Targeted depletion of uterine glandular Foxa2 induces embryonic diapause in mice
Abstract
Embryonic diapause is a reproductive strategy in which embryo development and growth is temporarily arrested within the uterus to ensure the survival of neonates and mothers during unfavorable conditions. Pregnancy is reinitiated when conditions become favorable for neonatal survival. The mechanism of how the uterus enters diapause in various species remains unclear. Mice with uterine depletion of Foxa2, a transcription factor, are infertile. In this study, we show that dormant blastocysts are recovered from these mice on day 8 of pregnancy with persistent expression of uterine Msx1, a gene critical to maintaining the uterine quiescent state, suggesting that these mice enter embryonic diapause. Leukemia inhibitory factor (LIF) can resume implantation in these mice. Although estrogen is critical for implantation in progesterone-primed uterus, our current model reveals that FOXA2-independent estrogenic effects are detrimental to sustaining uterine quiescence. Interestingly, progesterone and anti-estrogen can prolong uterine quiescence in the absence of FOXA2. Although we find that Msx1 expression persists in the uterus deficient in Foxa2, the complex relationship of FOXA2 with Msx genes and estrogen receptors remains to be explored.
Editor's evaluation
This manuscript reports the complex interactions that take place in the uterus between the endometrium and the blastocyst during and after embryonic diapause, a period of suspended animation that occurs in some mammals including the mouse, the model used here. The authors showed that one gene, Foxa2, interacts with two other genes, Msx1 and LIF, to control the success and duration of diapause. This will be of broad interest to researchers in the field of developmental biology and reproduction. It is a carefully done study, providing new information on the complex process that is diapause in which an embryo goes into suspended animation until it receives appropriate signaling from the uterine endometrial secretions to reactivate.
https://doi.org/10.7554/eLife.78277.sa0Introduction
Embryonic diapause is a reproductive strategy in which embryo development and growth is temporarily arrested within the uterus, but is reinitiated when conditions are favorable for neonatal and maternal survival (Cha and Dey, 2014; Fenelon and Renfree, 2018). During diapause, blastocyst growth, DNA synthesis, mitosis, and metabolic activity are temporarily downregulated in the uterus to achieve a quiescent state to support blastocyst survival.
The triggers for diapause vary widely across species, ranging from photoperiod, temperature, metabolic stress, lactation, or nutrition (Lopes et al., 2004). It is known to occur in more than 100 species spanning over seven orders. In mice, experimental ovariectomy early on the morning of day 4, before preimplantation estrogen (E2) secretion, induces embryonic diapause (Yoshinaga and Adams, 1966); alternatively, embryonic diapause can be induced by injections of estrogen receptor (ER) antagonists on days 3 and 4 of pregnancy to neutralize E2 function (Cha et al., 2013). Blastocyst reactivation can be rapidly initiated by a single injection of E2 in an ovariectomized dormant uterus (Yoshinaga and Adams, 1966). Preimplantation E2 secretion on day 4 morning induces leukemia inhibitory factor (LIF) to initiate implantation. Interestingly, blastocysts in Lif-/- females undergo diapause (Stewart et al., 1992). In spite of these recognized factors, the molecular mechanism which initiates embryonic diapause is still not fully understood.
FOXA2 (forkhead box protein A2), which is expressed in glandular epithelia in the mouse uterus, plays a key role in uterine gland development and implantation. Neonatal depletion of uterine Foxa2 causes a significant reduction in the number of glands (Jeong et al., 2010). Female mice with uterine glandular depletion of Foxa2 after puberty have implantation/decidualization failure due to compromised LIF induction on day 4 of pregnancy (Kelleher et al., 2017).
In this study, we show that depletion of Foxa2 in mouse uterine glands causes embryonic diapause. Dormant embryos were retrieved from uteri on day 8 of pregnancy. Msx1 expression, which appears to be critical to maintain a quiescent uterine environment (Cha et al., 2013), was maintained in Foxa2 deficient mice in our studies. Although implantation was triggered by a single LIF injection on day 8 of pregnancy in Foxa2 deficient mice, these mice are not able to support pregnancy to full term. Furthermore, we found that balancing of estrogenic effects by either progesterone (P4) supplement or application of an ER antagonist significantly improves survival of embryos in Foxa2 deficient mice. Our study reveals that Foxa2 plays an important role in mammalian embryonic diapause and that FOXA2-independent E2 effects are detrimental to uterine quiescence during diapause.
Results
Uterine depletion of Foxa2 results in female infertility due to disrupted Lif induction during implantation
Previous reports have shown that Foxa2 is expressed in the glandular epithelium before and during pregnancy (Jeong et al., 2010; Wang et al., 2018). Uterine glandular Foxa2 is critical to normal implantation (Kelleher et al., 2017). Using mice with uterine-specific (Foxa2f/fPgrCre/+) and uterine epithelial-specific depletion of Foxa2 (Foxa2f/fLtfCre/+), we confirmed that these mice are infertile (Figure 1a) due to the lack of Lif induction on day 4 of pregnancy (Figure 1d). Since the timing and domain of Cre activity driven by an Ltf or Pgr promoter differs (Figure 1b), Foxa2f/fLtfCre/+ females maintain FOXA2 expression in uterine glands before puberty (Figure 1c), whereas Foxa2f/fPgrCre/+ females have a minimal number of glands on postnatal day 30 (Figure 1c) due to early depletion of Foxa2 (Figure 1b). In mature pregnant females, no positive signals of FOXA2 are observed in either of the two mouse models on day 4 of pregnancy (Figure 1c). The efficient depletion of Foxa2 RNA in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+uteri is confirmed by quantitative PCR (Figure 1—figure supplement 1).
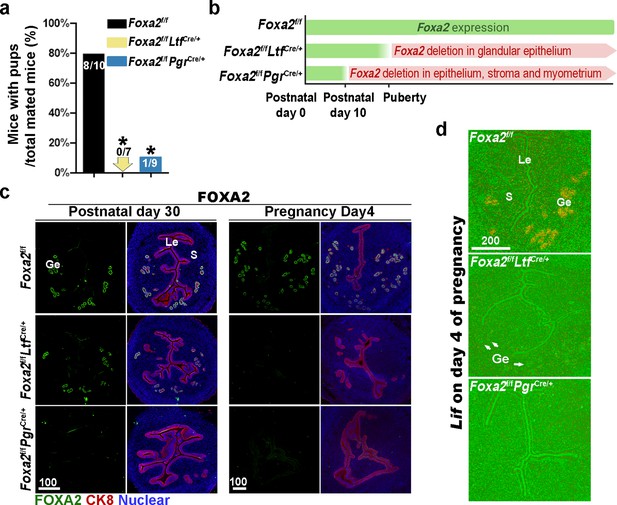
Uterine conditional depletion of Foxa2 causes loss of leukemia inhibitory factor (LIF) secretion and female infertility.
