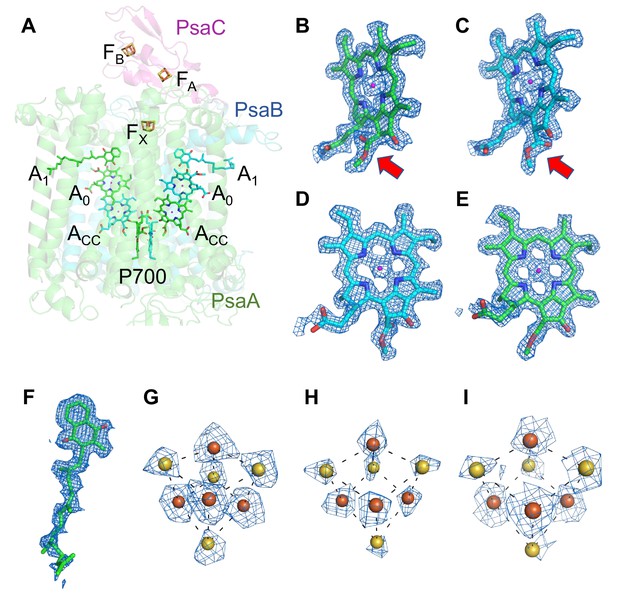
Cofactors involved in the electron-transfer reaction of the Gloeobacter photosystem I (PSI).
(A) Arrangement of cofactors involved in the electron-transfer reaction. P700, special pair Chls; Acc, accessory Chl; A0, primary electron acceptor; A1, secondary electron acceptor menaquinone-4; FX, FA, and FB, iron–sulfur clusters. P700 serves as a primary electron donor, and the electron produced is transferred to A1 via A0 (and ACC) in each branch, followed by sequential transfer to FX → FA → FB. However, whether the electrons are transferred through both branches or proceed preferentially through one branch is still under debate. (B–I) The cryo-electron microscopy (cryo-EM) density maps of cofactors and their refined models. (B) Chl a′ in P700; (C) Chl a in P700; (D) Acc; (E) A0; (F) A1; (G–I), FX, FA, and FB. Red arrows indicate structural differences between Chl a and Chl a′ (B, C). The densities for Chls, quinone, and iron–sulfur clusters were depicted at 5 σ, 3 σ, and 15 σ, respectively.
Subunit structures of the PSI monomer
PSI of Gloeobacter is a homo-trimeric complex, and its overall architecture is similar to those of the PSI trimer isolated from other cyanobacteria (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). Each monomer of the Gloeobacter PSI contains 10 subunits (Figure 1B), 9 of which are found in the genome of Gloeobacter (psaA, psaB, psaC, psaD, psaE, psaF, psaL, psaM, and psaZ) (Nakamura et al., 2003). PsaZ is positioned at the same location of PsaI in the PSI structure of Synechocystis (Malavath et al., 2018), although the Gloeobacter PsaZ has low sequence identity (20.0%) with the Synechocystis PsaI (Appendix 1—figure 3A, B; Inoue et al., 2004). The other eight subunits are located at similar positions of PSI from other cyanobacteria (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). An additional subunit is found at the position of PsaJ, which was modeled as polyalanines (Appendix 1—figure 3C), because the psaJ gene is not found in the Gloeobacter genome (Nakamura et al., 2003) but a subunit is clearly visible in the map at the same position as PsaJ (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). This subunit is named Unknown in the Gloeobacter structure. The cryo-EM map of Gloeobacter PSI indicates the absence of PsaG, PsaH, PsaK, and PsaX found in other PSIs, consistent with the absence of their genes in the Gloeobacter genome (Nakamura et al., 2003). While PsaG and PsaH are also absent in other cyanobacteria and appear in the green-lineage PSI cores, PsaK and PsaX are found in other cyanobacterial PSI structures (Suga and Shen, 2020; Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). The absence of these subunits in the Gloeobacter PSI suggests that the PSI core from Gloeobacter is in an early stage of evolution before PsaK and PsaX are incorporated into PSI, and is consistent with the primordial nature of this cyanobacterium during the evolutionary process.
The root mean square deviations (RMSDs) between the monomer unit of Gloeobacter PSI and that of cyanobacterial PSI with the same set of subunits from T. elongatus (PDB: 1JB0) (Jordan et al., 2001), Synechocystis (PDB: 5OY0) (Malavath et al., 2018), Thermosynechococcus vulcanus (PDB: 6K33) (Akita et al., 2020), Halomicronema hongdechloris (PDB: 6KMW) (Kato et al., 2020), and Acaryochloris marina MBIC11017 (PDB: 7COY) (Hamaguchi et al., 2021), are 0.85 Å for 2,008 Cα atoms, 0.82 Å for 2,019 Cα atoms, 0.90 Å for 2,011 Cα atoms, 0.88 Å for 1,785 Cα atoms, and 1.10 Å for 1,896 Cα atoms, respectively. This suggests that the overall structures of PSI are largely similar among the cyanobacteria, if we do not include some subunits that are absent or different between different cyanobacteria. However, some regions of the structure of Gloeobacter PSI display significant differences compared with other cyanobacterial PSI (Figure 3 and Appendix 1—figures 4–6). The Gloeobacter PSI possesses four types of characteristic loop structures which are named Loop1 (Tyr515–Gln529) and Loop2 (Asn652–Ser665) in PsaA (Appendix 1—figure 4), Loop3 (Pro717–Ile727) in PsaB (Appendix 1—figure 5), and Loop4 (Gln31–Asp36) in PsaF (Appendix 1—figure 6). These four types of loops do not exist in other cyanobacterial PSI trimers (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021). In contrast, the motif of Pro237–Gln248 in the Synechocystis PsaB is absent in the Gloeobacter PSI (Appendix 1—figure 5). All these insertions and deletions are located at the periplasmic side of the PSI monomer (Figure 3A).
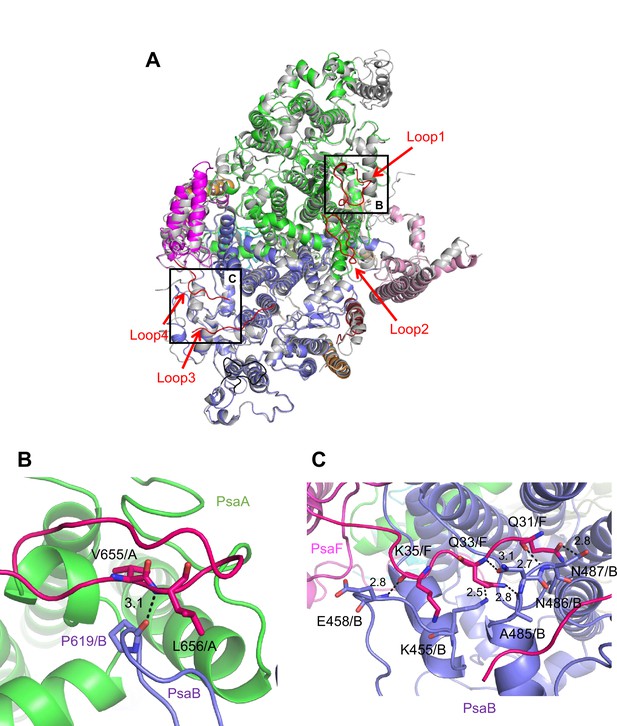
Characteristic structure of the Gloeobacter photosystem I (PSI).
(A) Superposition of the structure of Gloeobacter PSI (colored) with that of the Synechocystis PSI (gray) (PDB: 5OY0) viewed along the membrane normal from the periplasmic side. Structural differences of loop insertions are colored in red. The RMSD is 0.82 Å for 2,019 Cα atoms from subunits observed commonly between Gloeobacter and Synechocystis PSIs. (B, C) Closeup views of the loop insertions in the regions of Asn652–Ser665 of PsaA (Loop2) in the Gloeobacter PSI (B) and of Gln31–Asp36 of PsaF (Loop4) in the Gloeobacter PSI (C).
Both Loop1 of PsaA and Loop3 of PsaB do not interact with other subunits, whereas Loop2 of PsaA and Loop4 of PsaF are elongated so that both interact with PsaB. In Loop2, Leu656 of PsaA is hydrogen bonded with Pro619 of PsaB at a distance of 3.1 Å, and Val655 of PsaA is in hydrophobic interactions with PsaB-Pro619 (Figure 3B). In Loop4, Gln31, Gln33, and Lys35 of PsaF interact with Lys455, Glu458, Ala485, Asn486, and Asn487 of PsaB at distances of 2.5–3.1 Å (Figure 3C). Since our Gloeobacter PSI structure is solved at a high resolution, these structural differences cannot be ascribed to uncertainties due to lower resolutions, and the structural features of Gloeobacter PSI appear to contribute to the stability and assembly of the PSI complex in the membranes lacking thylakoids.
