Correction: The recycling endosome protein Rab25 coordinates collective cell movements in the zebrafish surface epithelium
Main text
Willoughby PM, Allen M, Yu J, Korytnikov R, Chen T, Liu Y, So I, Macpherson N, Mitchell JA, Fernandez-Gonzalez R, Bruce AEE. 2021. The recycling endosome protein Rab25 coordinates collective cell movements in the zebrafish surface epithelium . eLife 10:e66060. doi: 10.7554/eLife.66060.
Published 23 March 2021
After publication we became aware that an earlier version of the Materials and methods section, which was unedited and incomplete, was inadvertently included in the final draft. In addition, a supplemental analysis used three plasmids to disrupt actin specifically in the yolk cell. These plasmids were incorrectly referenced to a previous publication from the lab, but they have not been published. Haoyu Wan generated the plasmids and provided key input on the experimental design for the data presented in Figure 2—figure supplement 2B. We are therefore formally correcting the eLife paper to include Haoyu Wan as a co-author on this paper. Haoyu Wan has reviewed the manuscript and supports the conclusions and agrees to be responsible for all parts of the paper in its published form. These omissions do not impact any of the results reported in the paper.
We have corrected the manuscript as follows:
#1
Original author list:
Willoughby PM, Allen M, Yu J, Korytnikov R, Chen T, Liu Y, So I, Macpherson N, Mitchell JA, Fernandez-Gonzalez R, Bruce AEE.
Corrected author list:
Willoughby PM, Allen M, Yu J, Korytnikov R, Chen T, Liu Y, So I, Wan H, Macpherson N, Mitchell JA, Fernandez-Gonzalez R, Bruce AEE.
Details for omitted author:
Haoyu Wan
Department of Cell and Systems Biology
University of Toronto, Toronto, Canada
Contribution: Resources, methodology, Writing – reviewing and editing
Competing interests: None
#2
Original section of text from the Results:
Rab25a and Rab25b are required for normal epiboly movements
To explore the functions of Rab25a and Rab25b, CRISPR/Cas9 gene editing was used to generate maternal-zygotic (MZ) mutant lines. Guide RNAs were designed to target exon two which encodes the GTPase domain, the functional domain of Rab proteins (Mitra et al., 2017). We characterized two rab25a mutant alleles from two founder fish, a 13-base pair (bp) deletion (2.3) and 29 bp insertion (4).
Corrected text:
Rab25a and Rab25b are required for normal epiboly movements
To explore the functions of Rab25a and Rab25b, CRISPR/Cas9 gene editing was used to generate maternal-zygotic (MZ) mutant lines. Guide RNAs were designed to target exon two which encodes the GTPase domain, the functional domain of Rab proteins (Mitra et al., 2017). We characterized two rab25a mutant alleles from two founder fish, a 13-base pair (bp) deletion (2.3) and a 24 bp insertion / 3 bp deletion (4).
#3
Original section of text from Materials and Methods:
CRISPR/Cas9 mutant generation
To determine Cas9 target sites against rab25a and rab25b (ZFIN ID: ZDB-Gene-041212–69; ZDB-Gene-050706–113), the CHOPCHOP tool was used (https://chopchop-cbu-uib-no.myaccess.library.utoronto.ca).
Corrected text:
To determine Cas9 target sites against rab25a and rab25b (ZFIN ID: ZDB-Gene-041212–69; ZDB-Gene-050706–113), the CHOPCHOP tool was used (https://chopchop.cbu.uib.no)
#4
Original section of text from Materials and Methods (CRISPR/Cas9 Mutant Generation):
The Ambion MegaScript T7 kit was used to transcribe sgRNA in vitro. gRNA (50 pg) was coinjected with cas9 mRNA (300 pg) (pT3TS-nCas9 [Xba1 digest] Addgene plasmid: #46,757 Jao et al., 2013; transcribed using mMESSAGE mMachine T3 kit Life technologies [AM1348]).