(a) Percentage of mice with pups per total mated mice.Numbers on bars indicate mice with pups over total number of mated mice. *p < 0.05 by Student's t-test. (b) Cre recombinase activity starts differently in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ females. (c) Immunostaining of FOXA2 in the uteri on postnatal day 30 and day 4 pregnancy of Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+females. Epithelial cells are outlined by cytokeratin 8 (CK8) staining. Scale bars: 100 μm. (d) In situ hybridization of Lif in day 4 of pregnant uteri from Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+ females. White arrows point to uterine glands. Scale bar: 200 μm. Le, luminal epithelia; Ge, glandular epithelia; S, stroma.
Blastocysts enter embryonic diapause in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice
Suppression of preimplantation E2 secretion on day 4 of pregnancy renders mouse uteri quiescent to implantation. This uterine status can be extended by a daily supplement of P4. Unimplanted blastocyst development in quiescent uteri is arrested (Renfree and Shaw, 2000; Fenelon et al., 2014; Sherman and Barlow, 1972) in Lif-/- females, blastocysts recovered on day 7 of pregnancy retained implantation capabilities once transferred to wild-type surrogate uteri (Stewart et al., 1992). This result suggests that Lif-/- uteri are able to maintain the quiescent phase in spite of presumed E2 secretion on day 4 of pregnancy. Given the absence of LIF induction in Foxa2 deficient uteri, we next examined whether Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri enter diapause after day 4 of pregnancy.
Day 8 uteri were examined for implantation in Foxa2f/fLtfCre/+, Foxa2f/fPgrCre/+, and control (Foxa2f/f) females. Foxa2f/f uteri show implantation sites with apparently normal morphology (Figure 2a). In contrast, the numbers of implantation sites were significantly lower in Foxa2f/fLtfCre/+ or Foxa2f/fPgrCre/+ females as compared to Foxa2f/f females (Figure 2a and Supplementary file 1, Table 1). The frequency of mated females with implantation sites in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice was 16.7% (1/6) and 42.9% (3/7), respectively, significantly lower than those in Foxa2f/f mice (100%, 6/6) (Supplementary file 1, Table 1).
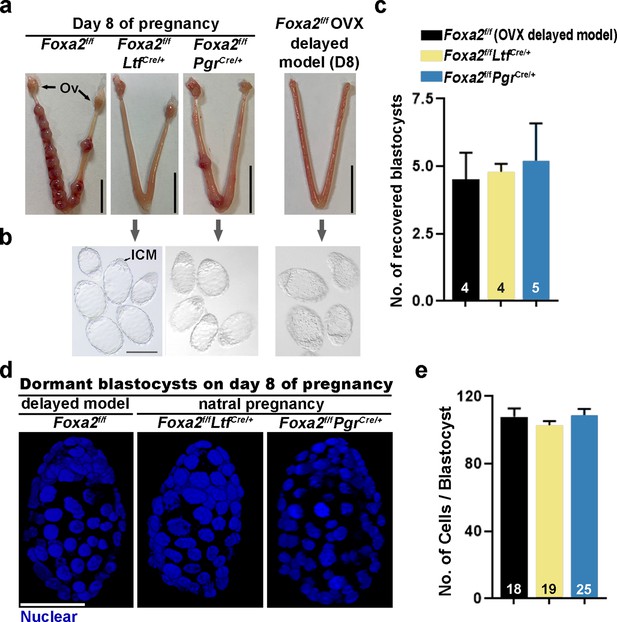
Dormant blastocysts are present in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+uteri on day 8 pregnancy.
(a) Representative photographs of day 8 pregnant uteri from Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+ females. An ovariectomy-induced delayed model of Foxa2f/f mice served as a prototypical control in maintaining dormant blastocysts. Scale bar: 10 mm. Ov, ovary. (b) Blastocysts recovered from Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+uteri on day 8. Blastocysts retrieved from ovariectomized Foxa2f/f mice in delay served as controls. ICM, inner cell mass. Scale bar: 100 µm. Quantification of blastocyst numbers were shown in panel c. Numbers on bars indicate numbers of animals examined. Values are expressed as mean + SEM. (d) Representative photographs of nuclear staining of dormant blastocysts recovered from mice without implantation sites. Scale bar: 50 µm. (e) Average cell numbers per blastocyst. Numbers of embryos examined are shown on bars. Values are expressed as mean + SEM.
Dormant blastocysts were recovered by flushing Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uterine horns on day 8 of pregnancy (Figure 2b). A diapause model created by ovariectomy on day 4 of pregnancy was used as a prototypical control (Figure 2—figure supplement 1), in which ~4.5 dormant blastocysts were retrieved. A similar number of blastocysts was recovered from Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uterine horns on day 8 of pregnancy (Figure 2c), and they morphologically resembled the dormant blastocysts from the ovariectomy delayed model (Figure 2b). It is known that dormant blastocysts cease mitotic activity and cell proliferation (Cha et al., 2013). Using DAPI staining (Figure 2d), we found that blastocysts retrieved from Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri have comparable cell numbers to those recovered from diapausing uteri achieved by ovariectomy (Figure 2e).
Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri show characteristics of uterine quiescence
Uterine quiescence apparently depends on the presence of muscle segment homeobox (Msx) genes. In mice and other diapausing animals, such as in mink and Tamar Wallaby, Msx1 and Msx2 genes persist during diapause, but their levels are quickly suppressed with blastocyst reactivation and implantation (Cha et al., 2013). However, mice with uterine conditional depletion of both Msx1 and Msx2 fail to achieve diapause and reactivation (Cha et al., 2013; Cha et al., 2020). Since dormant blastocysts were recovered from Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri on day 8 of pregnancy, we suspected that Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri remain quiescent in the absence of LIF induction. We examined Msx1 expression in the uterus on days 4 and 8 of pregnancy by fluorescence in situ hybridization. Msx1 signals were observed in epithelial cells before the E2 surge on day 4 in Foxa2f/f uteri, whereas luminal epithelial Msx1 signals were suppressed after the E2 secretion (Figure 3a; Daikoku et al., 2011). Remarkably, Msx1 expression persisted in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ luminal epithelial cells on day 8 of pregnancy (Figure 3a).