Cofactors of the PSI monomer
The cofactors identified in the monomer unit of Gloeobacter PSI are summarized in Appendix 1—table 2. There are 89 Chls a, 20 β-carotenes, 3 [4Fe–4S] clusters, 2 menaquinone-4s, and 4 lipid molecules in each monomer. The location of these molecules is similar to that in other cyanobacterial PSI structures (Jordan et al., 2001; Malavath et al., 2018; Kato et al., 2020; Gisriel et al., 2020; Hamaguchi et al., 2021; Xu et al., 2021); however, six Chls are less in the Gloeobacter PSI than those in the T. vulcanus PSI (Akita et al., 2020) and Synechocystis PSI (Malavath et al., 2018). These Chls include one Chl in PsaA (Chl1A), one Chl in PsaF (Chl1F), two Chls in PsaJ (Chl1J, 2J), and two Chls in PsaK (Chl1K, 2K) (Figure 4A, B). Both Chl1K and Chl2K do not exist in the Gloeobacter PSI because of the absence of PsaK in the genome and the structure. Both Chl1J and Chl2J are located in the periphery of PsaJ, whose amino acid residues cannot be assigned in the Gloeobacter PSI structure. The map quality around Chl1F in the Gloeobacter PSI is very high (Figure 4C), indicating that Chl1F is absent in the Gloeobacter PSI. Chl1A is difficult to bind to the Gloeobacter PSI, because the conserved His residue near Chl1A in other cyanobacteria is changed to Phe243 in the Gloeobacter PSI (Appendix 1—figure 4 and Appendix 1—figure 7A). This causes a steric hindrance between Phe243 and Chl1A in the Gloeobacter PSI (Figure 4D, Appendix 1—figure 7A), making the Chl molecule unable to bind. In addition, it is interesting to note that both T. vulcanus and H. hongdechloris PSIs possess an extra Chl (Chl1B) in PsaB (Kato et al., 2020; Akita et al., 2020), which is absent in both Gloeobacter PSI and Synechocystis PSI (Malavath et al., 2018). This is due to the changes in the loop structures (Figure 4E, Appendix 1—figure 5), as suggested by Toporik et al., 2020.
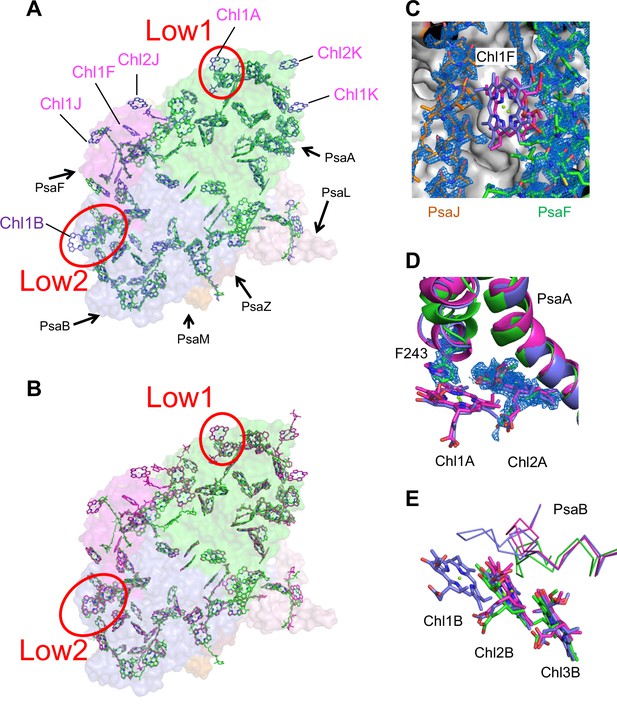
Comparison of pigments among the three types of photosystem I (PSIs).
(A, B) Superposition of the PSI-monomer structures viewed along the membrane normal from the periplasmic side, with protein subunits depicted in a surface model. (A) Gloeobacter (green) vs. T. vulcanus (purple) (PDB: 6K33); (B) Gloeobacter (green) vs. Synechocystis (magenta) (PDB: 5OY0). Red circles stand for the sites of Low1 and Low2. Closeup views of the environments near pigments of Chl1F (C) and Chl1A (D) with their densities depicted at 1.5 σ, and Chl1B (E), in the three species.
Identification of Chl clusters absent in the Gloeobacter PSI
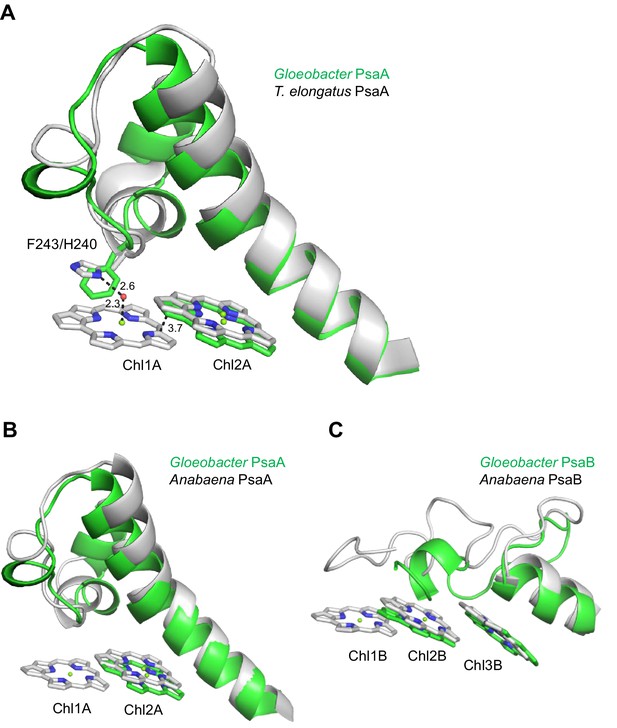
The energy levels of Chls tend to be lowered by the formation of dimeric or trimeric Chls; therefore, we focused on dimeric or trimeric Chl clusters through the structural comparisons. Among the six Chl molecules that are absent in the Gloeobacter PSI mentioned above, Chl1A forms a dimer with Chl2A in both Synechocystis PSI (Figure 5A) and T. vulcanus PSI (Figure 5B). Thus, this Chl dimer may represent one of the low-energy Chls in most cyanobacteria other than Gloeobacter. The Mg atom of Chl1A is coordinated by a water molecule in the PSI structures of T. elongatus (Jordan et al., 2001) and H. hongdechloris (Kato et al., 2020). In T. elongatus, this water molecule is hydrogen bonded to a neighboring His240 (Appendix 1—figure 7A), indicating that this hydrogen bond contributes to the stability of Chl1A. Sequence alignment showed that this His240 is conserved among most cyanobacteria (Appendix 1—figure 4); however, it is changed to Phe243 in the Gloeobacter PsaA (Appendix 1—figure 4 and Appendix 1—figure 7A). As mentioned above, this change leads to the loss of the water molecule that can coordinate the Chl molecule (Appendix 1—figure 7A). Thus, both the His residue and water molecule are responsible for the binding of Chl1A. Here, we name the Chl dimer of Chl1A and Chl2A found in the structure of most cyanobacterial PSI as Low1 (Figure 4A, B).
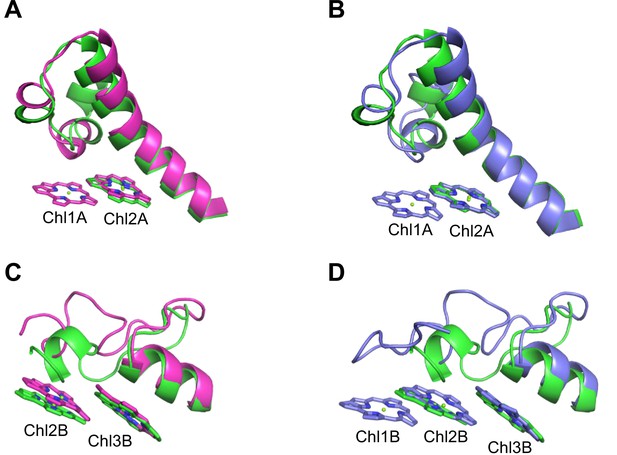
Closeup views of Low1 and Low2.
(A) Superposition of the Low1 site in the Gloeobacter photosystem I (PSI; green) and Synechocystis PSI (magenta) (PDB: 5OY0). (B) Superposition of the Low1 site in the Gloeobacter PSI (green) and T. vulcanus PSI (purple) (PDB: 6K33). (C) Superposition of the Low2 site in the Gloeobacter PSI (green) and Synechocystis PSI (magenta). (D) Superposition of the Low2 site in the Gloeobacter PSI (green) and T. vulcanus PSI (purple).
Further structural comparisons of T. vulcanus PSI with Gloeobacter PSI and Synechocystis PSI show a trimeric Chl cluster of Chl1B/Chl2B/Chl3B present in the T. vulcanus PSI but absent in the Gloeobacter and Synechocystis PSIs. The difference is due to the absence of Chl1B in the latter two cyanobacteria (Figure 5C, D). Therefore, this Chl trimer is also a probable candidate for low-energy Chls, which is supported by the spectroscopic analyses of PSI trimers (Schlodder et al., 2007) and the cryo-EM structure of PSI mutant complexes (Toporik et al., 2020). Based on these findings, we name the Chl trimer of Chl1B/Chl2B/Chl3B as Low2 (Figure 4A, B).