Corrected text:
The Ambion MegaScript T7 kit was used to transcribe sgRNA in vitro. gRNA (50 pg) was coinjected with cas9 mRNA (300 pg)(pT3TS-nCas9 [Xba1 digest] Addgene plasmid: #46,757 Jao et al., 2013; transcribed using mMESSAGE mMachine T3 kit Life technologies [AM1348]). The Zebrafish Genetics and Disease Models Core Facility at the Hospital for Sick Children generated founder Rab25a CRISPR zebrafish using the guide RNA that we designed.
#5
Original section of text from Materials and Methods (CRISPR/Cas9 Mutant Generation):
This led to the identification of two rab25a mutations from two different founder fish and one
rab25b mutation: rab25a2.3–13 BP Deletion 5’-TTGCTTTCAGTGGTTTTAATTGGAGAA———————————
AG-3’ rab25a4- 29 BP Insertion
5’TTGCTTTCAGTGGTTTTAATTGGAGAATTGGAAAGTTGGAAAGCGCAACTTTGGG
TTGGGTTGGAAAG-3’ rab25b- 9 BP Deletion 5’-TG———————CCATCACCTCTGCG
TGAGTTTG-3’
Corrected Text:
This led to the identification of two rab25a mutations from two different founder fish and one
rab25b mutation: rab25a2.3–13 bp deletion 5’-TTGCTTTCAGTGGTTTTAATTGGAGAA———————————
AG-3’ rab25a4 – 24 bp insertion / 3 bp deletion
5’TTGCTTTCAGTGGTTTTAATTGGAGAATTGGAAAGTTGGAAAGCGCAACTTTGGG
TTGGGTTGGAAAG-3’ rab25b –18 bp deletion 5’-TG———————CCATCACCTCTGCG
TGAGTTTG-3’
#6
Original section of text from Materials and Methods (Cloning): rab25b PCR products were gel extracted and recombined with pDONR221 using BP clonase. Clones were validated by sequencing. Fusion proteins and transgenic vectors were generated by gateway recombination using LR Clonase.
Corrected text:
Rab25b fusion proteins
To generate Rab25b fusion proteins, forward and reverse primers containing attB1 and attB2 BP Clonase recognition arm sequences were used to PCR amplify rab25b. attb-tagged rab25b PCR Products were gel extracted and recombined into a pDONR221 entry vector using BP Clonase (Invitrogen 11789013). Clones were identified via kanamycin selection and validated by sequencing.
attB1rab25b Forward Primer: 5’GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGGGTCTGATGAGGCCTA-3’
attB2rab25b Reverse Primer: 5’GGGGACCACTTTGTACAAGAAAGCTGGGTGTCACAAGTTTTTACAGCAGG-3’
pDONR221-rab25b vectors were recombined into a pCS2 +SP6 promoter destination vector by Gateway LR Clonase reaction (Invitrogen 11791020). Destination vectors contained a 5’ SP6 promoter sequence, followed by either eGfp or mCherry with Rab25 integrated in the 5’ to 3’ direction downstream, resulting in a N-terminally tagged Rab25b fusion protein. Sequencing was used to confirm integration and reading frame.
#7
Original section of text from Materials and Methods (Cloning): Text missing
Corrected Text:
FP2 Constructs
The constructs pFP2-DeAct-SpvB, pFP2-DeAct-GS1 and pFP2-DN-RhoA were generated to block actin polymerization/contraction in the yolk cell. pCMV-DeAct-SpvB (Addgene plasmid 89446) and pCMV-DeAct-GS1 (Addgene plasmid 89445) were gifts from Bradley Zuchero (Harterink et al., 2017) and pFP2 was kindly provided by Arne Lekven (Narayanan and Lekven, 2012). Both DeActs were PCR amplified and cloned into pCS2+. SpvB was amplified using primers containing Cla1/Stu1 restriction sequences for forward and reverse primers, respectively. PCR fragments were digested with either Cla/Stu1, and ligated into pCS2+. GS1 was amplified using primers containing BamHI/StuI restriction sequences for forward and reverse primers, respectively. PCR fragments were digested with either BamHI/StuI, and ligated into pCS2+. SpvB, GS1 and DN-Rho were cloned from pCS2 +into pFP2 using the Gibson assembly method (Gibson, et al. 2009).