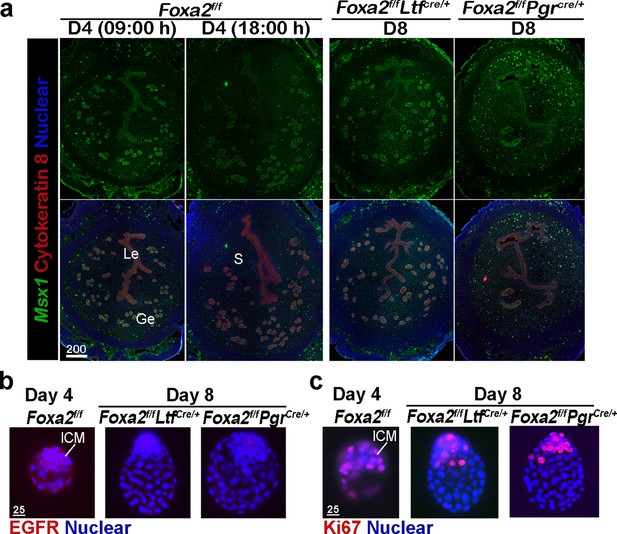
Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ females maintain uterine quiescence when examined on day 8 of pregnancy.
(a) Fluorescence in situ hybridization of Msx1 in days 4 and 8 pregnant uteri from Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+ females. Scale bar: 200 μm. (b) Epidermal growth factor receptor (EGFR) immunostaining on dormant blastocysts. Positive signals were observed in activated blastocysts recovered from Foxa2f/f uteri on day 4 of pregnancy. Scale bar: 25 μm. (c) Ki67 immunostaining on dormant blastocysts collected from day 8 Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ females. Scale bar: 25 μm. ICM, inner cell mass.
Epidermal growth factor receptor (EGFR) is present in day 4 blastocysts, but becomes suppressed during dormancy (Paria et al., 1993a). This is consistent with our current findings that EGFR expression is significantly lower in blastocysts recovered from Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri on day 8 of pregnancy as compared to those retrieved from Foxa2f/f uteri in the evening of day 4 (Figure 3b). The mitotic activity (Ki67 staining) in the trophectoderm of the recovered blastocysts in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice on day 8 is also arrested (Figure 3c). This result is consistent with a study by Fujimori’s group (Kamemizu and Fujimori, 2019). Collectively, the results suggest that Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri remain quiescent until at least day 8 of pregnancy, providing a uterine environment suitable for embryonic diapause.
Uterine activation in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice deteriorates during diapause
Embryonic diapause and uterine quiescence are reversible with a single injection of E2 or LIF in mice (Yoshinaga and Adams, 1966; Chen et al., 2000). Implantation failure has been shown to be rescued in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females by LIF administration on day 4 (Kelleher et al., 2017). No further analysis was carried out. Since dormant blastocysts are recovered from Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri on day 8 of pregnancy in our studies, we examined if the diapausing blastocysts can be rejuvenated. Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females received one injection of recombinant LIF (20 μg/mouse) on day 4 or 8, and the uteri were examined 2 days later (Figure 4a). Consistent with the previous report (Kelleher et al., 2017), implantation sites were observed in both Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri if LIF was given on day 4 of pregnancy (Figure 4b). Further histological evaluations reveal that normal-looking implantation chambers formed similar to those in Foxa2f/fmice on day 6 of pregnancy (Figure 4b and c). Almost all Foxa2f/fLtfCre/+ females (five of six) possessed implantation sites, whereas less than half (four of nine) Foxa2f/fPgrCre/+ females had implantation sites (Figure 4d), although the number of implantation sites was comparable in pregnant females.
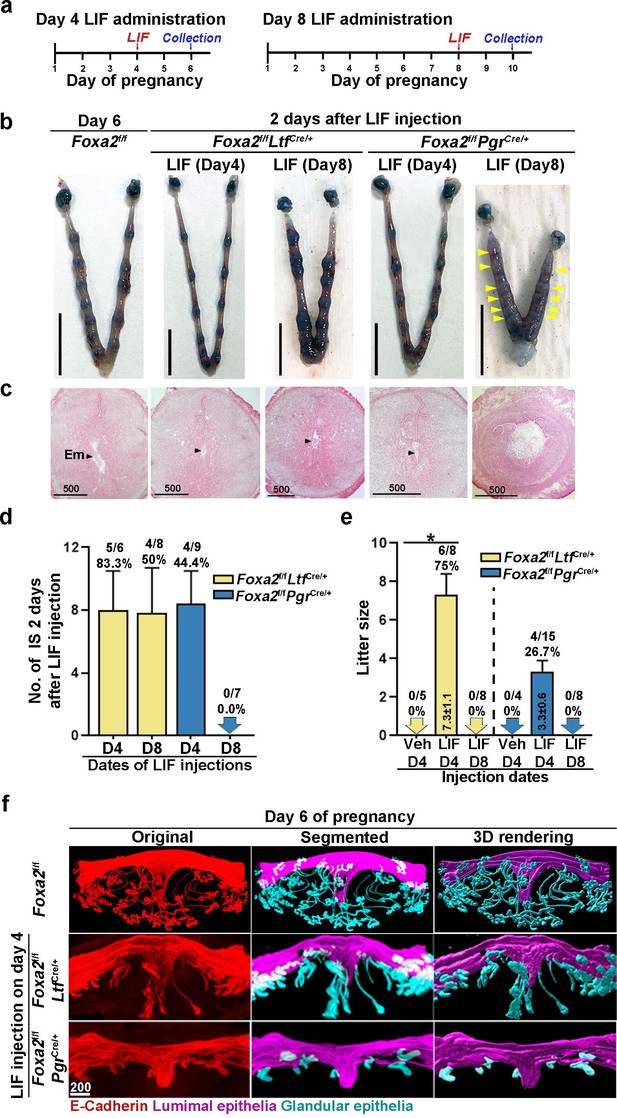
Pregnancy in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ females with leukemia inhibitory factor (LIF) treatment.
(a) Schematic outline of sample collection. LIF, LIF administration (20 μg). (b) Representative photograph of uteri from Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ females (days 6 and 10) with LIF treatment. Foxa2f/f uteri on day 6 serve as control. Scale bar: 10 mm. Histological pictures of implantation sites in panel b were presented in panel c. Arrowheads point to embryos. Em, embryo. Scale bar: 500 μm. (d) Average number of implantation sites in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice treated with LIF (20 μg) on day 4 or 8 of pregnancy. Numbers and percentage on bars indicate mice with implantation sites over total number of mated mice. (e) Litter sizes of Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+mice treated with LIF (20 μg) or vehicle on days 4 or 8 of pregnancy. Numbers and percentage on bars indicate mice with pups over total number of mated mice. *p<0.05. (f) 3D visualization of day 6 implantation sites in Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+females. Images of E-cadherin immunostaining, segmented, and 3D rendered images of day 6 implantation sites in each genotype show defects in Foxa2f/fPgrCre+ females with a LIF injection on day 4 of pregnancy. Scale bar: 200 μm.
