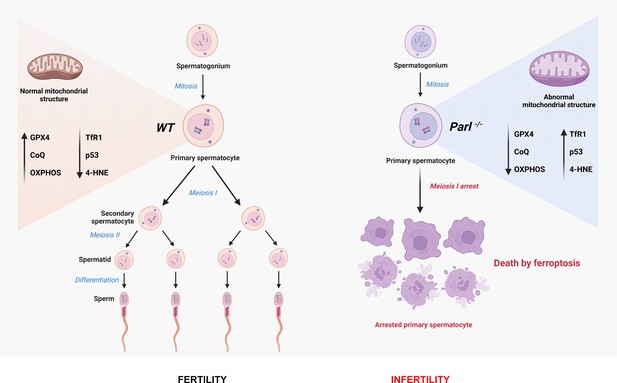
Mitochondrial defects caused by PARL deficiency lead to arrested spermatogenesis and ferroptosis
Figures
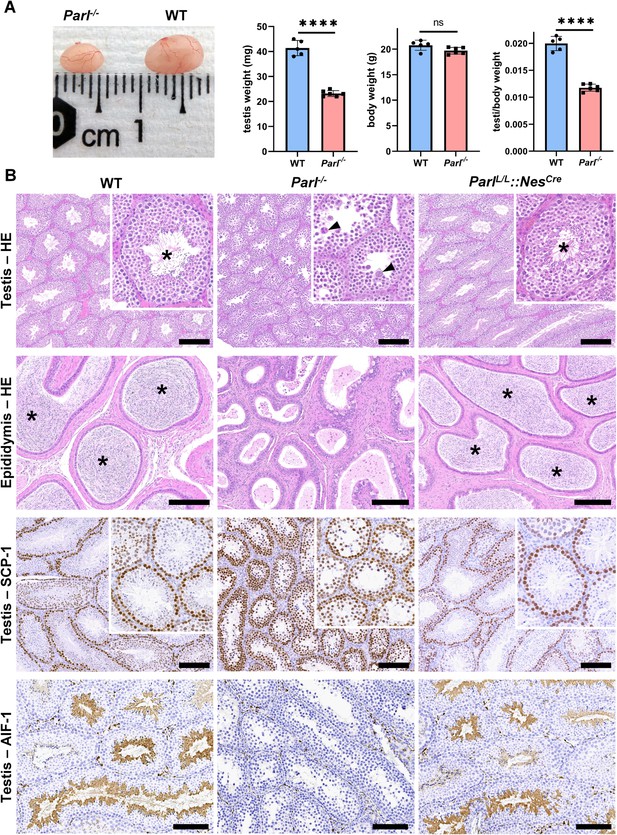
Severe testis atrophy in Parl-/- mice is caused by arrested spermatogenesis.
(A) Reduced testicular size and weight in 5-week-old Parl-/- mice (n = 5) compared to WT littermates (n = 6; unpaired two-tailed t-test, p-value<0.0001). The reduction in testicular weight is not explained by body weight differences (p=0.0598). (B) Histological assessment of testes from 6-week-old Parl-/- and WT mice reveals reduced diameter of Parl-/- seminiferous tubules with impaired germ cell maturation and complete spermatogenesis arrest at the level of primary spermatocytes (testis HE stain, n = 10 for each genotype). Parl-/- seminiferous tubules also exhibit intraluminal exfoliation of degenerated spermatocytes often in the form of multinucleated syncytia (testis HE stain inset, arrowheads). The complete arrest of spermatogenesis leads to total absence of sperm in Parl-/- seminiferous tubules and epididymis compared to WT littermates (testis and epididymis HE stain, n = 10 for each genotype; asterisks indicate mature spermatozoa in the WT). Immunohistochemistry for synaptonemal complex protein 1, SCP-1, confirms complete spermatogenesis arrest at the level primary spermatocytes in Parl-/- testis (testis SCP-1, n = 10 for each genotype). The distribution of SCP-1 expression is confined to primary spermatocytes and is lost in postmeiotic germ cells as they undergo maturation in WT seminiferous tubules. Immunohistochemistry for allograft inflammatory protein 1, AIF-1, reveals the complete absence of spermatids in Parl-/- testis while WT seminiferous tubules are densely populated by AIF-1-positive spermatids at different levels of maturation (testis AIF-1, n = 10 for each genptype). 8-week-old mice with conditional Parl deletion driven by the Nes promoter in the nervous system and Leydig cells (Parl L/L::NesCre) display a normal testicular and epididymal histology as well as SCP-1 and AIF-1 immunohistochemistry comparable to WT mice (right column, n = 4). Scale bars, 200 µm.
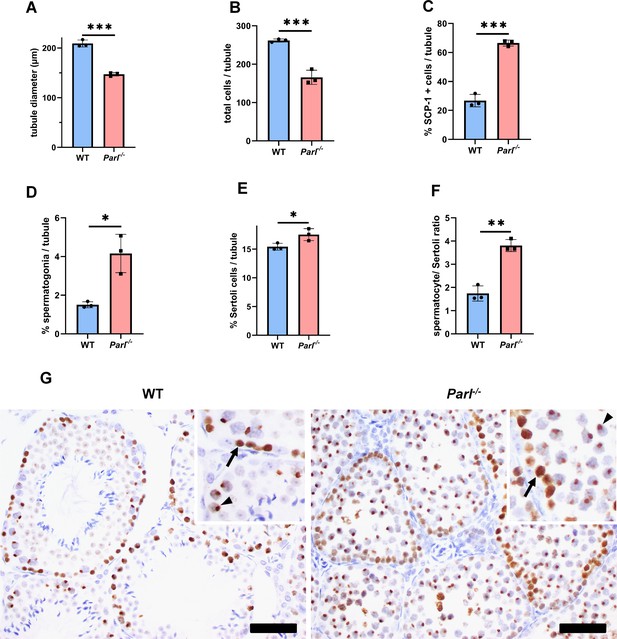
Quantitative morphometry, cell composition, and meiotic stage evaluation in 5-week-old WT and Parl-/- seminiferous tubules.
(A) Tubule diameters (n = 3 mice for each genotype; 8–12 tubules considered per mouse; p=0.0002). (B) Number of total cells/tubule (n = 3 mice for each genotype; 8–12 tubules considered per mouse;. p=0.0009). (C) Ratio between SCP-1-positive spermatocytes/total tubular cells (n = 3 mice for each genotype; 8–12 tubules considered per mouse; p=0,0001). (D) Percentage of spermatogonia/tubule quantified using c-KIT immunohistochemistry (n = 3 mice for each genotype; 8–12 tubules considered per mouse; p=0.0103). (E) Percentage of Sertoli cells/total tubular cells (n = 3 mice for each genotype; 8–12 tubules considered per mouse;. p=0.0382). Sertoli cells were identified by means of WT1 immunohistochemistry. (F) Spermatocyte/ Sertoli cells ratio determined as in (C) and (E) (n = 3 for each genotype). p=0.0021. Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test. (G) Immunohistochemistry for γH2AX (n = 3 for each genotype) shows prominent accumulation of pachytene spermatocytes, characterized by the distinct XY body positivity (insets, arrowheads), in Parl-/- tubules. On the contrary, WT testis are populated by less γH2AX-positive spermatocytes with a prevalence of zygotene spermatocytes characterized by the dispersed nuclear immunoreactivity (insets, arrows). Scale bars, 50 µm.
