Exploring the K+ binding site and its coupling to transport in the neurotransmitter:sodium symporter LeuT
eLife assessment
The bacterial neurotransmitter:sodium symporter homoglogue LeuT is a well-established model system for understanding the fundamental basis for how human monoamine transporters, such as those for dopamine and serotonin, couple ions with neurotransmitter uptake. Here the authors provide convincing data to show that K+ binding on the intraceullular side catalyses the return step of the transport cycle in LeuT by binding to one of the two sodium sites. The mechansitic consequences of K+ binding could either facilitate LeuT re-setting and/or prevent the rebinding and possible efflux of Na+ and substrate.
https://doi.org/10.7554/eLife.87985.3.sa0Fundamental: Findings that substantially advance our understanding of major research questions
- Landmark
- Fundamental
- Important
- Valuable
- Useful
Convincing: Appropriate and validated methodology in line with current state-of-the-art
- Exceptional
- Compelling
- Convincing
- Solid
- Incomplete
- Inadequate
During the peer-review process the editor and reviewers write an eLife Assessment that summarises the significance of the findings reported in the article (on a scale ranging from landmark to useful) and the strength of the evidence (on a scale ranging from exceptional to inadequate). Learn more about eLife Assessments
Abstract
The neurotransmitter:sodium symporters (NSSs) are secondary active transporters that couple the reuptake of substrate to the symport of one or two sodium ions. One bound Na+ (Na1) contributes to the substrate binding, while the other Na+ (Na2) is thought to be involved in the conformational transition of the NSS. Two NSS members, the serotonin transporter (SERT) and the Drosophila dopamine transporter (dDAT), also couple substrate uptake to the antiport of K+ by a largely undefined mechanism. We have previously shown that the bacterial NSS homologue, LeuT, also binds K+, and could therefore serve as a model protein for the exploration of K+ binding in NSS proteins. Here, we characterize the impact of K+ on substrate affinity and transport as well as on LeuT conformational equilibrium states. Both radioligand binding assays and transition metal ion FRET (tmFRET) yielded similar K+ affinities for LeuT. K+ binding was specific and saturable. LeuT reconstituted into proteoliposomes showed that intra-vesicular K+ dose-dependently increased the transport velocity of [3H]alanine, whereas extra-vesicular K+ had no apparent effect. K+ binding induced a LeuT conformation distinct from the Na+- and substrate-bound conformation. Conservative mutations of the Na1 site residues affected the binding of Na+ and K+ to different degrees. The Na1 site mutation N27Q caused a >10-fold decrease in K+ affinity but at the same time a ~3-fold increase in Na+ affinity. Together, the results suggest that K+ binding to LeuT modulates substrate transport and that the K+ affinity and selectivity for LeuT is sensitive to mutations in the Na1 site, pointing toward the Na1 site as a candidate site for facilitating the interaction with K+ in some NSSs.
Introduction
The family of neurotransmitter:sodium symporters (NSSs) include the transporters responsible for the reuptake of neurotransmitters from the extracellular space following synaptic transmission. Of pronounced interest to neuropharmacology is the subclass of monoamine transporters (MATs), including the dopamine transporter (DAT), the norepinephrine transporter (NET), and the serotonin transporter (SERT), which are molecular targets for a range of psychopharmaceuticals, including drugs against depression, anxiety, neuropathic pain, attention-deficit hyperactivity disorder (ADHD), and narcolepsy (Kristensen et al., 2011; Aggarwal and Mortensen, 2017). They are also targets for psychostimulant drugs of abuse, such as cocaine and amphetamine (Jayanthi and Ramamoorthy, 2005; Di Giovanni et al., 2016). Thus, understanding the molecular basis underlying their ligand binding and transport are of physiological and pharmacological interest.