Primers used to amplify DeActs were:
SpvB Forward primer:
5’- ACGATCGATGCCACCATGGGAGGTAATTCATCTCG-3’
SpvB Reverse primer:
5’-GACAGGCCTTCATGAGTTGAGTACCCTCA-3’.
GS1 Forward primer:
5’-ACGGGATCCGCCACCATGGTGGTGGAACACCCCGA-3’
GS1 Reverse primer:
5’-CCGAGGCCTTCAGAATCCTGATGCCACAC-3’.
Primers used to clone DeActs and DN-RhoA from pCS2 +into pFP2 were:
Forward primer:
5’-GGTCACTCACGCAACAATACAAGCTACTTGTTCTTTTTG-3’
Reverse primer:
5’-CATGTCTGGATCATCATTACGTAATACGACTCACTATAG-3’
#8
Original section of text from Materials and Methods: Text missing
Corrected text:
Transgenic Lines
Rab25b Transgenic rescue line
To generate a transgenic rescue construct for Rab25b, forward and reverse primers containing attB1 and attB2 BP Clonase recognition arm sequences were used to PCR amplify rab25b. attb-tagged rab25b PCR Products were gel extracted and recombined into a pDONR221 entry vector using BP Clonase (Invitrogen 11789013). Clones were identified via kanamycin selection and validated by sequencing.
attB1rab25b Forward Primer: 5’GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGGGTCTGATGAGGCCTA-3’
attB2rab25b Reverse Primer: 5’GGGGACCACTTTGTACAAGAAAGCTGGGTGTCACAAGTTTTTACAGCAGG-3’
pDONR221-rab25b vectors were recombined into a Tol2 transgenic destination vector by Gateway LR Clonase reaction (Invitrogen 11791020). Destination transgenic vectors contained two Tol2 recognition sequences that flanked divergent ß-actin and myl7 promoter sequences. Rab25b was integrated in the 5’ to 3’ orientation downstream of the ß-actin promoter sequence. Sequencing was used to confirm integration and reading frame. The myl7 promoter sequences contained a downstream RFP expression cassette for screening purposes. To generate Tg(actb1:rab25b,myl7:RFP) fish, Tol2 mRNA (25 pg) and the destination vector Tol2-RFP-myl7:ß-actin-rab25b-Tol2 (50 pg) were injected into 1 cell stage embryos. Embryos were screened at 48 hpf to confirm Myl7 heart restricted fluorescence and grown to adulthood.
Rab25a Myosin Transgenic Line
Female Tg(actb2:myl12.1-eGFP) fish were crossed to rab25a4 homozygous males. Heterozygous transgenic embryos were screened for fluorescence at 24hpf and grown to adulthood. Tg:(actb2:myl12.1-eGFP, rab25a4 (+/-)) fish were in-crossed to generate Tg:(actb2:myl12.1-eGFP, rab25a4 (-/-)) adult fish, which were screened for fluorescence at 24hpf and genotyped for the rab25a mutation by PCR/Hinf1 restriction digest.
#9
Original section of text from Materials and Methods:
Whole-mount immunohistochemistry
Antibody staining was performed as previously described (Lepage, 2014). Dilutions were as follows: rhodamine-phalloidin (1:200), anti-E-cadherin (Abcam, 1:1000), anti-ZO-1 (1:500), anti-phospho-myosin-light chain 2 Ser 19 (cell signaling, 1:100). Embryos were mounted in either 80% glycerol or 0.05% low-melt agarose. Secondary antibodies used were goat-anti-mouse Alexa 488 (Invitrogen, 1:500) and goat anti-rabbit-Cy3 (Jackson immunoresearch,1:500). Sytox green (Invitrogen) was dilution to 0.5 mM in fixative.