Notably, implantation sites with a normal appearance were observed in Foxa2f/fLtfCre/+ uteri when LIF was given on day 8 of pregnancy (Figure 4b), albeit edematous uteri in Foxa2f/fPgrCre/+ with faint blue bands. Histology of implantation sites confirmed this observation. Implantation chambers form in Foxa2f/fLtfCre/+ implantation sites, but neither embryos nor implantation chambers were found in Foxa2f/fPgrCre/+ implantation sites (Figure 4c). The rate of Foxa2f/fLtfCre/+ females with implantation sites decreased from 83.3% to 50% in females receiving LIF injection on day 4 (Figure 4d). All Foxa2f/fPgrCre/+ females showed abnormal light blue bands without recognizable implantation chambers when examined 2 days after LIF injection, and the implantation chambers showed no further development (Figure 4d).
Glands have been shown to be essential for implantation and pregnancy success (Kelleher et al., 2017; Gray et al., 2001). FOXA2 plays a key role in mouse uterine glandular genesis, and neonatal depletion of Foxa2 in mouse uteri causes defects in gland development (Jeong et al., 2010). To examine glands, day 6 implantation sites of Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females with LIF injection on day 4 were stained with an E-cadherin antibody. Tridimensional images were acquired as previously described (Yuan et al., 2018). The number of glands in Foxa2f/fLtfCre/+ implantation sites was significantly reduced compared to those in natural day 6 implantation sites of Foxa2f/f females (Figure 4f). As previously reported (Jeong et al., 2010), glands were rarely observed in Foxa2f/fPgrCre/+ implantation sites. These data suggest that an increased number of glands is not required for uterine quiescence and embryonic diapause, but the presence of a minimal number of glands is critical for reactivation after diapause.
In mammalian embryonic diapause, arrest of blastocyst development and uterine quiescence are transitory. Upon reactivation, the uterine environment becomes competent to support embryo development to term when conditions are favorable for neonatal survival. To study whether reactivated uteri in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females are able to support full-term pregnancy, litter sizes were counted. Six of eight Foxa2f/fLtfCre/+, but only 4 of 15 Foxa2f/fPgrCre/+ females injected with LIF on day 4 successfully delivered live pups, and Foxa2f/fPgrCre/+ females have reduced litter sizes (Figure 4e). Notably, neither Foxa2f/fLtfCre/+ nor Foxa2f/fPgrCre/+ females with day 8 LIF injection were able to support full-term pregnancy, in spite of implantation occurring 2 days after LIF injection in Foxa2f/fLtfCre/+ females (Figure 4e). These results suggest that uterine readiness for reactivation in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ deteriorates during diapause with preimplantation E2 secretion.
Progesterone supplement during diapause improves pregnancy outcomes in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females after reactivation
Progesterone is required to maintain uterine quiescence and blastocyst viability in mouse embryonic diapause. Embryonic diapause is also experimentally induced in the mouse by ovariectomy on day 4 of pregnancy before E2 secretion and maintained by daily P4 injections (Yoshinaga and Adams, 1966; Renfree and Fenelon, 2017). To examine if P4 levels decrease without implantation in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females, we evaluated serum concentration of P4 on days 4 and 8 of pregnancy and E2 on day 4 pregnancy in Foxa2f/fLtfCre/+, Foxa2f/fPgrCre/+, and Foxa2f/f females. P4 and E2 levels in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females were comparable to those in Foxa2f/f mice (Figure 5—figure supplement 1).
Although Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females have normal P4 and E2 levels, the uterine edema in Foxa2f/fPgrCre/+ females 2 days after LIF injection on day 8 (Figure 4b) suggests increased estrogenic effects during diapause. Therefore, we administered P4 on days 5, 7, and 9 with LIF injection on day 8 to counter the increased estrogenic effects in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females (Figure 5a). Embryo implantation was evaluated 2 days after LIF administration. All Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females had implantation sites with distinct blue bands (Figure 5b and d). Histological analysis identified embryos in the implantation chambers in mice of both genotypes (Figure 5c). Implantation rates and the numbers of implantation sites appear normal (Figure 5d). A comparable decidual response as revealed by Bmp2 RNA levels is observed between these P4 supplemented Foxa2f/fLtfCre/+ females 2 days after LIF injection and Foxa2f/f implantation sites on day 6 of natural pregnancy (Figure 5—figure supplement 2). Furthermore, around 40% of Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females successfully delivered progeny, although litter sizes were small (2~3 pups/litter) (Figure 5e). These data suggest that P4 supplementation improves uterine conditions during diapause in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice.
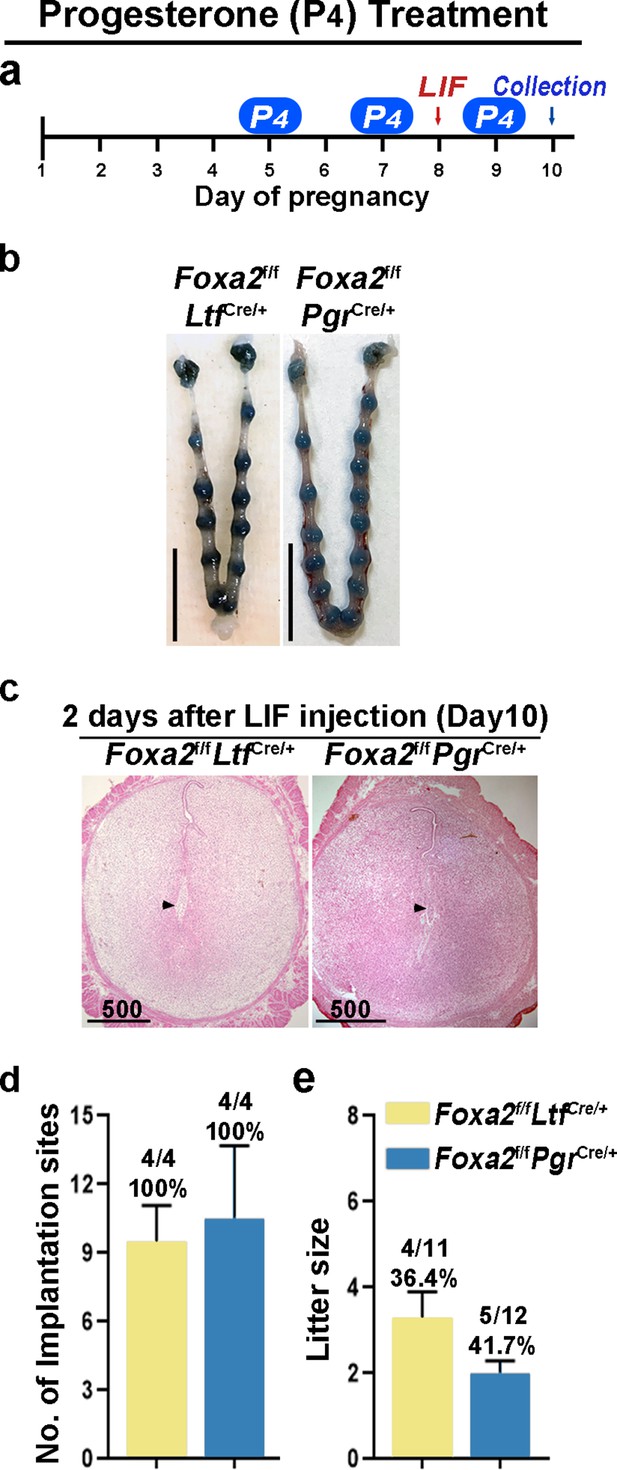
Counterbalance of estrogenic effects by P4 improves maintenance of diapause in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice.