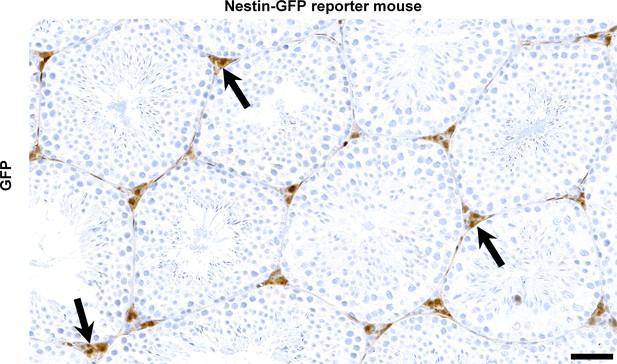
Nestin expression in Leydig cells.
Immunohistochemistry for GFP identifies diffuse signal in the Leydig cell population (arrows) of reporter mice with transgenic GFP expression under the Nes promoter (n = 3). Scale bar, 50 µm. Expression of Nes is expected to result in Cre-mediated deletion of Parl in the conditional knockout model Parl L/L::NesCre.
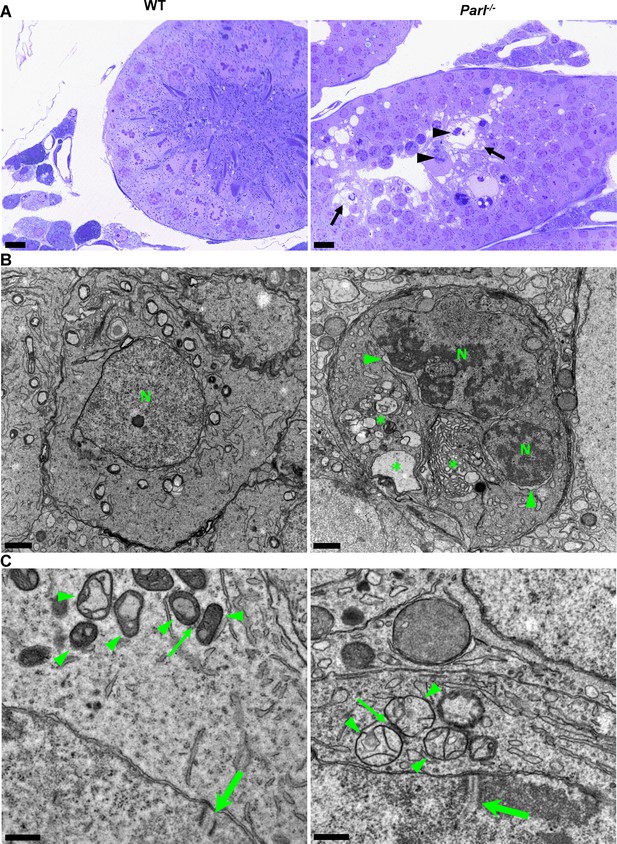
Impaired spermatogenesis in Parl-/- testis is associated with early mitochondrial morphological abnormalities and progressive degeneration of arrested spermatocytes.
(A) Toluidine blue-stained semithin sections of testis from 5-week-old WT and Parl-/- mice. Seminiferous tubules from Parl-/- mice show extensive degenerative changes in arrested spermatocytes including tortuous membrane infoldings, cytoplasmic vacuolation (arrows), irregular chromatin clumping, nuclear fragmentation (arrowheads), and absence of mature germ cells such as adluminal spermatids and spermatozoa (n = 3 for each genotype). A WT seminiferous tubule with normal germ cell maturation is shown for comparison (left panel). Scale bars, 20 µm. (B) Electron microscopy examination shows multifocal cisternae distention, disruption of the endoplasmic reticulum and Golgi apparatus, and abundant accumulation of damaged membranous material and organelles (asterisks) in Parl-/- spermatocytes. The nuclear envelope is diffusely distended (arrowheads) outlining a convoluted fragmented nucleus (N) with dense irregular clumps of chromatin. A WT spermatocyte at the end of pachytene is shown for comparison (left panel). Scale bars, 1 µm. (C) Electron microscopy analysis shows that mitochondria in Parl-/- primary spermatocytes are swollen with few thin irregular cristae and loss of normal matrix density (right panel, arrowheads) compared to WT (left panel, arrowheads). The thin arrows indicate the intermitochondrial cement (nuage) typically associated with mitochondria in primary spermatocytes. The large arrows indicate fully assembled synaptonemal complexes, structures that are only detectable during the zygotene and pachytene stages of meiotic prophase I (n = 3 for each genotype). Scale bars, 0.5 µm.
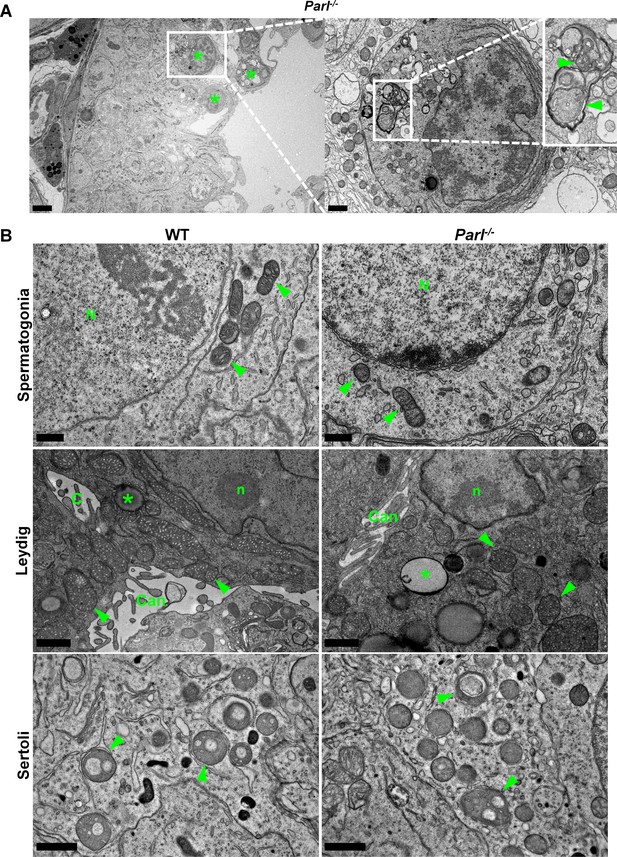
Ultrastructural abnormalities of mitochondria and other cell compartments are restricted to arrested spermatocytes and absent in other testis cell types.