Correlation of Low1 and Low2 with characteristic fluorescence bands
Our interest is how Low1 and Low2 identified here are related to Chls fluorescing at around 723 and 730 nm observed in the steady-state fluorescence-emission spectra (Appendix 1—figure 8A; van der Lee et al., 1993; Mimuro et al., 2010; Turconi et al., 1996; Pålsson et al., 1996). The spectrum of Synechocystis PSI (red line in Appendix 1—figure 8A) exhibits a fluorescence peak at around 723 nm, whereas that of T. vulcanus PSI (blue line in Appendix 1—figure 8A) displays a fluorescence peak at around 731 nm. In addition, the width of the fluorescence peak from the T. vulcanus PSI is broader than that from the Synechocystis PSI; therefore, it seems highly probable that the T. vulcanus PSI also possesses Chls fluorescing at around 723 nm in addition to those at around 730 nm. In contrast, the fluorescence spectrum of Gloeobacter PSI (black line in Appendix 1—figure 8A) exhibits a maximum peak at around 694 nm, which is significantly defferent from the fluorescence peaks at around 723 and 730 nm. The characteristic differences of fluorescence peaks in these cyanobacteria can even be observed in the absorption spectra measured at 77 K, which exhibit different intensities of relative absorbance over 700 nm (Appendix 1—figure 8B).
Based on these structural and fluorescence properties, it is proposed that Low1 and Low2 are Chls fluorescing at around 723 and 730 nm, respectively. In a previous study, Schlodder et al., 2007 listed nine groups of Chls as potential candidates for low-energy Chls in PSI based on their spectroscopic studies. Eight of the nine groups of Chls are well conserved in the PSI structures among the three cyanobacteria (Appendix 1—figure 9). Therefore, it is highly possible that these eight Chl groups are not Chls fluorescing at around 723 or 730 nm. In contrast, the remaining Chl group (Schlodder et al., 2007) is a triply stacked Chls, B1231/B1232/B1233 (the Chl numbering taken from Jordan et al., 2001), which is consistent with Low2 presented here. These observations support that the Chl cluster of Chl1B/Chl2B/Chl3B is Low2 which is present in T. vulcanus and T. elongatus but absent in Synechocystis and Gloeobacter.
In the previous study, the Low1 site was not listed as potential low-energy Chls (Schlodder et al., 2007). This may be due to the large number of Chls in the PSI core, resulting in difficulties in identifying individual low-energy Chls using spectroscopic methods. Our structure clearly indicates that the Low1 site is absent in the Gloeobacter PSI but present in other cyanobacteria, leading to the idea that Low1 is also a low-energy Chl site responsible for the long-fluorescence emission observed in other cyanobacteria but not in Gloeobacter.
Some cyanobacteria have tetrameric PSI complexes whose structures have been solved from the cyanobacterium Anabaena sp. PCC 7120 (hereafter referred to as Anabaena) (Kato et al., 2019; Zheng et al., 2019; Chen et al., 2020). Both Low1 and Low2 exist in the monomer units of the Anabaena PSI tetramer (Appendix 1—figure 7B, C). The fluorescence spectrum of Anabaena PSI tetramer showed a peak at around 730 nm under the liquid-nitrogen condition (Kato et al., 2019; Nagao et al., 2020b), which is similar to the spectrum of the T. vulcanus PSI trimer. These observations are in line with our proposal that Low1 and Low2 are responsible for Chls fluorescing at around 723 and 730 nm, respectively, in most cyanobacterial PSI monomers other than Gloeobacter, irrespective of the oligomeric states of PSI. However, it should be noted that the energy levels of Chls cannot be assigned only by the structural analysis of PSI. Further mutagenesis studies and theoretical calculations will be required for understanding the correlation of Low1 and Low2 with the fluorescence peaks at around 723 and 730 nm.
Low-energy Chls in interfaces among PSI-monomer units
Low-energy Chls including Chls fluorescing at around 730 nm have been thought to be located in interfaces among monomers in a PSI trimer from T. elongatus (El-Mohsnawy et al., 2010; Çoruh et al., 2021). The PSI monomer isolated from T. elongatus exhibited a fluorescence peak shifted to about 720 nm compared with a fluorescence peak at around 730 nm in the PSI trimer (Çoruh et al., 2021). However, the structure of PSI monomer displayed the lack of a Chl of B1233 at the Low2 site, in addition to several peripheral Chls (Çoruh et al., 2021). Thus, the fluorescence-peak shift observed in the T. elongatus PSI monomer may be due to the absence of Low2 but not Chls located in interfaces around the PSI-monomer units.
As for other cyanobacteria, Anabaena has both Low1 and Low2, while the fluorescence peak of PSI monomer at around 730 nm was virtually identical to that of PSI tetramer (Kato et al., 2019; Nagao et al., 2020b). Moreover, Synechocystis has Low1 without Low2, while the fluorescence peak of PSI monomer was consistent with that of PSI trimer (Turconi et al., 1996). These observations support the idea that Low1 and Low2 are responsible for Chls fluorescing at around 723 and 730 nm, respectively, and that these long-wavelength fluorescing Chls are not identical to Chls found in the interfaces among monomers, irrespective of the oligomeric states of PSI.
Evolution of Low1 and Low2 in oxyphototrophs
The overall structures of PSI supercomplexes in complex with light-harvesting complexes I have been solved from a red alga (Pi et al., 2018; Antoshvili et al., 2019), green algae (Su et al., 2019; Suga et al., 2019; Qin et al., 2019; Perez-Boerema et al., 2020), a diatom (Nagao et al., 2020a; Xu et al., 2020), a moss (Yan et al., 2021), and a higher plant (Qin et al., 2015; Mazor et al., 2017). Compared with the structure of Gloeobacter PSI (Figure 6A), Low1 is present in the PSI cores from a red alga Cyanidioschyzon merolae (PDB: 5ZGB), a green alga Chlamydomonas reinhardtii (PDB: 6JO5), a diatom Chaetoceros gracilis (PDB: 6L4U), a moss Physcomitrella patens (PDB: 6L35), and a higher plant Pisum sativum (PDB: 4XK8). The His residue corresponding to PsaA-His240 in T. elongatus is also conserved among the eukaryotes (Figure 6B), reflecting the presence of Low1 in eukaryotes.
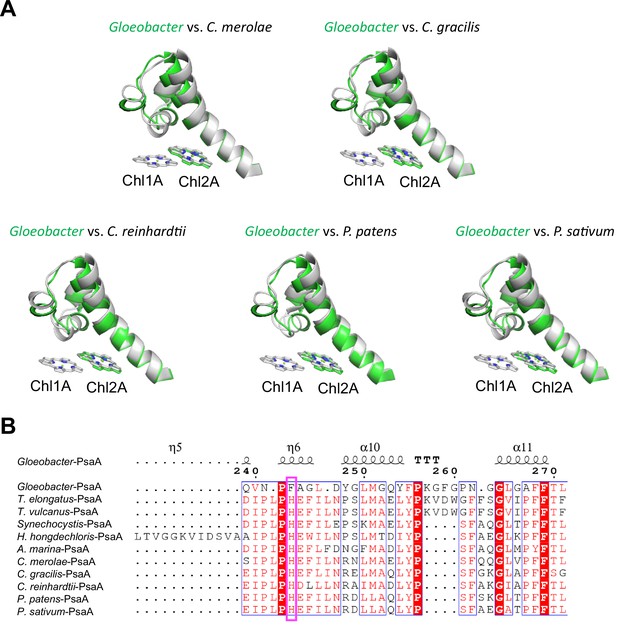
Structural comparisons of the Low1 site among oxyphototrophs.
(A) Superposition of the Low1 site of the Gloeobacter photosystem I (PSI; green) with PSI complexes of photosynthetic eukaryotes (gray): Cyanidioschyzon merolae (PDB: 5ZGB), Chaetoceros gracilis (PDB: 6L4U), Chlamydomonas reinhardtii (PDB: 6JO5), Physcomitrella patens (PDB: 6L35), and Pisum sativum (PDB: 4XK8). (B) Multiple sequence alignment of PsaA among oxyphototrophs using ClustalW and ESPript. The species shown are Gloeobacter violaceus PCC 7421, Thermosynechococcus elongatus BP-1, Thermosynechococcus vulcanus NIES-2134, Synechocystis sp. PCC 6803, Halomicronema hongdechloris C2206, Acaryochloris marina MBIC11017, C. merolae, C. gracilis, C. reinhardtii, P. patens, and P. sativum. The pink box displays the histidine residue involved in the binding of Chl1A.