Corrected Text:
Whole-mount immunohistochemistry
Antibody staining was performed as previously described (Lepage, 2014). Dilutions were as follows: rhodamine-phalloidin (1:200), anti-E-cadherin (Abcam, 1:1000), anti-RAB11B (Abcam 1:200), anti-ZO-1 (ThermoFisher Scientific, 1:500), anti-phospho-myosin-light chain 2 Ser 19 (Cell Signaling, 1:100). Embryos were mounted in either 80% glycerol or 0.05% low-melt agarose. Secondary antibodies used were goat-anti-mouse Alexa 488 (Invitrogen A11001, 1:500), goat anti-rabbit Alexa 488 (Invitrogen A11008, 1:500), goat anti-rabbit-Cy3 (Jackson ImmunoResearch AB-2338006,1:500). Sytox green (Invitrogen) was diluted to 0.5 mM in fixative. For embryos co-labelled with phalloidin and antibody, phalloidin was incubated at 1:200 dilution with primary antibody overnight at 4 °C in block solution.
#10
As a result of omissions in the Material and methods section, some key resources were omitted from the table and some information was incomplete.
Original Key Resources Table:
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| chemical compound | rhodamine-phalloidin | Invitrogen | Cat #R415 | (1:200) |
| chemical compound | sytox green | Invitrogen | Cat #S7020 | (1:1000) |
| chemical compound | pHrodo Red Dextran | Invitrogen | Cat# P10361 | (1 mg/ml) |
| antibody | anti-phospho-myosin-light chain 2 Ser 19 (Rabbit polyclonal) | Cell Signalling | Cat #3,671 | IF(1:100) |
| antibody | anti-Cdh1 (Rabbit polyclonal) | AnaSpec | Cat# 55,527 s | IF (1:1000) |
| commercial assay or kit | MEGAscript T7-Transcription Kit | Ambion | Cat#AMB1334 | |
| commercial assay or kit | MegaClear Clean Up Kit | Ambion | Cat#AM1908 | |
| commercial assay or kit | mMessage mMachine T3 Transcription Kit | ThermoFisher Scientific | Cat#AM1348 | |
| commercial assay or kit | NucAway Spin Column | Ambion | Cat#AM10070 | |
| strain (D. rerio) | AB wildtype | Zebrafish International Resource Centre | RRID:BDSC_5138 | |
| strain (D. rerio) | Tg:(XIEef1a1:dclk2DeltaK-GFP) | Sepich et al., 2011 | ||
| strain (D. rerio) | Tg:(XIEef1a:eGFP-tubα8I) | Fei, et al, 2019 | ||
| strain (D. rerio) | Tg:(actb2:myl12.1-eGFP) | Maitre et al., 2012 | ||
| strain (D. rerio) | MZrab25a4 | This study | https://zfin.org/ZDB-ALT-201221-11 | |
| strain (D. rerio) | MZrab25a2.3 | This study | https://zfin.org/ZDB-ALT-201221-10 | |
| strain (D. rerio) | MZrab25b | This study | https://zfin.org/ZDB-ALT-201221-12 | |
| strain (D. rerio) | Tg(actb1:rab25b) | This study | ||
| strain (D. rerio) | MZrab25a4 Tg:(actb1:myl12.1-eGFP) | This study | ||
| strain (D. rerio) | MZrab25b Tg:(actb1:myl12.1-eGFP) | This study | ||
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: TATTTATTCACCAAGCGGTTG (for genotyping) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: GAGTGGTTCTGGGTGTGAGTC (for genotyping) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: TGTTTGCAGTGGTTCTTATTGGAG (for genotyping) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: ATTACGTTCGCTTGCAGAATTT (for genotyping) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: ATGGGGACAGATTTAGCCTACAAC (for cDNA) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: CGAAGCTGCTGCAAAAACTCCTGA (for cDNA) |
| sequence-based reagent | rab25a_F | This study | PCR primers | Forward: GGATCCATGGGGACAGATTTAGCCTACAAC (ligation into pCS2 +via restriction digest via BamH1, Xho1) |