(a) Scheme of P4 treatment. Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice were treated with leukemia inhibitory factor (LIF) (20 μg) on day 8. Pregnancy was evaluated on day 10, 2 days after LIF administration. (b) Representative photographs of uteri in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice with P4 supplement 2 days after LIF administration. Scale bar: 10 mm. Histological pictures of implantation sites in panel b were presented in panel c. Scale bar: 500 μm. (d) Average number of implantation sites in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice with P4 supplement. Numbers and percentage on bars indicate mice with implantation sites over total number of mated mice. (e) Litter sizes of Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+mice with P4 supplement. Numbers and percentage on bars indicate mice with pups over total number of mated mice.
Suppression of estrogen action during diapause improves pregnancy outcomes in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females after reactivation
In mice, preimplantation E2 secretion on day 4 of pregnancy triggers a receptive phase followed by a uterine refractory phase on day 5 onward if implantation fails to occur. This refractory phase persists until P4 treatment is withdrawn (Wang et al., 2004). This activity suggests that E2 has a biphasic effect on embryo implantation: a positive effect to induce uterine receptivity and a negative effect in changing the receptive uterus to a nonreceptive state. In Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females, although E2-induced LIF expression was abolished, FOXA2-independent negative estrogenic effects may gradually induce the refractory phase in uteri. To test this possibility, we administrated an ER antagonist (ICI-182780, named ICI) on days 3, 5, and 7 before day 8 LIF injection in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females (Figure 6a). Of note, embryonic diapause can also be experimentally induced in mice via 50 mg ICI injections on days 3 and 4 (Cha et al., 2013). We have also confirmed this observation in our present study. To avoid the suppression of E2-induced LIF secretion on day 4, the dose of ICI was lowered to 25 mg per injection, the level at which implantation occurs normally in Foxa2f/ffemales (Figure 6b).
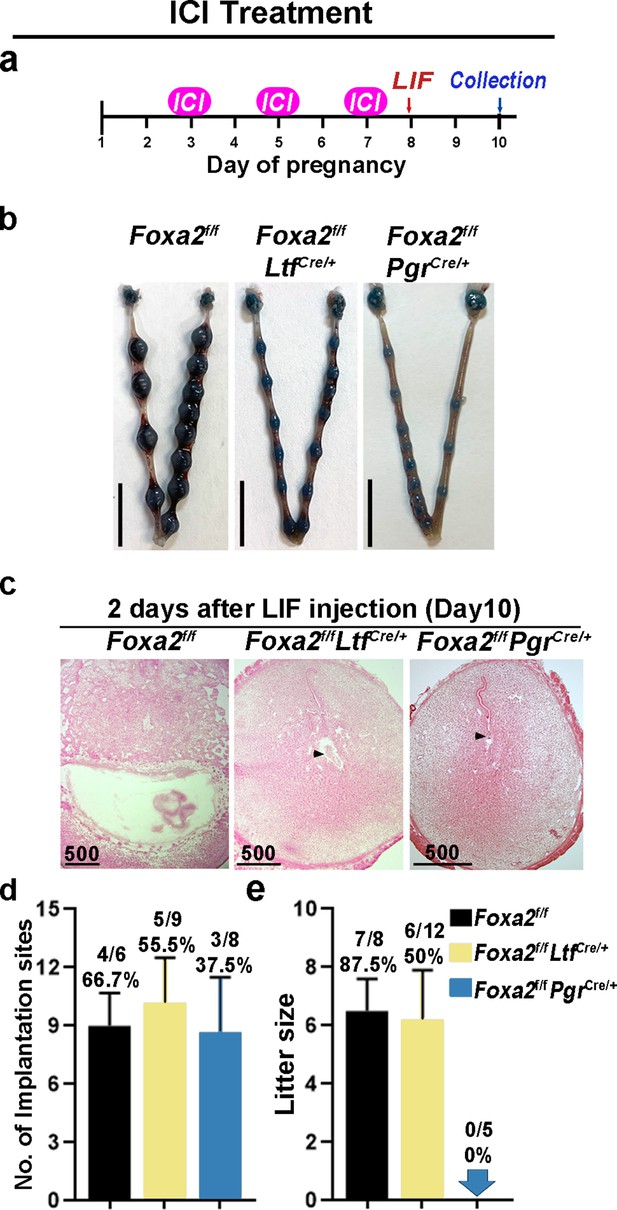
Neutralization of estrogenic effects by ICI improves diapause in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice.
(a) Scheme of ICI treatment. Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice were treated with leukemia inhibitory factor (LIF) (20 μg) on day 8. Pregnancy was evaluated on day 10, 2 days after LIF administration. (b) Representative photographs of uteri in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice with ICI treatment 2 days after LIF administration. Foxa2f/f mice have normal day 10 implantation sites, suggesting implantation occurs under 25 μg ICI treatment in Foxa2f/f mice. Scale bar: 10 mm. Histological pictures of implantation sites in panel b were presented in panel c. Scale bar: 500 μm. (d) Average number of implantation sites in Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+ mice with ICI treatment. Numbers and percentage on bars indicate mice with implantation sites over total number of mated mice. (e) Litter sizes of Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+mice with ICI treatment. Numbers and percentage on bars indicate mice with pups over total number of mated mice.
Similar to P4 supplement, ICI treatment improved uterine responses to LIF-induced reactivation of embryos in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females. Implantation sites with distinct blue bands were observed in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females 2 days after LIF injection (Figure 6b). Embryos were identified in implantation chambers in both Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ mice (Figure 6c). Quantitatively, 55.5% of Foxa2f/fLtfCre/+ females and 37.5% of Foxa2f/fPgrCre/+ females had implantation sites; the number of implantation sites in these mice is comparable to those in Foxa2f/f females (Figure 6d). A comparable decidual response as indicated by Bmp2 expression is observed between ICI-treated Foxa2f/fLtfCre/+ females 2 days after LIF injection and Foxa2f/f implantation sites on day 6 of natural pregnancy (Figure 5—figure supplement 2). Surprisingly, 50% of Foxa2f/fLtfCre/+ females supported pregnancy to full-term with a litter size comparable to those of Foxa2f/f females (Figure 6e). However, no delivery was observed in Foxa2f/fPgrCre/+ females. These results suggest that a low level of ICI suppressed adverse estrogenic effects on uterine quiescence during diapause.