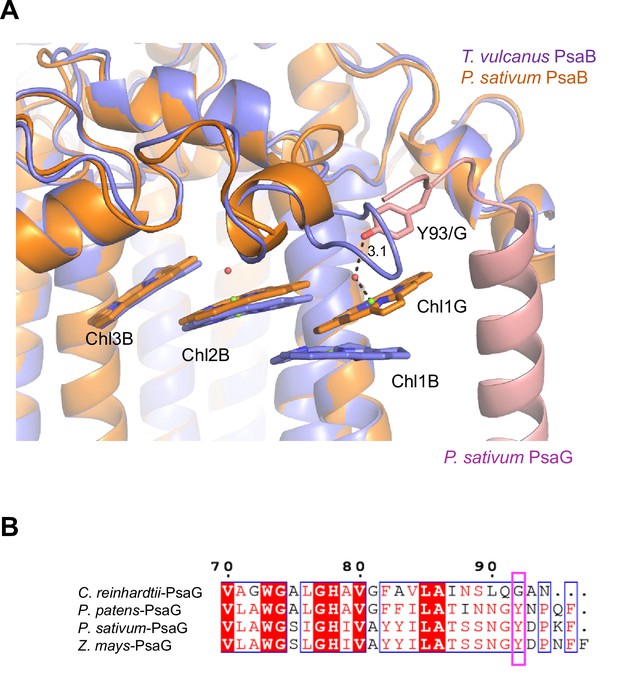
Low2 exists in PSIs of P. patens and P. sativum in addition to T. vulcanus (Figure 7A); however, the triple stacking interactions among these three Chls, especially the orientation of Chl1B and Chl1G, differ between cyanobacteria and the eukaryotes (Figure 7B). This is likely due to the different loop structures interacting with Chl1B between T. vulcanus and the eukaryotes (Figure 7B, C). In the P. sativum PSI, Chl1G corresponding to Chl1B is bound to Tyr93 of PsaG via a water molecule (Appendix 1—figure 10A). PsaG is absent in Gloeobacter but present in C. reinhardtii; however, the characteristic Tyr residue is changed to Gly in C. reinhardtii (Appendix 1—figure 10B), leading to the loss of this water molecule in C. reinhardtii. This suggests that the hydrogen-bond interaction between the water molecule and Tyr93 of PsaG is required for the formation of Low2 in eukaryotes. In contrast, the gene of psaG is not found in the red alga and diatom, resulting in the absence of Low2 in these organisms.
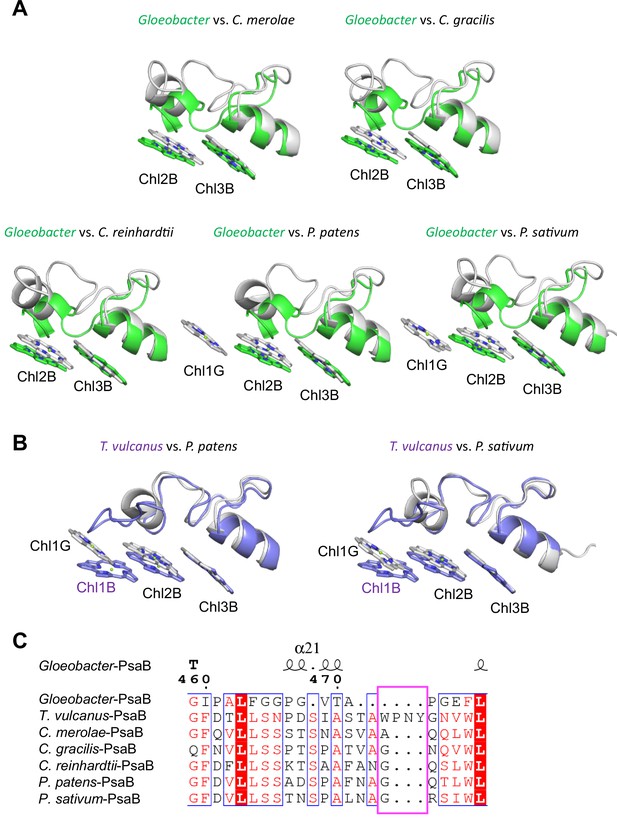
Structural comparisons of the Low2 site among oxyphototrophs.
(A) Superposition of the Low2 site of the Gloeobacter photosystem I (PSI; green) with PSI complexes of photosynthetic eukaryotes (gray): Cyanidioschyzon merolae (PDB: 5ZGB), Chaetoceros gracilis (PDB: 6L4U), Chlamydomonas reinhardtii (PDB: 6JO5), Physcomitrella patens (PDB: 6L35), and Pisum sativum (PDB: 4XK8). (B) Superposition of the Low2 site of T. vulcanus PSI (purple) (PDB: 6K33) with PSI complexes (gray) of P. patens and P. sativum. (C) Multiple sequence alignment of PsaB using ClustalW and ESPript. The pink box indicates the loop involved in the binding of Chl1B in T. vulcanus.
Conclusions
This study reveals the high-resolution structure of the PSI trimer from Gloeobacter at 2.04 Å by cryo-EM. Structural comparisons of the Gloeobacter PSI with the Synechocystis and T. vulcanus PSIs provide evidence for the locations of two types of characteristic Chl clusters, Low1 and Low2, present in other cyanobacteria but absent in Gloeobacter. Based on the structural and fluorescence properties, Low1 and Low2 may be responsible for Chls fluorescing at around 723 and 730 nm, respectively, in most cyanobacteria. In particular, Low1 is conserved in most oxyphototrophs from prokaryotes to eukaryotes other than Gloeobacter, implying that the lack of Low1 is a characteristic of primordial cyanobacteria occurring near the origin of oxyphototrophs.
Materials and methods
Purification and characterization of the PSI trimer from Gloeobacter
Request a detailed protocolThe cyanobacterium G. violaceus PCC 7421 was grown in BG11 medium (Stanier et al., 1971) supplemented with 10 mM 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES)–KOH (pH 8.0) and 1/1000 volume of KW21 (Daiichi Seimo) at a photosynthetic photon flux density (PPFD) of 5 µmol photons m−2 s−1 at 20°C with bubbling of air containing 3% (vol/vol) CO2. KW21 is helpful for enhancing the growth of photosynthetic organisms (Nagao et al., 2007; Nagao et al., 2013a). Cytoplasmic membranes were prepared after disruption of the cells with glass beads with a method similar to the preparation of thylakoid membranes as described previously (Nagao et al., 2017), and suspended in a buffer containing 0.2 M trehalose, 20 mM 2-morpholinoethanesulfonic acid (MES)–NaOH (pH 6.5), 5 mM CaCl2, and 10 mM MgCl2 (buffer A). The membranes were solubilized with 1% (wt/vol) n-dodecyl-β-D-maltoside (β-DDM) at a Chl concentration of 0.25 mg ml−1 for 30 min on ice in the dark with gentle stirring. After centrifugation at 50,000 × g for 30 min at 4°C, the resultant supernatant was loaded onto a Q-Sepharose anion-exchange column (2.5 cm of inner diameter and 6 cm of length) equilibrated with buffer A containing 0.03% β-DDM (buffer B). The column was washed with buffer B containing 100 mM NaCl (buffer C) until the eluate became colorless, and further washed with 60 ml of buffer B containing 150 mM NaCl. The PSI fraction was eluted with buffer B containing 200 mM NaCl, and subsequently loaded onto a linear trehalose gradient of 10–40% (wt/vol) in a medium containing 20 mM MES–NaOH (pH 6.5), 5 mM CaCl2, 10 mM MgCl2, 100 mM NaCl, and 0.03% β-DDM. After centrifugation at 154,000 × g for 18 hr at 4°C (P40ST rotor; Hitachi), a band containing the PSI trimers was collected and then concentrated using a 150 kDa cutoff filter (Apollo; Orbital Biosciences) at 4,000 × g. The concentrated PSI-trimer complexes were stored in liquid nitrogen until use.
Subunit composition of the PSI trimer was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis containing 16% acrylamide and 7.5 M urea according to a method (Ikeuchi and Inoue, 1988; Appendix 1—figure 1A, Source data 1). The samples (5 µg of Chl) were solubilized by 3% lithium lauryl sulfate and 75 mM dithiothreitol for 10 min at 60°C, and loaded onto the gel. A standard molecular weight marker (SP-0110; APRO Science) was used. The subunit bands were assigned by mass spectrometry according to a previous method (Nagao et al., 2019). Pigment compositions were analyzed as described in Nagao et al., 2013b, and the elution profile was monitored at 440 nm (Appendix 1—figure 1B).
Purification of the PSI trimer from Synechocystis
Request a detailed protocolThe cyanobacterium Synechocystis sp. PCC 6803 47H strain was grown in the BG11 medium supplemented with 10 mM HEPES–KOH (pH 8.0) at the PPFD of 30 µmol photons m−2 s−1 at 30°C with bubbling of air containing 3% (vol/vol) CO2. The 47H strain has a six-histidine tag at the C-terminus of the CP47 subunit (Ito and Tanaka, 2011). Thylakoid membranes were prepared as described previously (Nagao et al., 2017) and suspended in buffer A. The thylakoids were solubilized with 1% β-DDM at a Chl concentration of 0.50 mg ml−1 for 30 min on ice in the dark with gentle stirring. After centrifugation at 50,000 × g for 10 min at 4°C, the resultant supernatant was loaded onto a Ni2+ affinity column (2.5 cm of inner diameter and 10 cm of length) equilibrated with buffer C. The PSI-enriched fraction was collected by washing the column with buffer C, and subsequently diluted with an equal volume of buffer B. The diluted sample was applied onto a Q-Sepharose anion-exchange column (2.5 cm of inner diameter and 10 cm of length) equilibrated with buffer B. The column was washed with buffer B containing 150 mM NaCl until the eluate became colorless, and further washed with 50 ml of buffer B containing 200 mM NaCl and subsequently with 100 ml of buffer B containing 250 mM NaCl. The PSI fraction was eluted with buffer B containing 300 mM NaCl, and subsequently loaded onto a linear trehalose gradient of 10–40% (wt/vol) in a medium containing 20 mM MES–NaOH (pH 6.5), 5 mM CaCl2, 10 mM MgCl2, 100 mM NaCl, and 0.03% β-DDM. After centrifugation at 154,000 × g for 18 hr at 4°C (P40ST rotor; Hitachi), a band containing the PSI trimers was collected and then concentrated using a 150 kDa cutoff filter (Apollo; Orbital Biosciences) at 4,000 × g. The concentrated PSI-trimer complexes were stored in liquid nitrogen until use.