| sequence-based reagent | rab25a_R | This study | PCR primers | Reverse: CTGGAGCGAAGCTGCTGCAAAAACTCCTGA (ligation into pCS2 +via restriction digest via BamH1, Xho1) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: CTCGAGGGCGCCACCATGGGGACAGATTTAGCCTACAAC (ligation with into pCS2 +venus via Xho1 restriction digest) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: CTCGAGCGAAGCTGCTGCAAAAACTCCTGA (ligation with into pCS2 +venus via Xho1 restriction digest) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: GGGGACAAGTTTGTACAAAAAAGCAGGCT TCATGGGGTCTGATGAGGCCTA (rab25b with attb1 for recombination into pDONR221) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: GGGGACCACTTTGTACAAGAAAGCTGGGT GTCACAAGTTTTTACAGCAGG (rab25b with attb1 for recombination into pDONR221) |
| sequence-based reagent | rab11a_F | This study | PCR Primers | Forward: AGAAAAACGGTCTGTCCTTC (qPCR) |
| sequence-based reagent | rab11a_R | This study | PCR Primers | Reverse: TCAGGATGGTCTGAAAAGCA (qPCR) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: GAAGTGACCAGAGGCTCGAT (qPCR) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: GGAGTTTTTGCAGCAGCTT (qPCR) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: TCGGAGCTCTGCTGGTTTAT (qPCR) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: GCGTGATCGTAGAGCTCCTT (qPCR) |
| sequence-based reagent | Lsm12_F | This study | PCR Primers | Forward: AGTTGTCCCAAGCCTATGCAATCAG (qPCR) |
| sequence-based reagent | Lsm12_R | This study | PCR Primers | Reverse: CCACTCAGGAGGATAAAGACGAGTC (qPCR) |
| recombinant DNA reagent | pCS2+ (plasmid) | Rupp et al., 1994 | SP6/T7 based backbone | |
| recombinant DNA reagent | pCS2 +egfp-rab25b (plasmid) | This study | egfp-Rab25b version of pCS2+ | |
| recombinant DNA reagent | pCS2 +mcherry-rab25b (plasmid) | This study | mcherry-rab25b version of pCS2+ | |
| recombinant DNA reagent | pCS2 +venus-rab25a (plasmid) | This study | venus-rab25a version of pCS2+ | |
| recombinant DNA reagent | pCS2+-mcherry-Rab11a (plasmid) | Rathbun et al. ,2020 | mcherry-Rab11a version of pCS2+ | |
| recombinant DNA reagent | pCS2+-mcherry-Mklp1 (plasmid) | Rathbun et al., 2020 | mCherry-Mklp1 version of pCS2+ | |
| recombinant DNA reagent | pCS2 +lyn-eGfp | A gift from Brian Ciruna | lyn-eGfp version of pCS2+ | |
| recombinant DNA reagent | pTol2-actb1 | A gift from Brian Ciruna | Tol2 transgenics | |
| recombinant DNA reagent | pTol2-actb1:rab25b | This study | rab25b version of pTol2-actb1 | |
| recombinant DNA reagent | pCS2+- Gfp-Utrophin | Addgene | RRID: Addgene_26737 | Gfp-Utrophin version of pCS2+ |
| recombinant DNA reagent | pFP2 | Narayanan et al., 2012 | Wnt8a enhancer based backbone | |
| recombinant DNA reagent | pFP2-GS1 | Fei et al, 2019 | GS1 version of FP2 | |
| recombinant DNA reagent | pFP2-SPVB | Fei et al, 2019 | SPVB version of FP2 | |
| recombinant DNA reagent | pFP2-DN-RhoA | This study | DN-RhoA version of FP2 | |
| recombinant DNA reagent | pT3TS-nCas9 | Jao et al., 2013 | RRID: Addgene_46757 | T3 based backbone |
Corrected Key Resources Table:
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| chemical compound | rhodamine-phalloidin | Invitrogen | Cat #R415 | (1:200) |