Diapause requires a favorable uterine environment to maintain dormant embryos. A putative idea of the E2 secretion on day 4 mornings is to induce LIF and initiate the implantation process in a P4-primed mouse uterus. In the present studies, we have used mouse models conditionally deficient in Foxa2, and demonstrate that LIF suppression is not sufficient to maintain long-term uterine quiescence like in ovariectomized mice maintained on a P4 supplement. Our study reveals that E2 has an adverse impact on uterine quiescence independent of FOXA2/LIF (Figure 7).
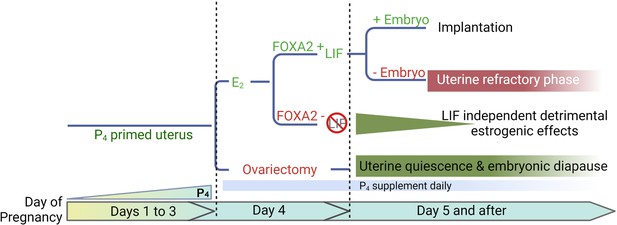
A representative scheme depicting roles of E2, FOXA2 (forkhead box protein A2), and leukemia inhibitory factor (LIF) in mouse diapause.
In natural pregnancy, a uterus enters a prereceptive phase before E2 secretion in the morning of day 4. In the presence of FOXA2, LIF is induced by E2 which renders the uterus into a receptive phase. In the event of implantation failure, the uterus becomes refractory on day 5 of pregnancy. The transition to the receptive phase is stopped if the E2 secretion on day 4 is prevented by ovariectomy. The uterus remains quiescent as long as P4 is supplemented; the embryonic development is arrested. A similar extension of prereceptive phase can be achieved by deleting FOXA2 or LIF. But the uterine quiescence is gradually compromised indicating that a LIF-independent estrogenic effect is detrimental to uterine quiescence.
Discussion
Over 130 mammalian species experience diapause. The triggers for diapause across species vary widely including sucking stimuli, photoperiod, the availability of nutrition, and so forth (Fenelon et al., 2014). The uterus is perhaps a determining factor for embryonic diapause in that a non-diapausing embryo undergoes dormancy in a diapausing uterus (Ptak et al., 2012). During diapause, mammals temporarily arrest blastocyst development and metabolic activity within the uterus. In normal pregnancy, uterine sensitivity to implantation is classified into three phases in mice: prereceptive, receptive, and nonreceptive (refractory) (Wang and Dey, 2006). Mouse uteri attain quiescence in diapause directly from the prereceptive phase via suppression of preimplantation E2 secretion or LIF on day 4 of pregnancy (Yoshinaga and Adams, 1966; Stewart et al., 1992; Paria et al., 1993b; Song et al., 2000). However, the mechanism to induce embryonic diapause in mice is not clearly understood. In the current study, we show that depletion of uterine Foxa2 triggers the mouse uterus to enter a quiescent status, which supports the arrest of embryonic development. A previous study showed that Foxa2f/fLtfCre/+ females have implantation failure due to LIF suppression prior to implantation (Kelleher et al., 2017), which potentially explains why Foxa2f/fLtfCre/+ uteri are quiescent, since a single injection of LIF is sufficient to initiate embryo implantation. Dormant blastocysts were recovered from Lif-/- females on day 7 of pregnancy (Stewart et al., 1992).
Uterine depletion of Foxa2 in either Foxa2f/fLtfCre/+ or Foxa2f/fPgrCre/+ females is not sufficient to maintain complete uterine quiescence by suppressing all uterine metabolic activities. In diapause, quiescent uteri are readily reactivated by an injection of E2 or LIF. However, our current study showed that only Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females with day 4 LIF injection delivered progeny; mice with day 8 LIF injection were unable to carry to term. Although Msx1 persists in Foxa2f/fLtfCre/+ or Foxa2f/fPgrCre/+ uteri on day 8 of pregnancy, mice with day 8 LIF injections failed to continue pregnancy to full term, indicating the reactivation of the uteri had been compromised from day 4 to day 8. Compared with ovariectomy-induced diapause, Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females still have E2 secretion on day 4 morning. Although E2-induced LIF expression is diminished, it is possible that E2 continues to have some effects, independent of FOXA2 that slowly compromises uterine readiness for reactivation in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females. Furthermore, we show that detrimental estrogenic effects on diapause could be countered by P4 supplement or ICI treatment.
The exact role of Foxa2 in LIF induction by E2 is not clearly understood. FOXA2 is a nuclear transcription factor and is involved in cell commitment, differentiation, and gene transcription in various organs including the lung, liver, pancreas, and gastrointestinal tract (Lantz et al., 2004; Wolfrum and Stoffel, 2006). Since both FOXA2 and ERs are transcription factors, it is possible that FOXA2 and ER synergistically turn on LIF activity. On the other hand, ER is activated by E2 secretion in the morning of day 4, whereas Foxa2 is constantly expressed in uterine glands, suggesting an alternative possibility that FOXA2 primes transcriptional regulatory regions of Lif, thus enabling ER binding. In fact, previous reports showed that FOXA2 is required for chromatin opening during endoderm differentiation (Cernilogar et al., 2019) and for proper chromatin remodeling in human pancreas specification (Lee, 2019).
Estrogen is harmful to uterine quiescence in mouse diapause. Estrogen is critical for the transition from the prereceptive to the receptive phase in P4-primed uteri (Wang and Dey, 2006). Without implantation, mouse uteri enter the refractory phase after a short receptive phase (implantation window), suggesting that E2 terminates the uterine receptive phase. There is evidence that E2 concentration determines the duration of the uterine receptive phase, wherein a high dose of E2 shortens the receptive period (Ma et al., 2003). Conversely, mouse uteri in diapause are ready to be reactivated by a shot of E2 as in the prereceptive phase. Ovariectomy-induced embryonic diapause could last for weeks in mice with continued P4 treatment (Ma et al., 2003; Weitlauf and Greenwald, 1968), suggesting that uterine quiescence can be maintained for significant periods of time in the absence of E2. In Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females, LIF induction is suppressed, which avoids a quick switch to the refractory phase. However, the FOXA2-independent E2 effect remains in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ uteri, slowly compromising the arrest of embryonic development and uterine quiescence. This dysfunction is further supported by our finding that P4 or a low dose of ICI, which suppress E2 function, improves the diapause condition in Foxa2f/fLtfCre/+ and Foxa2f/fPgrCre/+ females. These results indicate that estrogenic effects are not favorable to maintain diapause in mice.