The NSS member LeuT, a hydrophobic amino acid transporter originating from the thermophile bacterium Aquifex aeolicus, was the first NSS for which the structure solved (Yamashita et al., 2005). LeuT has served as a structural and mechanistic model for NSS proteins (Yamashita et al., 2005; Loland, 2015). All NSS proteins are thought to share the LeuT-fold structure, comprising 10 transmembrane (TM) segments ordered in a pseudo-symmetry in the plane of the membrane between the first and the second five TMs. The LeuT-fold is mostly followed by two C-terminal TMs. Comparing the available structures stabilized in different states of the transport cycle suggests an overall similar transport mechanism, substantiating LeuT as a valid model protein (Krishnamurthy and Gouaux, 2012; Wang et al., 2015; Yang and Gouaux, 2021; Shahsavar et al., 2021; Motiwala et al., 2022). The substrate binding site is located halfway through the core of the protein and consists of coordinating residues from TM1, -3, -6, and -8 (Yamashita et al., 2005). It is flanked by two Na+ binding sites, Na1 and Na2. The location and the residues forming the Na1 and Na2 sites are quite conserved between LeuT and the MATs. In the Na1 site, only one of the four coordinating residues differ by a serine to threonine substitution. In the Na2 site, two of the five residues are substituted between LeuT and MATs. A conserved feature shaping the Na+ binding sites is the helical unwinding in TM1 and TM6, which exposes backbone-carbonyl oxygen atoms to partake in the ion coordination (Yamashita et al., 2005). In LeuT, the Na1 ion is also coordinated by the carboxyl-group of the substrate, yielding substrate binding highly Na+-dependent (Yamashita et al., 2005).
The sodium gradient across the cell membrane is essential for driving substrate uptake in NSS proteins through occupation of the Na+ sites. In addition, it has for long been recognized that SERT antiports K+ as studies have shown that serotonin uptake was accelerated by an outward-directed K+ gradient (Nelson and Rudnick, 1979). Consequently, K+ antiport was suggested to increase the rate of the return step, which is thought to be the rate-limiting step of the transport cycle (Hasenhuetl et al., 2016; Fitzgerald et al., 2019). Cryo-EM reconstruction of SERT obtained in KCl revealed an inward-open conformation, suggesting this to be the most prevalent structure with KCl (Yang and Gouaux, 2021). The resolution of this structure, however, did not allow unambiguous identification of densities for bound ions. Thus, while the conformational details of the K+-bound state of SERT are emerging, the location of the K+ binding site remains unknown and the antiport mechanism not fully understood. We have shown that K+ is antiported also by DAT from Drosophila melanogaster (dDAT) by a mechanism that shares similarities with K+ antiport in SERT (Schmidt et al., 2022).
Previously, we have reported that LeuT interacts with K+ and that the binding favors an outward-closed conformation (Billesbølle et al., 2016). Intra-vesicular K+ also increased the concentrative capacity of [3H]alanine by LeuT, possibly by decreasing substrate efflux, but the details of K+’s interaction with LeuT were not fully explored (Billesbølle et al., 2016). Here, we use purified LeuT, either in detergent micelles or reconstituted into proteoliposomes, to characterize the molecular components of how K+ binds to LeuT, and how it influences the kinetics of substrate transport and the underlying conformational dynamics. Moreover, we mutate Na1 site residues and observe the effect on Na+ and K+ binding. Analogous to how LeuT for long has served as a structural model for the NSS family of proteins, it here serves as a functional model to suggest a role for K+ in the transport mechanism.
Results
We have previously shown that K+ inhibits Na+-dependent [3H]leucine binding to LeuT and changes its conformational equilibrium (Billesbølle et al., 2016). However, fundamental questions remain to be addressed: Is the effect of K+ the result of direct binding to a specific binding site in LeuT? If so, where is this site located? What are the kinetic mechanisms underlying the effect of K+ on [3H]alanine transport? To address this, we expressed His-tagged LeuT in Escherichia coli, harvested and solubilized the membranes in n-dodecyl-β-D-maltoside (DDM) and purified the protein by immobilized metal affinity chromatography. Purified LeuT was then used for radioligand binding assays, to analyze the consequences of ion binding on conformational dynamics by transition metal ion FRET (tmFRET), as well as for reconstitution into proteoliposomes for transport assays.