| chemical compound | sytox green | Invitrogen | Cat #S7020 | (1:1000) |
| chemical compound | pHrodo Red Dextran | Invitrogen | Cat# P10361 | (1 mg/ml) |
| antibody | anti-phospho-myosin-light chain 2 Ser 19 (Rabbit polyclonal) | Cell Signalling | Cat #3,671 | IF(1:100) |
| antibody | anti-Cdh1 (Rabbit polyclonal) | AnaSpec | Cat# 55,527 s | IF (1:1000) |
| antibody | anti-RAB11B (Rabbit polyclonal) | Abcam | Cat#3,612 | IF (1:200) |
| antibody | anti-ZO-1 | ThermoFisher Scientific | Cat#33–9100 ZO1-1A12 | IF (1:500) |
| commercial assay or kit | MEGAscript T7-Transcription Kit | Ambion | Cat#AMB1334 | |
| commercial assay or kit | MegaClear Clean Up Kit | Ambion | Cat#AM1908 | |
| commercial assay or kit | mMessage mMachine T3 Transcription Kit | ThermoFisher Scientific | Cat#AM1348 | |
| commercial assay or kit | NucAway Spin Column | Ambion | Cat#AM10070 | |
| strain (D. rerio) | AB wildtype | Zebrafish International Resource Centre | RRID:BDSC_5138 | |
| strain (D. rerio) | Tg:(XIEef1a1:dclk2DeltaK-GFP) | Sepich et al., 2011 | https://zfin.org/ZDB-TGCONSTRCT-090702-3 | |
| strain (D. rerio) | Tg:(XIEef1a:eGFP-tubα8I) | Fei, et al, 2019 | https://zfin.org/ZDB-ALT-170215-4 | Line: uot3Tg |
| strain (D. rerio) | Tg:(actb2:myl12.1-eGFP) | Maitre et al., 2012 | https://zfin.org/ZDB-ALT-130108-2 | Line: e2212Tg |
| strain (D. rerio) | MZrab25a4 | This study | https://zfin.org/ZDB-ALT-201221-11 | Line: uot10 |
| strain (D. rerio) | MZrab25a2.3 | This study | https://zfin.org/ZDB-ALT-201221-10 | Line: uot9 |
| strain (D. rerio) | MZrab25b | This study | https://zfin.org/ZDB-ALT-201221-12 | Line: uot11 |
| strain (D. rerio) | Tg(actb1:rab25b,myl7:RFP) | This study | https://zfin.org/ZDB-ALT-220311-4 | Line: uot16Tg |
| strain (D. rerio) | MZrab25a4 Tg:(actb1:myl12.1-eGFP) | This study | ||
| strain (D. rerio) | MZrab25b Tg:(actb1:myl12.1-eGFP) | This study | ||
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: TATTTATTCACCAAGCGGTTG (for genotyping) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: GAGTGGTTCTGGGTGTGAGTC (for genotyping) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: TGTTTGCAGTGGTTCTTATTGGAG (for genotyping) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: ATTACGTTCGCTTGCAGAATTT (for genotyping) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: ATGGGGACAGATTTAGCCTACAAC (for cDNA) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: CGAAGCTGCTGCAAAAACTCCTGA (for cDNA) |
| sequence-based reagent | rab25a_F | This study | PCR primers | Forward: GGATCCATGGGGACAGATTTAGCCTACAAC (ligation into pCS2 +via restriction digest via BamH1, Xho1) |
| sequence-based reagent | rab25a_R | This study | PCR primers | Reverse: CTGGAGCGAAGCTGCTGCAAAAACTCCTGA (ligation into pCS2 +via restriction digest via BamH1, Xho1) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: CTCGAGGGCGCCACCATGGGGACAGATTTAGCCTACAAC (ligation with into pCS2 +venus via Xho1 restriction digest) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: CTCGAGCGAAGCTGCTGCAAAAACTCCTGA (ligation with into pCS2 +venus via Xho1 restriction digest) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: GGGGACAAGTTTGTACAAAAAAGCAGGCT TCATGGGGTCTGATGAGGCCTA (rab25b with attb1 for recombination into pDONR221) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: GGGGACCACTTTGTACAAGAAAGCTGGGT GTCACAAGTTTTTACAGCAGG (rab25b with attb1 for recombination into pDONR221) |