(A) Degenerating/dying spermatocytes from 5-week-old Parl-/- mice are mainly observed across the adluminal compartment of the seminiferous tubule (left panel, asterisks). At higher magnification (right panel), the cytoplasm of the degenerating spermatocyte shows multifocal cisternae distention and disruption of the endoplasmic reticulum with abundant accumulation of irregular coils of membranous material wrapped around damaged organelles including mitochondria (inset, arrowheads). Irregular nuclear infoldings and chromatin clumping are also evident (n = 3). Scale bars, 5 µm (left panel) and 1 µm (right panel). (B) Ultrastructural abnormalities in 5-week-old Parl-/- mice are not evident in spermatogonia, Leydig cells, and Sertoli cells. Spermatogonia (top panel) characterized by large round nuclei (N) and scant cytoplasm with scattered small oval mitochondria with lamellar cristae (arrowheads); scale bar, 0.5 µm. Leydig cells (middle panel) typically characterized by nuclei with a single prominent nucleolus (n), intercellular canaliculi with rudimentary microvillus processes (Can), large round to elongated mitochondria with dense tubular crista (arrowheads), and scattered cytoplasmic lipid droplets (asterisks); scale bar, 1 µm. Cytoplasmic projections of Sertoli cells (bottom panel) with typical round mitochondria characterized by few often dilated tubular cristae (arrowheads) (n = 3 for each genotype). Scale bar, 0.5 µm.
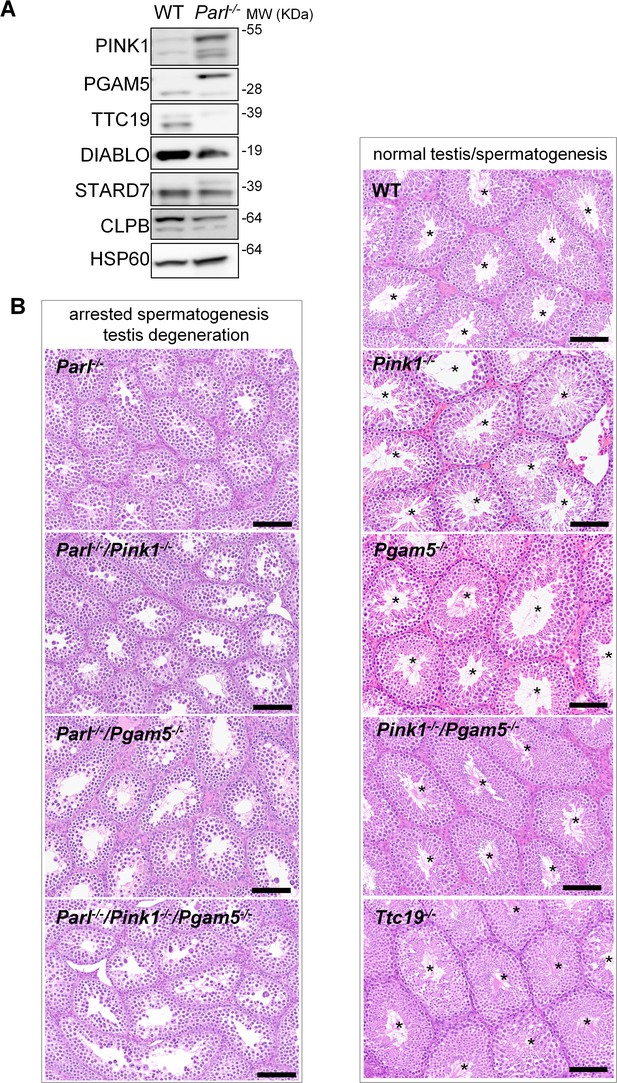
Mice with genetic manipulation of the PARL substrates PINK1, PGAM5, and TTC19 do not reproduce or modify Parl-/- testis phenotype.
(A) Immunoblots of testis mitochondria from 6-week-old WT and Parl−/− mice with antibodies for the established PARL substrates PINK1, PGAM5, TTC19, DIABLO, STARD7, and CLPB. Severe accumulation of unprocessed PINK1 and PGAM5, as well as severe decrease in the mature processed form of TTC19 are evident in Parl−/− testis. HSP60 is the loading control. (B) Histology of testes from 7-week-old mice of the indicated genotypes (HE stain, n = 3 for each genotype). Parl-/-/Pink1-/-, Parl-/-/Pgam5-/-, and Parl-/-/Pink1-/-/Pgam5-/- show complete lack of sperm production and no modification of the testicular phenotype compared to Parl-/- mice. Ttc19-/-, Pink1-/-, Pgam5-/-, and Pink1-/-/Pgam5-/- mice have no evident testis pathology and show normal sperm production (mature spermatozoa are indicated by asterisks), and are fertile. Scale bar, 145 µm.
-
Figure 3—source data 1
Original images for Figure 3A.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig3-data1-v2.pdf
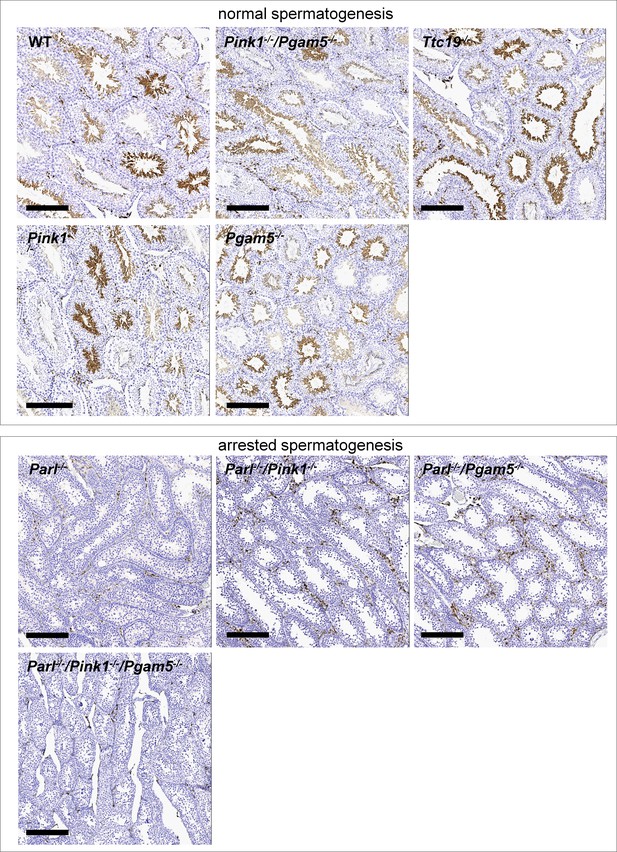
Similar to what has been described in Parl-/- mice, immunohistochemistry for AIF-1 confirms spermatogenesis arrest with complete absence of spermatids in Parl-/-/Pink1-/-, Parl-/-/Pgam5-/-, and Parl-/-/Pink1-/-/Pgam5-/- mice.