K+ binding is competing with Na+ and saturable
To characterize the relationship between Na+ and K+ binding to LeuT, we investigated the effect of K+ on Na+-dependent [3H]leucine binding using the scintillation proximity assay (SPA) (Quick and Javitch, 2007). Accordingly, we performed a Na+ titration with 100 nM [3H]leucine (Figure 1A). Choline (Ch+), seemingly inert to LeuT (Billesbølle et al., 2016), was used as counter ion to maintain the ionic strength. Na+ promoted the binding of [3H]leucine to LeuT with an EC50 for Na+ of 7.7 mM, in line with previous studies (Zhao et al., 2011; Shi et al., 2008). In the presence of K+, however, the EC50 right-shifted while the Bmax remained unchanged, consistent with a competitive mechanism of inhibition between Na+ and K+ as suggested previously (Billesbølle et al., 2016).
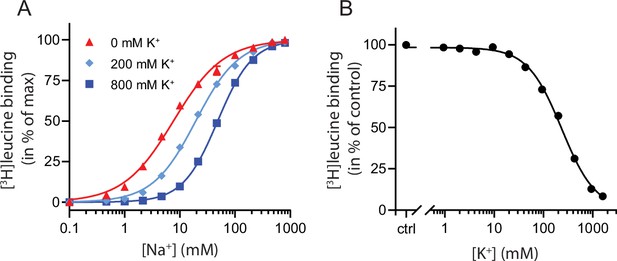
K+ competitively inhibits Na+-mediated [3H]leucine binding in LeuT.
(A) Na+-mediated [3H]leucine (10× Kd) binding to purified LeuT in the absence (red triangles) or presence of 200 mM (light blue diamond) or 800 mM (blue squares) K+. Data are normalized to Bmax and fitted to a Hill equation, yielding EC50 values in 0, 200, and 800 mM K+ of 7.7 [7.3; 8.1], 19.4 [19.1; 19.8], and 48.5 [47.5; 49.5] mM, respectively. (B) K+-dependent displacement of Na+-mediated [3H]leucine binding in the presence of 7.7 mM Na+, which corresponds to the EC50 determined in (A). Data are normalized to a control with 0 mM K+ and fitted to a Hill equation with an IC50 value of 234.8 [224.8; 243.1] mM. All data points are shown as mean ± standard error of the mean (s.e.m.), n=3–6 conducted in triplicates. Error bars often smaller than data points. EC50 and IC50 values are reported as mean [s.e.m. interval]. The ionic strength was maintained by substituting Na+ and K+ with Ch+. All data is provided in the source data file.
-
Figure 1—source data 1
Excel file containing data for (A) Na+-mediated [3H]leucine binding to purified LeuT in the absence of K+, and (B) K+-dependent displacement of Na+-mediated [3H]leucine binding.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig1-data1-v1.zip
To determine whether the inhibition of [3H]leucine binding by K+ was saturable, we titrated in K+ in the presence of Na+ and [3H]leucine. Displacement of [3H]leucine binding by K+ is concentration-dependent, with an IC50 for K+ of 235 [225; 243] mM (mean [s.e.m. interval]) (Figure 1B). To ensure that the displacement of [3H]leucine by K+ was not caused by artifacts originating from the high total salt concentrations (1.6 M), we repeated the displacement assay with a total ionic strength of 208 mM salt using either Ch+ or N-methyl-D-glucamine (NMDG+), both of which are inert to LeuT, as counter ions. Again, K+ dose-dependently displaced [3H]leucine binding with an inhibition constant not significantly different from that measured in the high salt conditions (Figure 1—figure supplement 1). Although the approach is indirect with respect to K+, this saturable, ionic strength-independent displacement of [3H]leucine binding is indicative of a specific, low affinity K+ binding site in LeuT.