| sequence-based reagent | rab11a_F | This study | PCR Primers | Forward: AGAAAAACGGTCTGTCCTTC (qPCR) |
| sequence-based reagent | rab11a_R | This study | PCR Primers | Reverse: TCAGGATGGTCTGAAAAGCA (qPCR) |
| sequence-based reagent | rab25a_F | This study | PCR Primers | Forward: GAAGTGACCAGAGGCTCGAT (qPCR) |
| sequence-based reagent | rab25a_R | This study | PCR Primers | Reverse: GGAGTTTTTGCAGCAGCTT (qPCR) |
| sequence-based reagent | rab25b_F | This study | PCR Primers | Forward: TCGGAGCTCTGCTGGTTTAT (qPCR) |
| sequence-based reagent | rab25b_R | This study | PCR Primers | Reverse: GCGTGATCGTAGAGCTCCTT (qPCR) |
| sequence-based reagent | SpvB-F | This study | PCR primers | Forward: ACGATCGATGCCACCATGGGAGGTAATTCATCTCG |
| sequence-based reagent | SpvB-R | This study | PCR primers | Reverse: GACAGGCCTTCATGAGTTGAGTACCCTCA |
| sequence-based reagent | GS1-F | This study | PCR primers | ACGGGATCCGCCACCATGGTGGTGGAACACCCCGA |
| sequence-based reagent | GS1-R | This study | PCR primers | CCGAGGCCTTCAGAATCCTGATGCCACAC |
| sequence-based reagent | Gibson-F | This study | PCR primers | GGTCACTCACGCAACAATACAAGCTACTTGTTCTTTTTG (for cloning from pCS2 +into pFP2) |
| sequence-based reagent | Gibson-R | This study | PCR primers | CATGTCTGGATCATCATTACGTAATACGACTCACTATAG (for cloning from pCS2 +into pFP2) |
| sequence-based reagent | Lsm12_F | This study | PCR Primers | Forward: AGTTGTCCCAAGCCTATGCAATCAG (qPCR) |
| sequence-based reagent | Lsm12_R | This study | PCR Primers | Reverse: CCACTCAGGAGGATAAAGACGAGTC (qPCR) |
| recombinant DNA reagent | pCS2+ (plasmid) | Rupp et al., 1994 | SP6/T7 based backbone | |
| recombinant DNA reagent | pCS2 +egfp-rab25b (plasmid) | This study | egfp-Rab25b version of pCS2+ | |
| recombinant DNA reagent | pCS2 +mcherry-rab25b (plasmid) | This study | mcherry-rab25b version of pCS2+ | |
| recombinant DNA reagent | pCS2 +venus-rab25a (plasmid) | This study | venus-rab25a version of pCS2+ | |
| recombinant DNA reagent | pCS2+-mcherry-Rab11a (plasmid) | Rathbun et al. ,2020 | mcherry-Rab11a version of pCS2+ | |
| recombinant DNA reagent | pCS2+-mcherry-Mklp1 (plasmid) | Rathbun et al., 2020 | mCherry-Mklp1 version of pCS2+ | |
| recombinant DNA reagent | pCS2 +lyn-eGfp | A gift from Brian Ciruna | lyn-eGfp version of pCS2+ | |
| recombinant DNA reagent | pTol2-actb1 | A gift from Brian Ciruna | Tol2 transgenics | |
| recombinant DNA reagent | pTol2-actb1:rab25b | This study | rab25b version of pTol2-actb1 | |
| recombinant DNA reagent | pCS2+- Gfp-Utrophin | Addgene | RRID: Addgene_26737 | Gfp-Utrophin version of pCS2+ |
| recombinant DNA reagent | pFP2 | Narayanan et al., 2012 | Wnt8a enhancer based backbone | |
| recombinant DNA reagent | pFP2-GS1 | This study | GS1 version of FP2 | |
| recombinant DNA reagent | pFP2-SPVB | This study | SPVB version of FP2 | |
| recombinant DNA reagent | pFP2-DN-RhoA | This study | DN-RhoA version of FP2 | |
| recombinant DNA reagent | pT3TS-nCas9 | Jao et al., 2013 | RRID: Addgene_46757 | T3 based backbone |
| recombinant DNA reagent | pSK-H2B-Rfp:5xUAS:Gfp-Dcx | Distel et al., 2010 |
#11
We have identified a typo in Figure 2—figure supplement 1F. The label sox19 should be sox17.