Purification of the PSI trimer from T. vulcanus
Request a detailed protocolThe cyanobacterium T. vulcanus NIES-2134 was grown and its thylakoid membranes were prepared as described previously (Kawakami and Shen, 2018). The PSI-enriched fraction was obtained as precipitation by centrifugation at 100,000 × g for 60 min at 4°C after second-round treatments of the thylakoids with N,N-dimethyldodecylamine-N-oxide (Kawakami and Shen, 2018), and suspended with a 30 mM MES–NaOH (pH 6.0) buffer containing 5% (wt/vol) glycerol, 3 mM CaCl2, and 0.03% β-DDM. The PSI fraction was loaded onto a linear trehalose gradient of 10–40% (wt/vol) in a medium containing 20 mM MES–NaOH (pH 6.5), 5 mM CaCl2, 10 mM MgCl2, 100 mM NaCl, and 0.03% β-DDM. After centrifugation at 154,000 × g for 18 hr at 4°C (P40ST rotor; Hitachi), a band containing the PSI trimers was collected and then concentrated using a 150 kDa cutoff filter (Apollo; Orbital Biosciences) at 4,000 × g. The concentrated PSI-trimer complexes were stored in liquid nitrogen until use.
Absorption and fluorescence-emission spectra at 77 K
Request a detailed protocolAbsorption spectra of the three types of PSI trimers were measured at 77 K using a spectrometer equipped with an integrating sphere unit (V-650/ISVC-747, JASCO) (Hamada et al., 2012), and their steady-state fluorescence-emission spectra were recorded at 77 K using a spectrofluorometer (FP-8300/PMU-183, JASCO). The excitation wavelength was set to 440 nm.
Cryo-EM data collection
Request a detailed protocolFor cryo-EM experiments, 3 μl aliquots of the Gloeobacter PSI trimers (1.68 mg Chl ml−1) were applied to Quantifoil R1.2/1.3, Cu 200 mesh grids pretreated by gold sputtering. Without waiting for incubation, the excess amount of the solution was blotted off for 5 s with a filter paper in an FEI Vitrobot Mark IV at 4°C under 100% humidity. The grids were plunged into liquid ethane cooled by liquid nitrogen and then transferred into a CRYO ARM 300 electron microscope (JEOL) equipped with a cold-field emission gun operated at 300 kV. Zero-energy loss images were recorded at a nominal magnification of ×60,000 on a direct electron detection camera (Gatan K3, AMETEK) with a nominal defocus range of −1.8 to −0.6 μm. One-pixel size corresponded to 0.823 Å. Each image stack was exposed at a dose rate of 17.555 e− Å−2 s−1 for 4.0 s, and consisted of dose-fractionated 50 movie frames. In total 7,282 image stacks were collected.
Cryo-EM image processing
Request a detailed protocolThe movie frames thus obtained were aligned and summed using MotionCor2 (Zheng et al., 2017) to yield dose weighted images. Estimation of the contrast transfer function (CTF) was performed using CTFFIND4 (Mindell and Grigorieff, 2003). All of the following processes were performed using RELION3.1 (Zivanov et al., 2020). In total 961,960 particles were automatically picked up and used for reference-free 2D classification. Then, 946,827 particles were selected from good 2D classes and subsequently subjected to 3D classification without imposing any symmetry. An initial model for the first 3D classification was generated de novo from 2D classifications. As shown in Appendix 1—figure 2, the final PSI-trimer structure was reconstructed from 261,743 particles. The overall resolution of the cryo-EM map was estimated to be 2.04 Å by the gold standard FSC curve with a cutoff value of 0.143 (Appendix 1—figure 2D; Grigorieff and Harrison, 2011). Local resolutions were calculated using RELION (Appendix 1—figure 2F).
Model building and refinement
Request a detailed protocolThe cryo-EM map thus obtained was used for the model building of the PSI trimer. Each subunit of the homology models constructed using the Phyre2 server (Kelley et al., 2015) was first manually fitted into the map by using UCSF Chimera (Pettersen et al., 2004), and then inspected and manually adjusted with Coot (Emsley et al., 2010). The complete PSI-trimer structure was refined with phenix.real_space_refine (Adams et al., 2010) and REFMAC5 (Murshudov et al., 2011) with geometric restraints for the protein-cofactor coordination. The final model was validated with MolProbity (Chen et al., 2010), EMRinger (Barad et al., 2015), and Q-score (Pintilie et al., 2020). The statistics for all data collection and structure refinement are summarized in Appendix 1—Tables 1 and 2. All structural figures were made by PyMOL (Schrödinger, 2015) and UCSF ChimeraX (Goddard et al., 2018).
Appendix 1
Supplementary Information
Biochemical characterization of the Gloeobacter PSI trimer.
(A) SDS-PAGE analysis of the PSI trimer. Protein bands were identified by mass spectrometry. It should be noted that some of the subunits in the PSI trimer were not detected by SDS-PAGE, probably due to their poor staining by CBB, but were visualized by the present cryo-EM map. (B) HPLC analysis of pigments extracted from the PSI trimer monitored at 440 nm. Letters of a and β-Car indicate Chl a and β-carotene, respectively. Data are representative of three independent experiments.
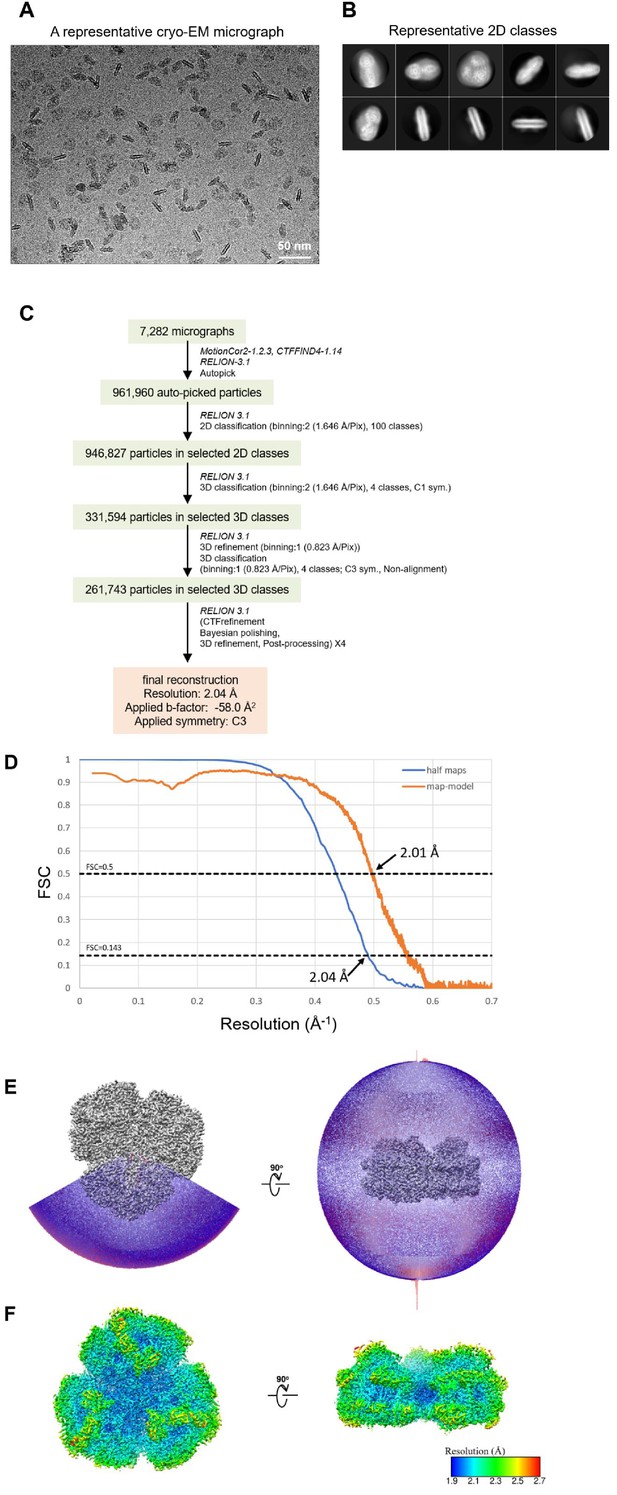
Cryo-EM data collection and processing of the Gloeobacter PSI trimer.
(A) Representative cryo-EM micrograph of the PSI trimer. (B) Representative 2D classes of the PSI trimer. The box size is 330 Å. (C) A flowchart showing the classification scheme of the PSI trimer. The overall structure of PSI was reconstructed at a 2.04-Å resolution from 261,743 particles. (D) FSC curves of the PSI trimer for independently refined half maps (half maps) and full map vs. model (map-model). (E) Angular distribution of the particles used for reconstruction of the PSI trimer. Each cylinder represents one view, and its height is proportional to the number of particles. (F) Local resolution map of the PSI trimer.