On the contrary, as observed in WT mice, normal seminiferous tubules in Pink1-/-, Pgam5-/-, Ttc19-/- and Pink1-/-/Pgam5-/- mice are populated by AIF-1-positive spermatids at different levels of maturation (n = 3 for each genotype).
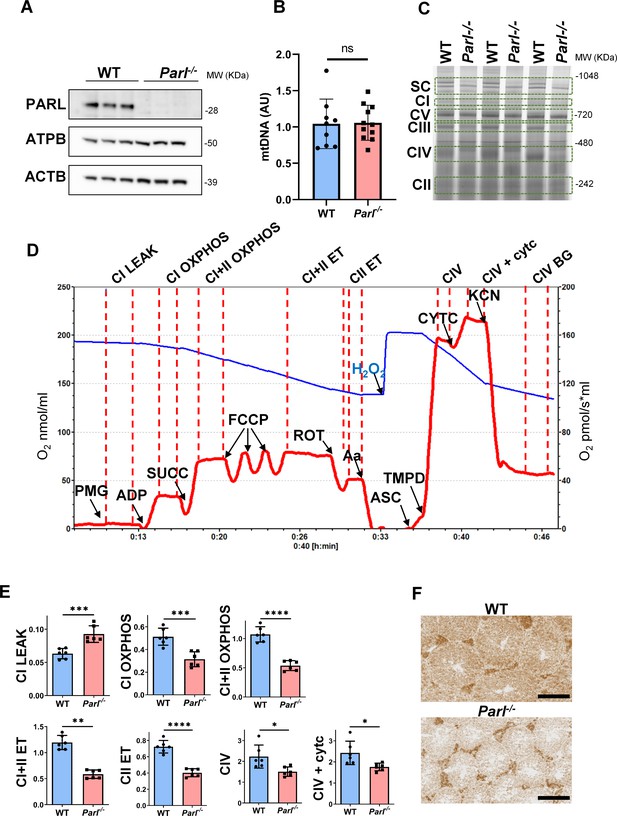
Severe mitochondrial electron transfer defects in Parl-/- testis mitochondria.
(A) Immunoblots of testis lysates from 6-week-old WT and Parl−/− mice with antibodies for PARL, ATPB, TOMM20, and ACTB (n = 3 for each genotype). ACTB is the loading control. (B) Quantification of mitochondrial DNA normalized to nuclear DNA in testis from 5-week-old WT and Parl-/- mice (n = 10 for each genotype). MtDNA was quantified by measuring the ratio (mtDNA/nDNA) between a target mitochondrial gene (Cox1) and a reference nuclear gene (B2m) using quantitative real-time PCR as detailed in the ‘Methods’ section. No significant difference is found between WT and Parl-/- testis (p=0.9146). (C) Blue native gel electrophoresis of testis mitochondria from 6-week-old WT and Parl-/- mice (n = 3 for each genotype). Mitochondrial complexes and supercomplex constituted by macromolecular assembly of complex I (CI), complex III (CIII) dimer, and complex IV (CIV) are visualized after staining with Instant Blue and marked by dotted lines. Assembly defects are evident for CI, CIII, CIV, and the supercomplex. (D) Representative trace illustrating the protocol for high-resolution respirometry in testis mitochondria. The blue trace indicates the O2 concentration (nmol/ml), and the red trace indicates its time derivative (pmol of O2 consumed/s*ml). Testis mitochondria (150 μg) were loaded in Miro6 buffer. Substrates are as follows: CI (PMG, pyruvate + malate + glutamate), CII (Succ, succinate), and CIV (ASC/TMPD, ascorbate + TMPD). The uncoupler is CCCP. The specific mitochondrial inhibitors are rotenone (ROT) for CI, antimycin a (Aa) for CIII, and cyanide (KCN) for CIV. Respiratory states are indicated between red dashed lines. CI LEAK, CI-driven leak respiration, in presence of CI substrates but no adenylates; CI OXPHOS, CI-driven phosphorylating respiration; CI+II OXPHOS, phosphorylating respiration driven by combined activation of CI and II; CI+II ET, electron transfer capacity driven by combined CI and II; CII ET, ET driven by CII; CIV, CIV-driven respiration; CIV+cytc: CIV-driven respiration after addition of exogenous cytochrome c to evaluate the integrity of the outer mitochondrial membranes; CIV BG: chemical background of CIV-driven respiration. H2O2 in the presence of catalase is used to reoxygenate the chamber. (E) Quantification of the respiratory states of testis mitochondria from 6-week-old WT and Parl-/- mice (n = 6 for each genotype) as from the protocol described in (D) and in the ‘Methods’ section. Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test: *p<0.05, **p<0.01,***p<0.001, and ****p<0.0001. (F) Cytochrome c oxidase histochemistry in frozen testis sections from 6-week-old WT and Parl-/- mice (n = 3 for each genotype).
-
Figure 4—source data 1
Original images for Figure 4A.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig4-data1-v2.pdf
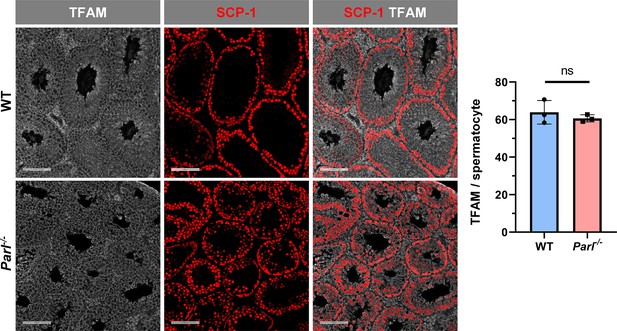
Unaltered TFAM expression in Parl-/- spermatocytes.
Normalized quantification of TFAM immunofluorescence in SCP1-positive primary spermatocytes does not reveal significant expression differences between 5-week-old WT and Parl-/- mice (n = 3 mice for each genotype, 500–1000 SCP-1-positive spermatocytes considered for each mouse; p=0.439). Scale bars, 100 µm. Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test.
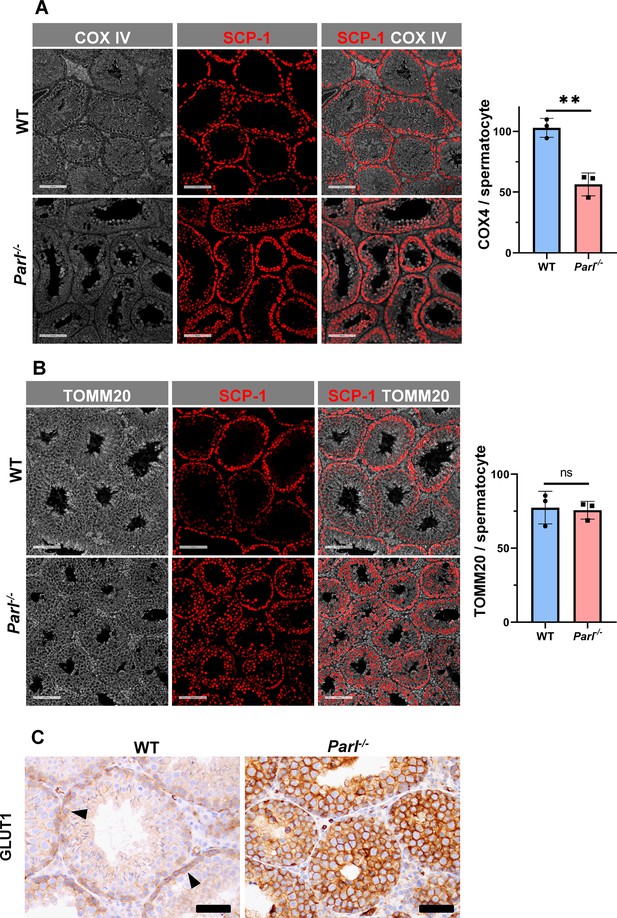
Severe loss of COX4 associated with increased expression of glucose intracellular transporter in Parl-/- spermatocytes.