Original Figure 2 - figure supplement 1F:
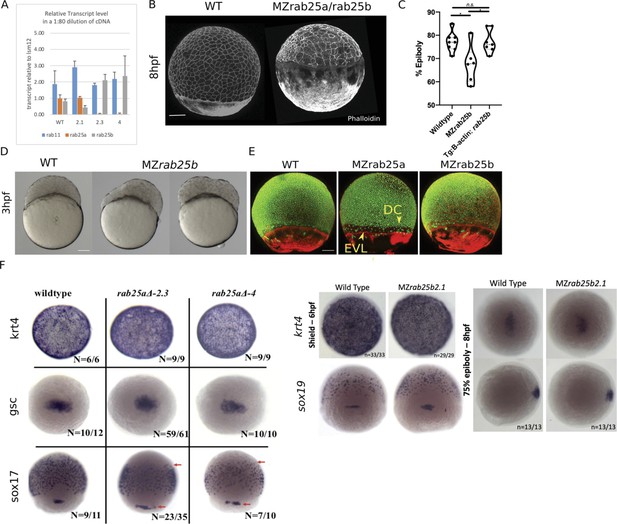
Corrected Figure 2 - figure supplement 1F:
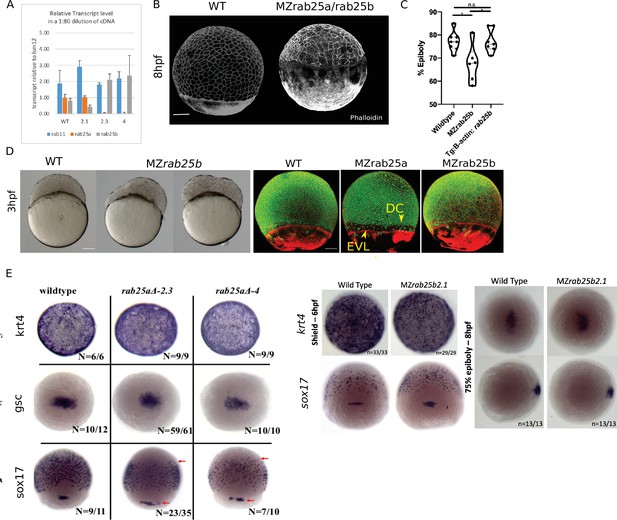
Typo in one label (sox19 instead of sox17)
#12
Additional Citations to be added to the reference section:
Distel M, Hocking JC, Volkmann K, Köster RW. 2010. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. Journal of Cell Biology 19:875–90. doi: 10.1083/jcb.201004154. PMID:21059852.
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods 6:343–5. doi: 10.1038/nmeth.1318. PMID:19363495.
Harterink M, da Silva ME, Will L, Turan J, Ibrahim A, Lang AE, van Battum EY, Pasterkamp RJ, Kapitein LC, Kudryashov D, Barres BA, Hoogenraad CC, Zuchero JB. 2017. DeActs: genetically encoded tools for perturbing the actin cytoskeleton in single cells. Nature Methods 14:479–482. doi: 10.1038/nmeth.4257 PMID:28394337.
The article has been corrected accordingly.
Article and author information
Author details
Version history
- Received:
- Accepted:
- Version of Record published:
Copyright
© 2022, Willoughby et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 239
- views
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.