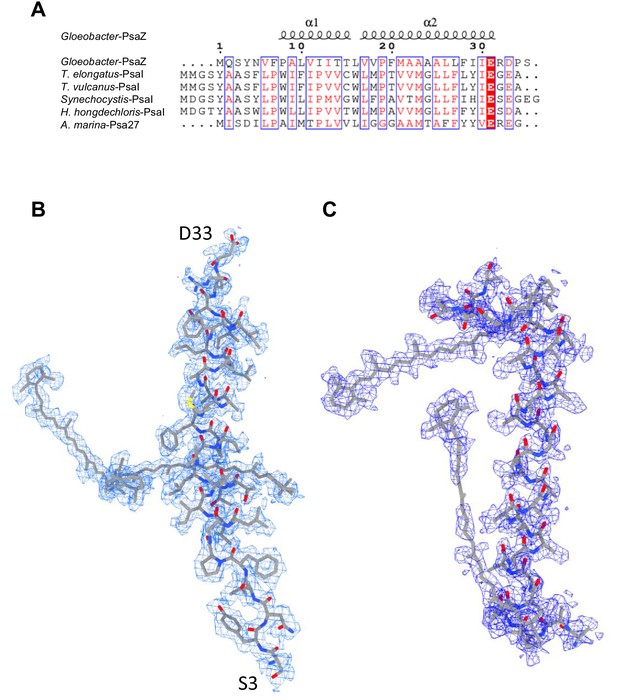
Identification of the Gloeobacter PSI subunits.
(A) Multiple sequence alignment (ClustalW and ESPript) of the Gloeobacter PsaZ and other cyanobacterial PsaI subunits. The species shown are Gloeobacter violaceus PCC 7421, Thermosynechococcus elongatus BP-1, Thermosynechococcus vulcanus NIES-2134, Synechocystis sp. PCC 6803, Halomicronema hongdechloris C2206, and Acaryochloris marina MBIC11017. (B) The cryo-EM density (blue meshes) of PsaZ and its corresponding model (gray sticks). (C) The cryo-EM density of the Unknown subunit (blue meshes) and its corresponding model (gray sticks).
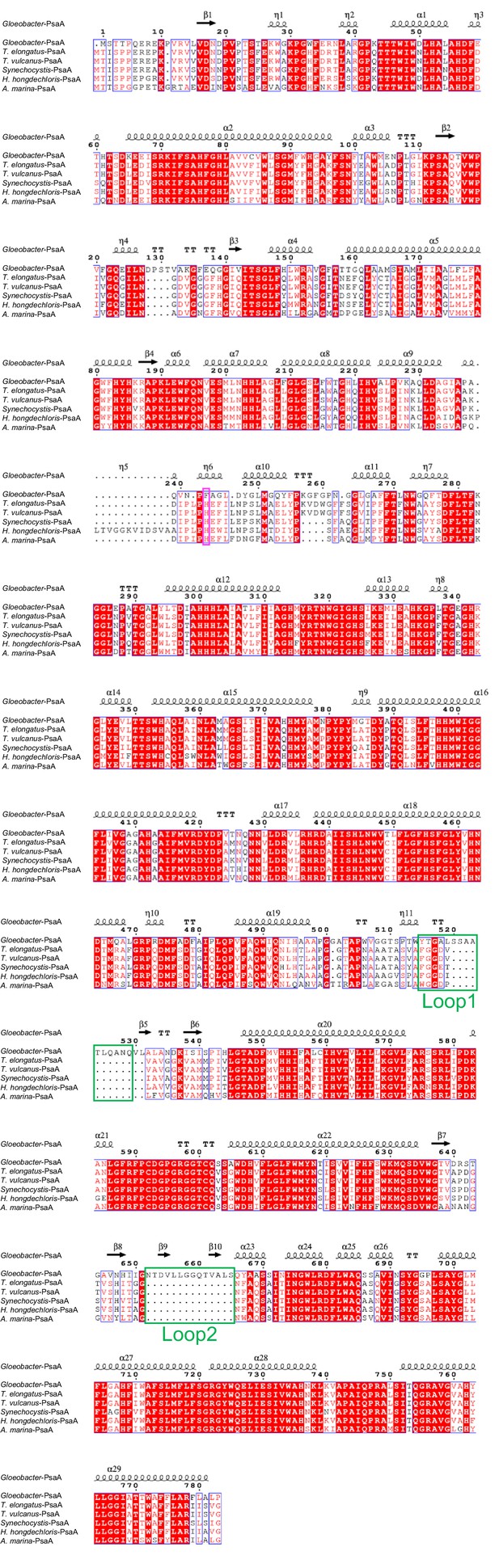
Comparison of the structurally known PsaA subunit among six species of cyanobacteria.
Multiple sequence alignment was carried out using ClustalW and ESPript. Secondary-structural elements are shown above the sequence. Completely conserved residues are highlighted in red. Loop1 (Tyr515-Gln529) and Loop2 (Asn652-Ser665) are labeled and indicated by green boxes. The pink box stands for the histidine residue involved in the binding of Chl1A. The species shown are Gloeobacter violaceus PCC 7421, Thermosynechococcus elongatus BP-1, Thermosynechococcus vulcanus NIES-2134, Synechocystis sp. PCC 6803, Halomicronema hongdechloris C2206, and Acaryochloris marina MBIC11017.
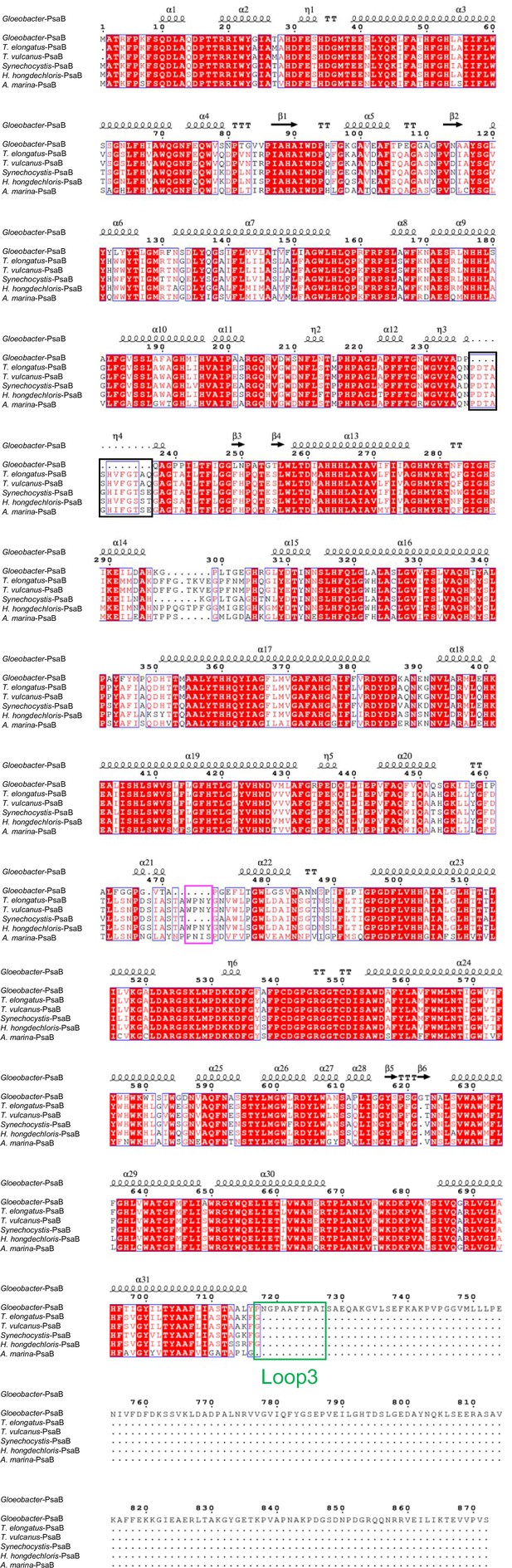
Comparison of the structurally known PsaB subunit among six species of cyanobacteria.
Multiple sequence alignment was carried out using ClustalW and ESPript. Secondary-structural elements are shown above the sequence. Completely conserved residues are highlighted in red. Loop3 (Pro717-Ile727) is labeled and indicated by a green box. The pink box indicates the loop involved in the binding of Chl1B. The black box stands for the deletion in the Gloeobacter PsaB. The species shown are Gloeobacter violaceus PCC 7421, Thermosynechococcus elongatus BP-1, Thermosynechococcus vulcanus NIES-2134, Synechocystis sp. PCC 6803, Halomicronema hongdechloris C2206, and Acaryochloris marina MBIC11017.
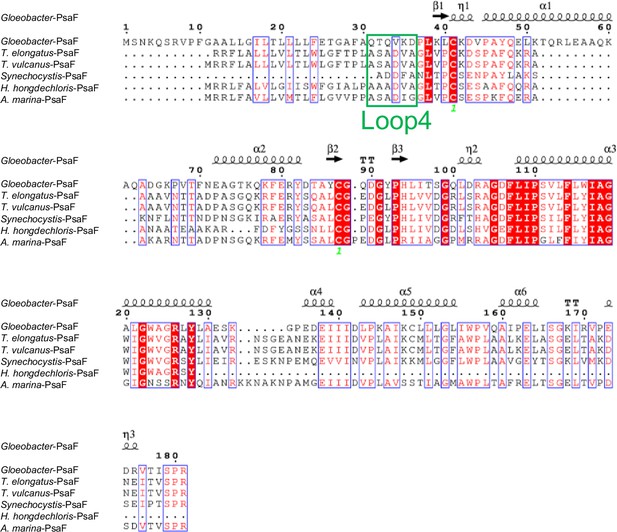
Comparison of the structurally known PsaF subunit among six species of cyanobacteria.