(A) Quantitative immunofluorescence shows decreased expression of COX4 in SCP-1-positive spermatocytes from 5-week-old Parl-/- mice compared to WT littermates (n = 3 for each genotype, 500–1000 SCP-1-positive spermatocytes for each mouse, two-sided Student’s t-test: p=0.0027). Scale bars, 100 µm. Bar graphs indicate average ± SD. (B) Normalized quantification of TOMM20 immunofluorescence in SCP-1-positive primary spermatocytes does not reveal significant differences in mitochondrial mass in the two different genotypes (n = 3 mice for each genotype, 500–1000 SCP-1-positive spermatocytes considered for each mouse; p=0.821). Scale bars, 100 µm. Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test. (C) GLUT1 immunohistochemistry of testis from 5-week-old mice shows prominent overexpression of GLUT1 in arrested Parl-/- spermatocytes, and low levels in WT (arrowheads) (n = 3 for each genotype). Scale bars, 50 µm.
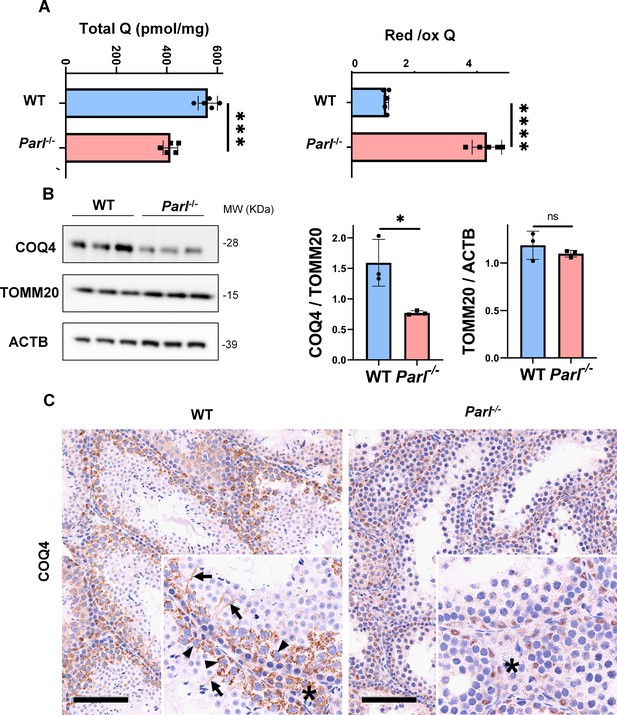
Severe alteration in coenzyme Q (CoQ) biosynthesis and redox state in Parl-/- testis.
(A) Concentration (left) and CoQ red/ox ratio (right) of total CoQ (Q9 + Q10) measured by HPLC in the testes of 5-week-old WT and Parl-/- mice (n = 5 for each genotype). Total CoQ levels are severely decreased in Parl-/- testis compared to WT littermates (p=0.0001 calculated by two-sided Student’s t-test). Moreover, the redox status is altered with drastic elevation in the reduced/oxidized CoQ ratio (p<0,0001 calculated by two-sided Student’s t-test). (B) Immunoblot analysis of total testis lysates from 5-week-old WT and Parl-/- mice with antibodies for COQ4, TOMM20, and ACTB (n = 3 for each genotype). ACTB is the total lysate loading control. TOMM20 is the mitochondrial content control. Quantification of COQ4/TOMM20 confirms a significant decrease in Parl-/- testis compared to WT littermates (n = 3; p=0,0212 calculated by two-sided Student’s t-test.) but unchanged TOMM20/ACTB (n = 3; p=0,368 calculated by two-sided Student’s t-test), indicating that the observed decrease in COQ expression is not explained by decreased mitochondrial mass. Bar graphs indicate average ± SD. (C) Immunohistochemistry for COQ4 shows severely decreased levels of testicular COQ4 expression in 5-week-old Parl-/- mice compared to WT controls (n = 3 for each genotype). The deficit is particularly prominent in Parl-/- arrested spermatocytes, almost devoid of COQ4 expression, compared to the high constitutive levels of COQ4 expression in WT spermatocytes (inset, stage II tubule, arrowheads). Decreased COQ4 expression is also evident in Parl-/- Leydig cells compared to WT mice (insets, asterisk). In addition, COQ4-positive Sertoli cell projections observed in WT mice (inset, stage II tubule, arrows) are not evident in the seminiferous tubules of Parl-/- mice. Scale bar, 100 µm.
-
Figure 6—source data 1
Original images for Figure 6B.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig6-data1-v2.pdf
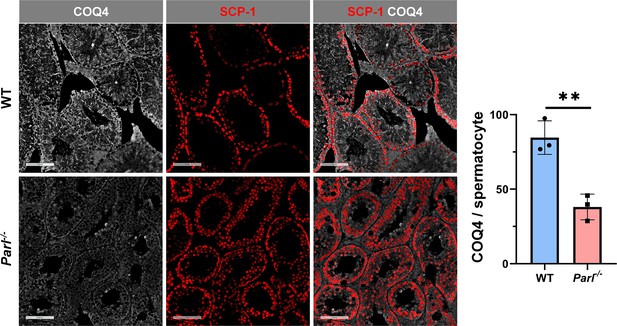
Severe loss of COQ4 in Parl-/- spermatocytes.
Normalized quantification of COQ4 immunofluorescence in SCP1-positive primary spermatocytes shows significantly higher levels of expression in 5-week-old WT compared to Parl-/- mice (n = 3 mice for each genotype, 500–1000 SCP-1-positive spermatocytes considered for each mouse, p=0.0047). Scale bars, 100 µm. Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test.