Materials and methods
Animals and treatment
Request a detailed protocolFoxa2f/f mice on a CD1 background were generated as described (Sund et al., 2000). This mouse line was originally obtained from Jeff Whitsett’s lab at our Institute. Foxa2f/fLtfCre+ and Foxa2f/fPgrCre+mice were generated by mating Foxa2f/f females with LtfCre/+ males (C57BL/6 and albino B6 mixed background) and PgrCre/+ mice. LtfCre/+ and PgrCre/+ mice on a C57BL/6 background were generated as described (Daikoku et al., 2014; Soyal et al., 2005). Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+mice were housed in the animal care facility at Cincinnati Children’s Hospital Medical Center according to the National Institute of Health and institutional guidelines for laboratory animals. All protocols were approved by the Cincinnati Children’s Animal Care and Use Committee. Mice were provided with autoclaved Laboratory Rodent Diet 5010 (Purina) and UV light-sterilized reverse osmosis/deionized constant circulation water ad libitum. All mice used in this study were housed under a 12:12 hr light:dark cycle. At least three mice from each genotype were used for each individual experiment.
Analysis of pregnancy events
Request a detailed protocolThree adult (3 months of age) females from each genotype were randomly chosen and housed with a Foxa2f/f fertile male overnight in separate cages; the morning of finding the presence of a vaginal plug was considered successful mating (day 1 of pregnancy), and these females are designated as mated females which were selected for pregnancy experiments. For analysis of parturition, parturition events were monitored from day 18 through day 27 by observing mice daily, morning, noon, and evening.
Litter size, pregnancy rate, gestation length, and outcomes were monitored. Implantation sites were examined on pregnancy day 6 or day 8. Blue reaction was performed by intravenous injection of a blue dye solution (Chicago Blue dye) 4 min before mice were sacrificed. Distinct blue bands along the uterus indicated implantation sites. For confirmation of pregnancy in mice showing no blue bands, one uterine horn was flushed with saline and checked for the presence of blastocysts. If blastocysts were present, the contralateral horn was used for experiments; mice without any blastocysts were excluded. ICI (Fulvestrant, Sigma-Aldrich, 25 μg/mouse/day) or progesterone (P4, Sigma-Aldrich, 2 mg/mouse/day) was administered in the morning (0900 hr). To induce implantation, a single injection of recombinant LIF (20 μg per mouse) was administrated in the morning (0900 hr). Embryo implantation sites were examined 2 days after LIF injection by intravenous injection of a blue dye solution.
Histology
Request a detailed protocolTissue sections from control and experimental groups were processed on the same slide. Frozen sections (12 μm) were fixed in 4% paraformaldehyde (PFA) in PBS for 10 min at room temperature and then stained with hematoxylin and eosin (H&E) for light microscopy analysis. Images presented are representative of three independent experiments.
Immunostaining
Request a detailed protocolStaining for FOXA2 (1:300, WRAB-FOXA2, Seven Hills Bioreagents), E-cadherin (1:300, 3195s, Cell Signaling Technology), EGFR (1:100, 4267, Cell Signaling Technology), Ki67 (1:200, MA5-14520, Invitrogen), and CK8 (1:100, TROMA-1, Hybridoma Bank, Iowa) was performed using secondary antibodies conjugated with Alexa 488 or Alexa 594 (1:300, Jackson Immuno Research). Nuclear staining was performed using Hoechst 33342 (4 µg/ml, H1399, Thermo Scientific). Tissue sections from control and experimental groups were processed on the same slide for each experiment. Images presented are representative of three independent experiments.
Whole-mount immunostaining for 3D imaging
Request a detailed protocolTo reveal the tridimensional visualization of implantation sites, whole-mount immunostaining with 3DISCO clearing was performed as previously described (Yuan et al., 2018). Anti-E-cadherin antibody (1:100, 3195s, Cell Signaling Technology) was used to stain the luminal epithelium. 3D images were acquired by a Nikon upright confocal microscope (Nikon A1R). To construct the 3D structure of the tissue, the surface tool in Imaris (Bitplane) was used.
Fluorescence in situ hybridization
Request a detailed protocolDigoxigenin (DIG)-labeled probes were generated according to the manufacturer’s protocol (Roche). PFA-fixed frozen sections from control and experimental groups were hybridized with DIG-labeled cRNA probes.Frozen sections (12 µm) from each genotype and treatment group were processed on the same slide for each probe. Briefly, following fixation (in 4% PFA/PBS) and acetylation, slides were hybridized at 55°C with DIG-labeled Lif and Msx1 probe. Anti-DIG-peroxidase was applied onto hybridized slides following washing and peroxide quenching. Color was developed by TSA (Tyramide Signal Amplification) fluorescein according to the manufacturer’s instructions (PerkinElmer). Nuclear staining was performed using Hoechst 33342 (4 µg/ml, H1399, Thermo Scientific). Images presented are representative of three independent experiments.
In situ hybridization using radioactive probes
Request a detailed protocolIn situ hybridization using radioactive (35S GTP) labeled Lif probes was performed as previously described (Tan et al., 1999). In brief, frozen sections (12 μm) were mounted onto poly-L-lysine-coated slides and fixed in cold 4% PFA in PBS. The sections were prehybridized and hybridized at 45°C for 4 hr in 50% (vol/vol) formamide hybridization buffer containing 35S-labeled anti-sense RNA probes (PerkinElmer). RNase A-resistant hybrids were detected by autoradiography. All sections were post-stained with H&E. Images presented are representative of three independent experiments.
Progesterone (P4) and estradiol-17b (E2) assays
Request a detailed protocolMouse blood samples were collected at 9:00 am on days 4 and 8 of pregnancy. Serum was separated by centrifugation and stored at –80°C until analysis. Serum hormonal levels in the serum were measured by P4 or E2 EIA kit (Cayman Chemical) as previously described (Daikoku et al., 2011).
Quantitative RT-PCR
Request a detailed protocolRNAs from Foxa2f/f, Foxa2f/fLtfCre+, and Foxa2f/fPgrCre+mice uterine samples were analyzed as described previously (Sun et al., 2014; Das et al., 1995). In brief, total RNA was extracted with Trizol (Invitrogen, Waltham, MA) according to the manufacturer’s protocol. After DNase treatment (Ambion, Austin, TX), 1 µg of total RNA was reverse-transcribed with Superscript II (Invitrogen). Real-time PCR was performed using primers 5’-GACATACCGACGCAGCTACA-3’ (sense) and 5’- GCCGGTAGAAAGGGAAGAGG-3’ (anti-sense) for mouse Foxa2; 5’- GCAGATGTACCGCACTGAGATTC-3’ (sense) and 5’-ACCTTTGGGCTTACTCCATTGATA-3’ (anti-sense) for mouse Rpl7.