Multiple sequence alignment was carried out using ClustalW and ESPript. Secondary-structural elements are shown above the sequence. Completely conserved residues are highlighted in red. Loop4 (Gln31-Asp36) is labeled and indicated by a green box. The species shown are Gloeobacter violaceus PCC 7421, Thermosynechococcus elongatus BP-1, Thermosynechococcus vulcanus NIES-2134, Synechocystis sp. PCC 6803, Halomicronema hongdechloris C2206, and Acaryochloris marina MBIC11017.
Structural comparisons of the Low1 and Low2 sites in PsaA between Gloeobacter and other organisms.
(A) Superposition of the Low1 site in the Gloeobacter PSI (green) with that of the T. elongatus PSI (gray) (PDB: 1JB0). Phe243 of the Gloeobacter PsaA and His240 of the T. elongatus PsaA are shown in stick models. Dashed lines indicate interactions with distances labeled in Å. (B, C) Superposition of the Low1 (B) and Low2 (C) sites in the Gloeobacter PSI (green) with those in the Anabaena PSI (gray) (PDB: 6JEO).
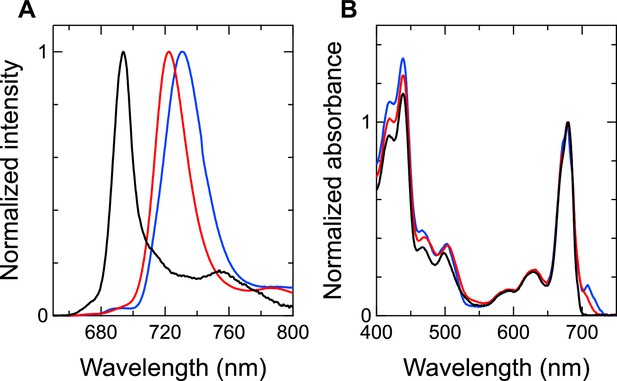
Fluorescence-emission and absorption spectra of the Gloeobacter PSI (black), Synechocystis PSI (red), and T. vulcanus PSI (blue).
(A) Fluorescence spectra were measured at 77 K and normalized by their maximum-peak intensities. (B) Absorption spectra were measured at 77 K and normalized by the Qy-peak intensities.
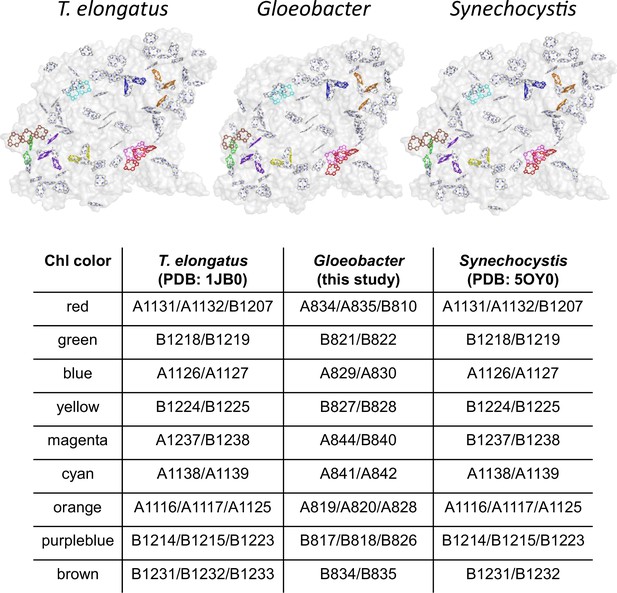
Possible low-energy Chls proposed by Schlodder et al., 2007.
Nine groups of Chls proposed as low-energy Chls were colored differently in the PSI structures of three cyanobacteria, whereas the other Chls and protein structures were colored gray. The nine groups were listed in the table, with the numbering of these Chls taken from each PDB data.
Structural comparisons of the Low2 site.
(A) Superposition of the Low2 site and its surrounding environment between the T. vulcanus PsaB (purple) (PDB: 6K33) and the P. sativum PsaB (orange) and PsaG (pink) (PDB: 4XK8). Tyr93 of the P. sativum PsaG involved in the binding of Chl1G is shown in a stick model. (B) Multiple sequence alignment of PsaG using ClustalW and ESPript. The species shown are Chlamydomonas reinhardtii, Physcomitrella patens, Pisum sativum, and Zea mays. The pink box displays the tyrosine residue involved in the binding of Chl1G.
Cryo-EM data collection and structural analysis statistics.
| Complex | PSI trimer |
|---|---|
| PDB ID | 7F4V |
| EMDB ID | EMD-31455 |
| Data collection and processing | |
| Magnification | 60,000 |
| Voltage (kV) | 300 |
| Electron exposure (e−/Å) | 70.22 |
| Defocus range (μm) | −1.8 to −0.6 |
| Pixel size (Å) | 0.823 |
| Symmetry imposed | C3 |
| Initial particle number | 961,960 |
| Final particle number | 261,743 |
| Map resolution (Å) | 2.04 |
| FSC threshold | 0.143 |
| Refinement | |
| Initial Model used (PDB code) | Homology modeling |
| Model resolution (Å) | 2.01 |
| FSC threshold | 0.5 |
| Map sharpening B factor (Å2) | −58.0 |
| Model composition (trimer) | |
| Non-hydrogen atoms | 67,641 |
| Protein | 49,899 |
| Ligand | 17,340 |
| Water | 402 |
| B factors (Å2) | |
| Protein | 39.2 |
| Ligand | 40.5 |
| Water | 27.3 |
| R.m.s deviations | |
| Bond lengths (Å) | 0.025 |
| Bond angles (°) | 2.45 |
| Validation | |
| MolProbity score | 1.78 |
| Clashscore | 4.77 |
| Poor rotamers (%) | 2.78 |
| EMRinger score | 6.48 |
| Ramachandran plot | |
| Favored (%) | 96.8 |
| Allowed (%) | 3.11 |
| Disallowed (%) | 0.09 |
Averaged Q-score in each subunit and cofactors in each monomer unit of the PSI core.
| Protein | Amino acid residues | Averaged Q-score | Chlorophylls | Carotenoids | Lipids | Others |
|---|---|---|---|---|---|---|
| PsaA | 11–782 | 0.85 | 43 Chl a 1 Chl a′ | 5 BCR | 2 LHG | 1 [4Fe-4S] cluster, 1 menaquinone-4 |
| PsaB | 3–727 | 0.87 | 41 Chl a | 7 BCR | 1 LMG 1 LHG | one menaquinone-4 |
| PsaC | 2–81 | 0.87 | - | - | - | 2 [4Fe-4S] cluster |
| PsaD | 11–143 | 0.81 | - | - | - | - |
| PsaE | 3–64 | 0.76 | - | - | - | - |
| PsaF | 31–181 | 0.76 | 1 Chl a | 1 BCR | - | - |
| PsaZ | 3–33 | 0.85 | - | 2 BCR | - | - |
| Unknown | 1–33 | 0.80 | - | 2 BCR | - | - |
| PsaL | 11–782 | 0.85 | 3 Chl a | 2 BCR | - | - |
| PsaM | 3–727 | 0.84 | - | 1 BCR | - | - |
| Total | 89 | 20 | 4 | 5 |
Data availability
The cryo-EM density map and atomic model have been deposited in the Electron Microscopy Data Bank and the Protein Data Bank (EMD ID code 31455 and PDB ID code 7F4V). Figs. 1-7 in the main text are made with these data by PyMOL and UCSF ChimeraX. The source data of Appendix 1—figure 1A is provided as a xlsx file, which is named Source Data 1.
-
Electron Microscopy Data BankID 31455. Cryo-EM Structure of a primordial cyanobacterial photosystem I.
-
RCSB Protein Data BankID 7F4V. Cryo-EM Structure of a primordial cyanobacterial photosystem I.
References
-
PHENIX: a comprehensive Python-based system for macromolecular structure solutionActa Crystallographica. Section D, Biological Crystallography 66:213–221.https://doi.org/10.1107/S0907444909052925
-
Structure-based calculations of optical spectra of photosystem I suggest an asymmetric light-harvesting processJournal of the American Chemical Society 132:3331–3343.https://doi.org/10.1021/ja9072222
-
Structure and function of photosystem I in Cyanidioschyzon merolaePhotosynthesis Research 139:499–508.https://doi.org/10.1007/s11120-018-0501-4
-
Electron transfer in photosystem IBiochimica et Biophysica Acta - Bioenergetics 1507:100–114.https://doi.org/10.1016/s0005-2728(01)00202-x
-
MolProbity: all-atom structure validation for macromolecular crystallographyActa Crystallographica. Section D, Biological Crystallography 66:12–21.https://doi.org/10.1107/S0907444909042073
-
Features and development of CootActa Crystallographica. Section D, Biological Crystallography 66:486–501.https://doi.org/10.1107/S0907444910007493
-
Structure of photosystem IBiochimica et Biophysica Acta, Bioenergetics 1507:5–31.https://doi.org/10.1016/S0005-2728(01)00195-5
-
Energy transfer and trapping in photosystem IBiochimica et Biophysica Acta - Bioenergetics 1507:80–99.https://doi.org/10.1016/s0005-2728(01)00203-1
-
Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopyCurrent Opinion in Structural Biology 21:265–273.https://doi.org/10.1016/j.sbi.2011.01.008
-
The structure of Gloeobacter violaceus and its phycobilisomesArchives of Microbiology 129:181–189.https://doi.org/10.1007/BF00425248
-
Excitation energy relaxation in a symbiotic cyanobacterium, Prochloron didemni, occurring in coral-reef ascidians, and in a free-living cyanobacterium, Prochlorothrix hollandicaBiochimica et Biophysica Acta - Bioenergetics 1817:1992–1997.https://doi.org/10.1016/j.bbabio.2012.06.008
-
A new cryo-EM system for single particle analysisJournal of Structural Biology 207:40–48.https://doi.org/10.1016/j.jsb.2019.04.011
-
The Plasticity of Photosystem IPlant & Cell Physiology 62:1073–1081.https://doi.org/10.1093/pcp/pcab046
-
BookPhotosystem I: the Light-Driven Plastocyanin:Ferredoxin OxidoreductaseIn: Golbeck JH, editors. The Long Wavelength Chlorophylls of Photosystem I. Dordrecht, The Netherlands: Springer. pp. 177–192.