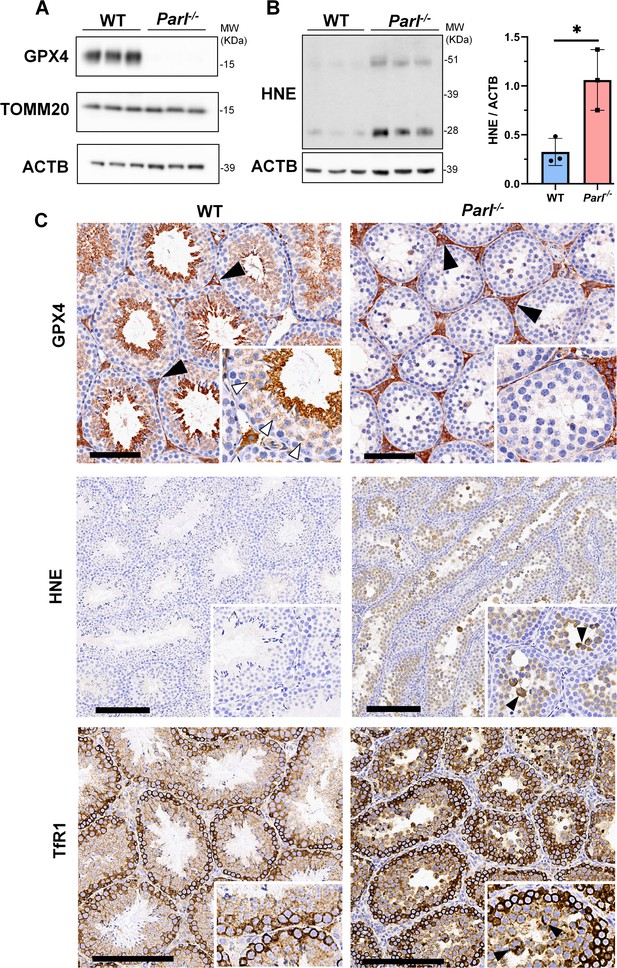
Massive ferroptosis activation in Parl-/- arrested spermatocytes.
(A) Immunoblot of total testis lysates obtained from 5-week-old WT and Parl-/- mice using antibodies for GPX4, TOMM20, and ACTB (n = 3 for each genotype). ACTB is the loading control. GPX4 expression is barely detectable in Parl-/- testis. (B) Immunoblot analysis of total testis lysates from 7-week-old WT and Parl-/- mice using anti-HNE and anti-ACTB antibodies (n = 3 for each genotype). ACTB is the loading control. Quantification of the HNE/ACTB ratio is shown on the right as a graph indicating average ± SD (n = 3 for each genotype). The statistically significant HNE/ACTB ratio increase in Parl-/- mice has been calculated by two-sided Student’s t-test (p=0.0199). (C) Immunohistochemistry for GPX4, HNE, and TfR1 in testis from 6-week-old WT and Parl-/- mice (n = 3 for each genotype). GPX4 expression is barely detectable in Parl-/- arrested spermatocytes compared to WT littermates (inset, stage X tubule, white arrowheads), while it is unaffected in interstitial Leydig cells (black arrowheads) (top panels, scale bar, 100 um). HNE immunohistochemistry shows gradual intensification of lipid peroxidation during spermatocyte degeneration culminating in adluminal/exfoliated spermatocytes (inset, arrowheads) (middle panels; scale bar, 200 µm). Similarly, TfR1 expression is abnormally increased in degenerating Parl-/- spermatocytes (bottom panels; scale bars, 200 µm; inset, arrowheads).
-
Figure 7—source data 1
Original images for Figure 7A.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-data1-v2.pdf
-
Figure 7—source data 2
Original images for Figure 7B.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-data2-v2.pdf
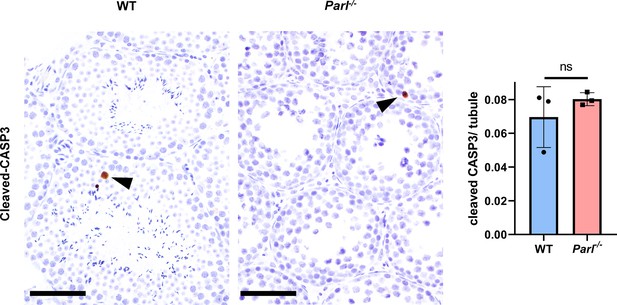
Unremarkable levels of apoptosis activation in degenerated Parl-/- testis.
Cleaved-caspase-3 immunohistochemistry on testis from 5-week-old Parl-/- mice and WT littermates (n = 3 for each genotype). The maturation defect and degenerative changes of PARL-deficient seminiferous tubules are not associated with a significant increase of caspase-dependent apoptotic cell death as confirmed by sporadic cleaved caspase-3 expression with no substantial differences among the two genotypes (left panel). Arrowheads indicate occasional apoptotic cells in the seminiferous tubules. Scale bar, 50 µm. Quantification of caspase 3-positive cells/tubule, does not show significant differences between 5-week-old Parl-/- and WT mice (right panel; p=0,374). Between 13,000 and 16,000 cells were analyzed from each animal (n = 3 mice for each genotype). Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test.
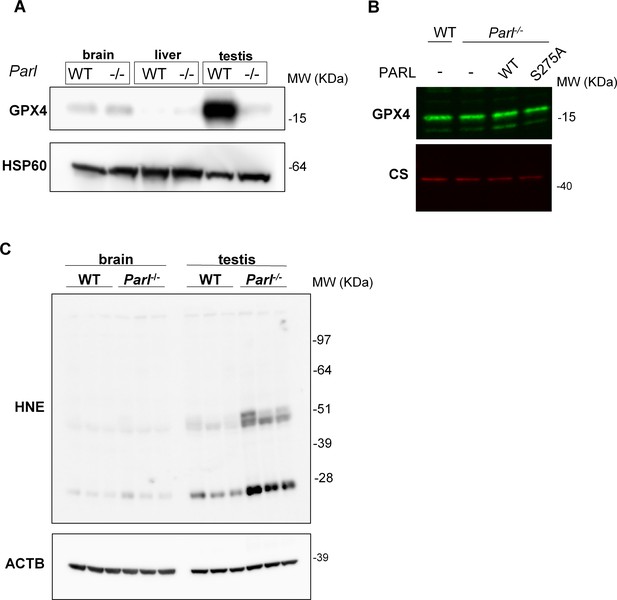
Lack of effect of PARL proteolytic activity on GPX4 expression in vitro and testis-specific induction of ferroptosis in PARL-deficient mice.
(A) Mitochondria isolated from brain, liver, and testis of 6-week-old WT and Parl-/- mice (n = 3 for each genotype) were immunoblotted with antibodies for GPX4 and HSP60. HSP60 is the loading controls. GPX4 deficiency is evident in mitochondria isolated from Parl-/- testis, but not from other organs. (B) 20 µg of total protein from WT and Parl-/- mouse embryonic fibroblasts (MEFs) complemented or not with WT or catalytic inactive PARL S275A were separated by SDS PAGE and immunoblotted with GPX4 antibody. Citrate synthase (CS) is the loading control. (C) Brain and testis total lysates obtained from 6-week-old WT and Parl-/- mice (n = 3 for each genotype) were immunoblotted with antibodies for HNE and ACTB. ACTB is the loading control. In absence of PARL, lipid peroxidation is specifically increased in testis but not in the brain.