Statistical analysis
Request a detailed protocolEach experiment was repeated at least three times using independent samples. Data are shown as mean ± SEM. Statistical analyses were performed using a two-tailed Student’s t-test. A value of p<0.05 was considered statistically significant.
Data availability
All data are included in the manuscript.
References
-
Pre-marked chromatin and transcription factor co-binding shape the pioneering activity of Foxa2Nucleic Acids Research 47:9069–9086.https://doi.org/10.1093/nar/gkz627
-
Cadence of procreation: orchestrating embryo-uterine interactionsSeminars in Cell & Developmental Biology 34:56–64.https://doi.org/10.1016/j.semcdb.2014.05.005
-
A role for Msx genes in mammalian embryonic diapauseBioscientifica Proceedings 10:44–51.https://doi.org/10.1530/biosciprocs.10.002
-
Embryonic diapause: development on holdThe International Journal of Developmental Biology 58:163–174.https://doi.org/10.1387/ijdb.140074bm
-
The history of the discovery of embryonic diapause in mammalsBiology of Reproduction 99:242–251.https://doi.org/10.1093/biolre/ioy112
-
Developmental biology of uterine glandsBiology of Reproduction 65:1311–1323.https://doi.org/10.1095/biolreprod65.5.1311
-
Foxa2 is essential for mouse endometrial gland development and fertilityBiology of Reproduction 83:396–403.https://doi.org/10.1095/biolreprod.109.083154
-
Distinct dormancy progression depending on embryonic regions during mouse embryonic diapause†Biology of Reproduction 100:1204–1214.https://doi.org/10.1093/biolre/ioz017
-
Foxa2 regulates multiple pathways of insulin secretionThe Journal of Clinical Investigation 114:512–520.https://doi.org/10.1172/JCI21149
-
Deoxyribonucleic acid content in delayed mouse blastocystsJournal of Reproduction and Fertility 29:123–126.https://doi.org/10.1530/jrf.0.0290123
-
Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient miceMolecular Endocrinology 14:1147–1161.https://doi.org/10.1210/mend.14.8.0498
-
Ovarian LGR5 is critical for successful pregnancyFASEB Journal 28:2380–2389.https://doi.org/10.1096/fj.13-248344
-
Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyteMolecular and Cellular Biology 20:5175–5183.https://doi.org/10.1128/MCB.20.14.5175-5183.2000
-
Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeupThe Journal of Biological Chemistry 279:10649–10658.https://doi.org/10.1074/jbc.M312203200
-
Roadmap to embryo implantation: clues from mouse modelsNature Reviews. Genetics 7:185–199.https://doi.org/10.1038/nrg1808
-
Generation of mouse for conditional expression of forkhead box A2Endocrinology 159:1897–1909.https://doi.org/10.1210/en.2018-00158
-
Survival of blastocysts in the uteri of ovariectomized miceJournal of Reproduction and Fertility 17:515–520.https://doi.org/10.1530/jrf.0.0170515
-
Delayed implantation in the spayed, progesterone treated adult mouseJournal of Reproduction and Fertility 12:593–595.https://doi.org/10.1530/jrf.0.0120593
Article and author information
Author details
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD103475)
- Sudhansu K Dey
Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD068524)
- Sudhansu K Dey
National Research Foundation of Korea (NRF-2021R1A6A3A03038446)
- Yeon Sun Kim
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Katie Gerhardt for her excellent editing of the manuscript. This work was supported in parts by NIH grants (HD103475 and HD068524 to SKD). YSK is supported by a National Research Foundation of Korea (NRF) grant (NRF-2021R1A6A3A03038446).
Copyright
© 2022, Matsuo, Yuan, Kim et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,327
- views
-
- 314
- downloads
-
- 15
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
Wing dimorphism is a common phenomenon that plays key roles in the environmental adaptation of aphid; however, the signal transduction in response to environmental cues and the regulation mechanism related to this event remain unknown. Adenosine (A) to inosine (I) RNA editing is a post-transcriptional modification that extends transcriptome variety without altering the genome, playing essential roles in numerous biological and physiological processes. Here, we present a chromosome-level genome assembly of the rose-grain aphid Metopolophium dirhodum by using PacBio long HiFi reads and Hi-C technology. The final genome assembly for M. dirhodum is 447.8 Mb, with 98.50% of the assembled sequences anchored to nine chromosomes. The contig and scaffold N50 values are 7.82 and 37.54 Mb, respectively. A total of 18,003 protein-coding genes were predicted, of which 92.05% were functionally annotated. In addition, 11,678 A-to-I RNA-editing sites were systematically identified based on this assembled M. dirhodum genome, and two synonymous A-to-I RNA-editing sites on CYP18A1 were closely associated with transgenerational wing dimorphism induced by crowding. One of these A-to-I RNA-editing sites may prevent the binding of miR-3036-5p to CYP18A1, thus elevating CYP18A1 expression, decreasing 20E titer, and finally regulating the wing dimorphism of offspring. Meanwhile, crowding can also inhibit miR-3036-5p expression and further increase CYP18A1 abundance, resulting in winged offspring. These findings support that A-to-I RNA editing is a dynamic mechanism in the regulation of transgenerational wing dimorphism in aphids and would advance our understanding of the roles of RNA editing in environmental adaptability and phenotypic plasticity.
-
- Developmental Biology
The evolutionarily conserved Hippo (Hpo) pathway has been shown to impact early development and tumorigenesis by governing cell proliferation and apoptosis. However, its post-developmental roles are relatively unexplored. Here, we demonstrate its roles in post-mitotic cells by showing that defective Hpo signaling accelerates age-associated structural and functional decline of neurons in Caenorhabditis elegans. Loss of wts-1/LATS, the core kinase of the Hpo pathway, resulted in premature deformation of touch neurons and impaired touch responses in a yap-1/YAP-dependent manner, the downstream transcriptional co-activator of LATS. Decreased movement as well as microtubule destabilization by treatment with colchicine or disruption of microtubule-stabilizing genes alleviated the neuronal deformation of wts-1 mutants. Colchicine exerted neuroprotective effects even during normal aging. In addition, the deficiency of a microtubule-severing enzyme spas-1 also led to precocious structural deformation. These results consistently suggest that hyper-stabilized microtubules in both wts-1-deficient neurons and normally aged neurons are detrimental to the maintenance of neuronal structural integrity. In summary, Hpo pathway governs the structural and functional maintenance of differentiated neurons by modulating microtubule stability, raising the possibility that the microtubule stability of fully developed neurons could be a promising target to delay neuronal aging. Our study provides potential therapeutic approaches to combat age- or disease-related neurodegeneration.