-
The Phyre2 web portal for protein modeling, prediction and analysisNature Protocols 10:845–858.https://doi.org/10.1038/nprot.2015.053
-
Structure and function of wild-type and subunit-depleted photosystem I in SynechocystisBiochimica et Biophysica Acta - Bioenergetics 1859:645–654.https://doi.org/10.1016/j.bbabio.2018.02.002
-
Variations in photosystem I properties in the primordial cyanobacterium Gloeobacter violaceus PCC 7421Photochemistry and Photobiology 86:62–69.https://doi.org/10.1111/j.1751-1097.2009.00619.x
-
Accurate determination of local defocus and specimen tilt in electron microscopyJournal of Structural Biology 142:334–347.https://doi.org/10.1016/s1047-8477(03)00069-8
-
REFMAC5 for the refinement of macromolecular crystal structuresActa Crystallographica. Section D, Biological Crystallography 67:355–367.https://doi.org/10.1107/S0907444911001314
-
Isolation and characterization of oxygen-evolving thylakoid membranes and Photosystem II particles from a marine diatom Chaetoceros gracilisBiochimica et Biophysica Acta - Bioenergetics 1767:1353–1362.https://doi.org/10.1016/j.bbabio.2007.10.007
-
High excitation energy quenching in fucoxanthin chlorophyll a/c-binding protein complexes from the diatom Chaetoceros gracilisThe Journal of Physical Chemistry B 117:6888–6895.https://doi.org/10.1021/jp403923q
-
pH-induced regulation of excitation energy transfer in the cyanobacterial photosystem I tetramerThe Journal of Physical Chemistry B 124:1949–1954.https://doi.org/10.1021/acs.jpcb.0c01136
-
Structure and function of photosystems I and IIAnnual Review of Plant Biology 57:521–565.https://doi.org/10.1146/annurev.arplant.57.032905.105350
-
UCSF Chimera - A visualization system for exploratory research and analysisJournal of Computational Chemistry 25:1605–1612.https://doi.org/10.1002/jcc.20084
-
P700+- and 3P700-induced quenching of the fluorescence at 760 nm in trimeric Photosystem I complexes from the cyanobacterium Arthrospira platensisBiochimica et Biophysica Acta - Bioenergetics 1706:53–67.https://doi.org/10.1016/j.bbabio.2004.08.009
-
Steady-state and transient polarized absorption spectroscopy of photosystem I complexes from the cyanobacteria Arthrospira platensis and Thermosynechococcus elongatusBiochimica et Biophysica Acta - Bioenergetics 1767:732–741.https://doi.org/10.1016/j.bbabio.2007.01.013
-
Fluorescence of the various red antenna states in photosystem I complexes from cyanobacteria is affected differently by the redox state of P700Biochimica et Biophysica Acta - Bioenergetics 1807:1423–1431.https://doi.org/10.1016/j.bbabio.2011.06.018
-
SoftwareThe PyMOL Molecular Graphics SystemVersion 1.8.
-
Efficient energy transfer from the long-wavelength antenna chlorophylls to P700 in photosystem I complexes from Spirulina platensisJournal of Photochemistry and Photobiology B 30:153–160.https://doi.org/10.1016/1011-1344(95)07173-Y
-
Purification and properties of unicellular blue-green algae (order Chroococcales)Bacteriological Reviews 35:171–205.https://doi.org/10.1128/br.35.2.171-205.1971
-
Structural variations of photosystem I-antenna supercomplex in response to adaptations to different light environmentsCurrent Opinion in Structural Biology 63:10–17.https://doi.org/10.1016/j.sbi.2020.02.005
-
Steady-state polarized light spectroscopy of isolated Photosystem I complexesPhotosynthesis Research 35:311–321.https://doi.org/10.1007/BF00016562
-
A unique photosystem I reaction center from A chlorophyll d-containing cyanobacterium Acaryochloris marinaJournal of Integrative Plant Biology 63:1740–1752.https://doi.org/10.1111/jipb.13113
Article and author information
Author details
Funding
Japan Society for the Promotion of Science (JP20H02914)
- Koji Kato
Japan Society for the Promotion of Science (JP21K19085)
- Ryo Nagao
Japan Society for the Promotion of Science (JP20K06528)
- Keisuke Kawakami
Japan Society for the Promotion of Science (JP16H06553)
- Seiji Akimoto
Japan Society for the Promotion of Science (JP17H06433)
- Jian-Ren Shen
JST-Mirai (JPMJMI20G5)
- Koji Yonekura
Takeda Science Foundation
- Koji Kato
the Cyclic Innovation for Clinical Empowerment (CiCLE) from the Japan Agency for Medical Research and Development, AMED
- Tasuku Hamaguchi
- Keisuke Kawakami
- Koji Yonekura
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Acknowledgements
This work was supported by JSPS KAKENHI grant nos. JP20H02914 (Koji.K.), JP21K19085 (R.N.), JP20K06528 (Keisuke.K), JP16H06553 (S.A.), and JP17H06433 (J.-R.S.), JST-Mirai Program Grant Number JPMJMI20G5 (K.Y.), Takeda Science Foundation (Koji.K.), and the Cyclic Innovation for Clinical Empowerment (CiCLE) from the Japan Agency for Medical Research and Development, AMED (T.H., Keisuke.K., and K.Y.).
Copyright
© 2022, Kato et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,427
- views
-
- 266
- downloads
-
- 30
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Plant Biology
- Structural Biology and Molecular Biophysics
The Calvin-Benson-Bassham cycle (CBBC) performs carbon fixation in photosynthetic organisms. Among the eleven enzymes that participate in the pathway, sedoheptulose-1,7-bisphosphatase (SBPase) is expressed in photo-autotrophs and catalyzes the hydrolysis of sedoheptulose-1,7-bisphosphate (SBP) to sedoheptulose-7-phosphate (S7P). SBPase, along with nine other enzymes in the CBBC, contributes to the regeneration of ribulose-1,5-bisphosphate, the carbon-fixing co-substrate used by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The metabolic role of SBPase is restricted to the CBBC, and a recent study revealed that the three-dimensional structure of SBPase from the moss Physcomitrium patens was found to be similar to that of fructose-1,6-bisphosphatase (FBPase), an enzyme involved in both CBBC and neoglucogenesis. In this study we report the first structure of an SBPase from a chlorophyte, the model unicellular green microalga Chlamydomonas reinhardtii. By combining experimental and computational structural analyses, we describe the topology, conformations, and quaternary structure of Chlamydomonas reinhardtii SBPase (CrSBPase). We identify active site residues and locate sites of redox- and phospho-post-translational modifications that contribute to enzymatic functions. Finally, we observe that CrSBPase adopts distinct oligomeric states that may dynamically contribute to the control of its activity.
-
- Plant Biology
Soil salinity is one of the major threats to agricultural productivity worldwide. Salt stress exposure alters root and shoots growth rates, thereby affecting overall plant performance. While past studies have extensively documented the effect of salt stress on root elongation and shoot development separately, here we take an innovative approach by examining the coordination of root and shoot growth under salt stress conditions. Utilizing a newly developed tool for quantifying the root:shoot ratio in agar-grown Arabidopsis seedlings, we found that salt stress results in a loss of coordination between root and shoot growth rates. We identify a specific gene cluster encoding domain-of-unknown-function 247 (DUF247), and characterize one of these genes as Salt Root:shoot Ratio Regulator Gene (SR3G). Further analysis elucidates the role of SR3G as a negative regulator of salt stress tolerance, revealing its function in regulating shoot growth, root suberization, and sodium accumulation. We further characterize that SR3G expression is modulated by WRKY75 transcription factor, known as a positive regulator of salt stress tolerance. Finally, we show that the salt stress sensitivity of wrky75 mutant is completely diminished when it is combined with sr3g mutation. Together, our results demonstrate that utilizing root:shoot ratio as an architectural feature leads to the discovery of a new stress resilience gene. The study’s innovative approach and findings not only contribute to our understanding of plant stress tolerance mechanisms but also open new avenues for genetic and agronomic strategies to enhance crop environmental resilience.