-
Figure 7—figure supplement 2—source data 1
Original images for Figure 7—figure supplement 2A.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-figsupp2-data1-v2.pdf
-
Figure 7—figure supplement 2—source data 2
Original images for Figure 7—figure supplement 2B.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-figsupp2-data2-v2.pdf
-
Figure 7—figure supplement 2—source data 3
Original images for Figure 7—figure supplement 2C.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-figsupp2-data3-v2.pdf
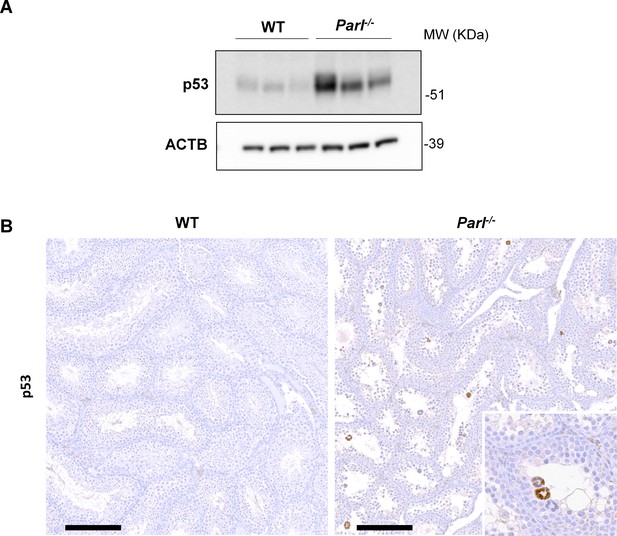
Increased activation of p53 in PARL-deficient spermatocytes undergoing ferroptosis.
(A) Immunoblot analysis of testis total lysates from 7-week-old WT and Parl-/- mice (n = 3 for each genotype) with antibodies for p53 and ACTB. ACTB is the loading control. Parl-/- testes shows increased levels of p53 compared to WT littermates. (B) Immunohistochemical analysis confirms increased p53 expression in the seminiferous tubules of 7-week-old Parl-/- mice compared to WT controls (n = 3 for each genotype). Nuclear immunolabeling is mainly detectable in the adluminal and exfoliated multinucleated spermatocytes (inset) suggesting that p53 upregulation in Parl-/- testis takes place during the late stages of degeneration. No p53 expression is detectable via immunohistochemistry in WT littermates. Scale bars, 200 µm.
-
Figure 7—figure supplement 3—source data 1
Original images for Figure 7—figure supplement 3A.
- https://cdn.elifesciences.org/articles/84710/elife-84710-fig7-figsupp3-data1-v2.pdf
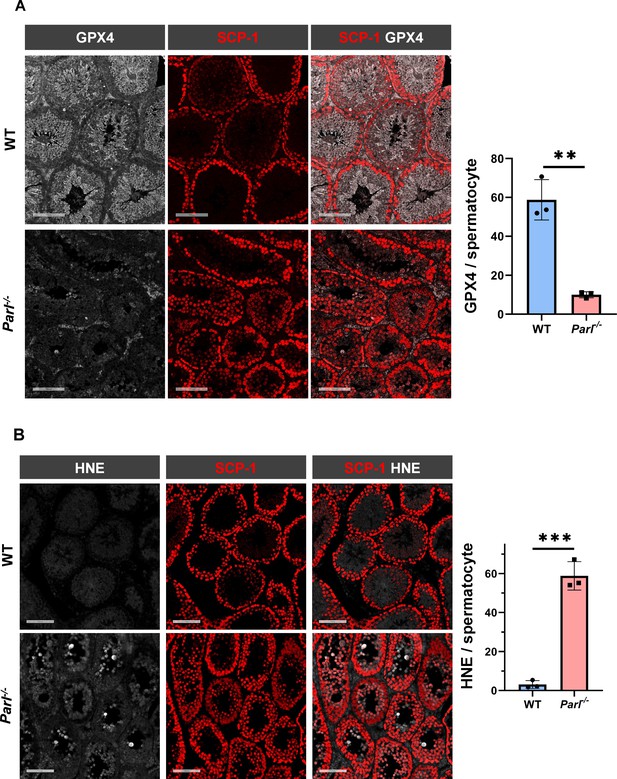
Hallmarks of ferroptosis in Parl-/- primary spermatocytes.
(A) Quantitative immunofluorescence shows severely reduced GPX4 expression in SCP-1-positive primary spermatocytes from 5-week-old Parl-/- mice compared to WT littermates (n = 3 mice for each genotype, 500–1000 SCP-1-positive spermatocytes considered for each mouse, p=0.0013). (B) Quantitative immunofluorescence shows increased HNE accumulation in Parl-/- SCP-1-positive spermatocytes compared to WT littermates (n = 3 mice for each genotype, 500–1000 SCP-1-positive spermatocytes considered for each mouse, p=0.0002). Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test. Scale bars, 100 µm.
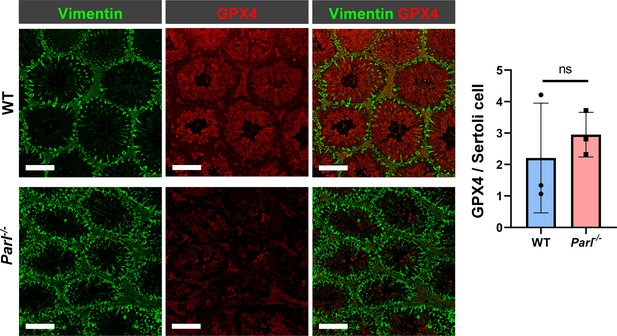
Unchanged GPX4 expression in Parl-/- Sertoli cells.
Quantitative immunofluorescence in WT and Parl-/- tubules shows very low expression of GPX4 in Vimentin-positive Sertoli cells of both genotypes. Normalized quantification of GPX4 immunofluorescence in Vimentin-positive Sertoli cells does not reveal significant expression differences between WT and Parl-/- 5-week-old mice (n = 3 mice for each genotype; 300–330 Vimentin-positive Sertoli cells considered for each mouse, p=0,5313). Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student’s t-test. Scale bars, 100 µm.
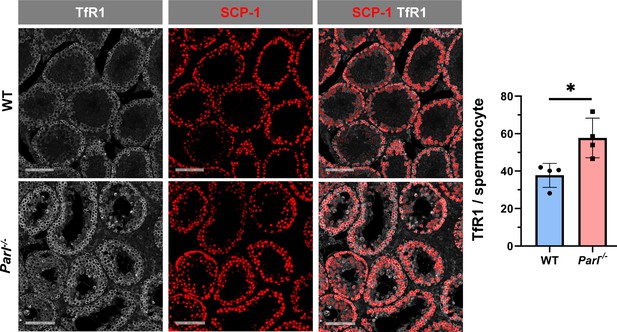
Increased transferrin receptor expression in Parl-/- spermatocytes undergoing ferroptosis.
Normalized quantification of TfR1 immunofluorescence in SCP1-positive spermatocytes shows significantly higher levels of expression in 5-week-old Parl-/- mice compared to WT littermates (n = 4 mice for each genotype, 500–100 SCP-1-positive spermatocytes considered for each mouse, p=0.0229). Bar graphs indicate average ± SD. Statistical significance calculated by two-sided Student t-test. Scale bars, 100 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | B6.129P2(Cg)-Parltm1.1Bdes/Ieg | PMID:16839884 | RRID:IMSR_EM:02075 | |
| Genetic reagent (M. musculus) | B6.129P2-Parltm1Bdes/Ieg | PMID:16839884 | RRID:IMSR_EM:02076 | |
| Genetic reagent (M. musculus) | Tg(Nes-cre)1Kln; Parltm1Bdes/Parltm1Bdes | PMID:10471508 | RRID:MGI:6280694 | |
| Genetic reagent (M. musculus) | Parltm1.1Bdes/Parltm1.1Bdes; Pgam5tm1d(EUCOMM)Wtsi/ Pgam5tm1d(EUCOMM)Wtsi | PMID:30578322 | RRID:MGI:6280688 | |
| Genetic reagent (M. musculus) | Parltm1.1Bdes/Parltm1.1Bdes; Pink1tm1.1Wrst/Pink1tm1.1Wrst | PMID:20049710 | RRID:MGI:6280687 | |
| Genetic reagent (M. musculus) | C57BL/6J-Ttc19em1Bds | PMID:30578322 | RRID:MGI:6280684 | |
| Cell line (M. musculus) | WT, Parl-/-, Parl-/-+ParlWT, Parl-/-+ParlS275A | PMID:30578322 | RRID:MGI:2159769 | |
| Antibody | Anti-SCP-1 (rabbit monoclonal) | Abcam | Cat#: ab175191 | IHC 1:200, IF 1:500 |
| Antibody | Anti-AIF1 (rabbit polyclonal) | Wako | Cat#: 019-19741; RRID:AB_839504 | IHC 1:200 |
| Antibody | Anti-COQ4 (rabbit polyclonal) | ProteinTech | Cat#: 16654-1AP; RRID:AB_2878296 | IF 1:800, IHC 1:200, WB 1:1000 |
| Antibody | Anti-GPX4 (rabbit polyclonal) | Sigma | Cat#: HPA047224; RRID:AB_2679990 | IHC 1:100, IF 1:500 |
| Antibody | Anti-HNE (rabbit polyclonal) | Alpha Diagnostic International | Cat#: HNE11-S; RRID:AB_2629282 | IF 1:10,000, IHC 1:3000 |
| Antibody | Anti-TFR1 (rabbit monoclonal) | Abcam | Cat#: ab214039; RRID:AB_2904534 | IF 1:3000, IHC 1:1000 |
| Antibody | Anti-p53 (rabbit polyclonal) | Leica/Novocastra | Cat#: NCL-L-p53-CM5p; RRID:AB_2895247 | IHC 1:300 |
| Antibody | Anti-p53 (mouse monoclonal) | Cell Signaling Technology | (1C12) Mouse mAb #2524; RRID:AB_331743 | WB 1:1000 |
| Antibody | Anti-Wilm’s tumor 1 (rabbit monoclonal) | Abcam | Cat#: ab89901; RRID:AB_2043201 | IF 1:1500 |
| Antibody | Anti-cKit (rabbit polyclonal) | Agilent/DAKO | Cat#: A4502; RRID:AB_2335702 | IHC 1:50 |
| Antibody | Anti-γH2AX (rabbit monoclonal) | Cell Signaling Technology | Cat#: 2577; RRID:AB_2118010 | IHC 1:1000 |
| Antibody | Anti-COX4 (rabbit polyclonal) | ProteinTech | Cat#:11242–1-AP; RRID:AB_2085278 | IF1:3000 |
| Antibody | Anti-GLUT1 (rabbit monoclonal) | Cell Signaling Technology | Cat#:73015 | IHC 1:600 |
| Antibody | Anti-TOMM20 (rabbit polyclonal) | ProteinTech | Cat#: 73015; RRID:AB_2207530 | IF 1:4000 |
| Antibody | Anti-TFAM (rabbit polyclonal) | Abcam | Cat#: ab307302 | IF 1:3000 |
| Antibody | Anti-PARL (rabbit polyclonal) | PMID:16839884 | Cat#: N/A | WB 1/1000 |
| Antibody | Anti-actin (mouse monoclonal) | Sigma | Cat#: A5441; RRID:AB_476744 | WB 1:200,000 |
| Antibody | Anti-HSP60 (mouse monoclonal) | BD Biosciences | Cat#: 611562; RRID:AB_399008 | WB 1:50,000 |
| Antibody | Anti-ATP5B (mouse monoclonal) | Abcam | Cat#: ab14730; RRID:AB_301438 | WB 1:50,000 |
| Antibody | Anti-TOMM20 (rabbit polyclonal) | Santa Cruz | Cat#: sc-11415; RRID:AB_2207533 | WB 1:5000 |
| Antibody | Anti-PINK1 (rabbit polyclonal) | Cayman | Cat#: 10006283; RRID:AB_10098326 | WB 1:500 |
| Antibody | Anti-PGAM5 (rabbit polyclonal) | Sigma | Cat#: HPA036979; RRID:AB_10960559 | WB 1:250 |
| Antibody | Anti-TTC19 (rabbit polyclonal) | Sigma | Cat#: HPA052380; RRID:AB_2681806 | WB 1:2000 |
| Antibody | Anti-CLPB (rabbit polyclonal) | ProteinTech | Cat#: 15743-1-AP; RRID:AB_2847900 | WB 1:1000 |
| Antibody | Anti-STARD7 (rabbit polyclonal) | ProteinTech | Cat#: 15689-1-AP; RRID:AB_2197820 | WB 1:2000 |
| Antibody | Anti-DIABLO (rabbit polyclonal) | Cell Signaling Technology | Cat#: 15108; RRID:AB_2798711 | WB 1:1000 |
| Antibody | Anti-GPX4 (mouse monoclonal) | R&D Systems | Cat#: MAB5457; RRID:AB_2232542 | WB 1:1000 |
| Antibody | Anti-GPX4 (mouse monoclonal) | Santa Cruz | Cat#: sc-166570; RRID:AB_2112427 | WB 1:1000 |
| Antibody | Anti-HNE (mouse monoclonal) | R&D Systems | Cat#: 198960; RRID:AB_664165 | WB 1:500 |
| Antibody | Anti-citrate synthase (mouse monoclonal) | Abcam | Cat#: ab96600; RRID:AB_10678258 | WB 1:1000 |
| Software, algorithm | GraphPad Prism software | GraphPad Prism (https://graphpad.com) | RRID:SCR_015807 | |
| Software | ImageJ software | ImageJ (http://imagej.nih.gov/ij/) | RRID:SCR_003070 |