The binding of ions is reflected in changes in the conformational equilibrium of LeuT
To obtain a more direct readout of K+ binding to LeuT, we turned to tmFRET. This method relies on the distance-dependent quenching of a cysteine-conjugated fluorophore (FRET donor) by a transition metal (FRET acceptor), here Ni2+, coordinated to an engineered α-helical His-X3-His site (Taraska et al., 2009a). In LeuT, we have inserted the His-X3-His site in extracellular loop (EL)4a (A313H-A317H) and a cysteine at the top of TM10 (K398C) that is labeled with fluorescein-5-maleimide (F5M). The distance between these FRET probes changes upon opening and closing of the extracellular gate in LeuT (Figure 2A and Figure 2—figure supplement 1A). Importantly, this construct, LeuT A313H-A317H-K398F5M (from here and on named LeuTtmFRET), retains WT activity with respect to ligand binding affinities (Billesbølle et al., 2016). Accordingly, changes in tmFRET intensity is a conformational readout for both ion and ligand binding.
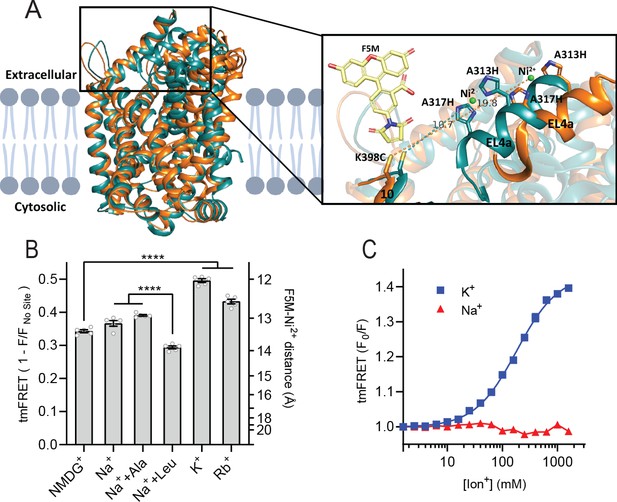
K+ shifts the conformational equilibrium toward an outward-closed state.
(A) Left: cartoon representation of the superimposed structures of LeuT in the outward-open Na+-bound state (orange; PDB-ID 3TT1) and inward-open state (turquoise; PDB-ID 3TT3), viewed parallel to the plane of the membrane. Right: enlarged view of the top of transmembrane (TM)10 and extracellular loop (EL)4a, carrying the fluorescein-5-maleimide (F5M) (yellow, shown adjacent to K398C) and the Ni2+-chelating His-X3-His site (A313H-A317H). Dashed lines indicate for both structures the distance between the coordinated Ni2+ ion (green) and the side chain sulfur of K398C (10.7 Å in 3TT3 and 19.8 Å in 3TT1). (B) Transition metal ion FRET (tmFRET) efficiencies (1 – F/Fno site) obtained for LeuT in 800 mM of the ions specified (±50 µM leucine or 200 µM alanine when indicated) upon saturation of the His-X3-His site with 10 mM Ni2+. Right vertical axis shows FRET efficiencies converted to distances by the FRET equation. ****p<0.0001 represents the significance levels from a Tukey multiple comparison-corrected one-way analysis of variance (ANOVA). (C) tmFRET (F0/F) as a function of Na+ (red triangles) or K+ (blue squares) performed on LeuTtmFRET in the presence of 750 µM Ni2+. The K+ response fitted to a Hill equation yields a Hill slope of 1.15±0.03 (mean ± s.e.m.) and an EC50 of 182.6 [176.4; 189.1] mM, mean [s.e.m. interval]. All data points represent mean ± s.e.m. (error bars often smaller than data points), n=3–5 conducted in triplicates. All data is provided in the source data file.
-
Figure 2—source data 1
Excel file containing data for Figure 2B and C providing transition metal ion FRET (tmFRET) efficiencies obtained for by the added ions in 10 mM Ni2+.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig2-data1-v1.zip
To determine the Ni2+ concentrations required to saturate the His-X3-His site, we first recorded the FRET efficiency as a function of increasing Ni2+ for LeuT incubated with NMDG+, K+, or Na+± leucine (Figure 2—figure supplement 1B). To ensure close to saturating conditions for both Na+ and K+ while preserving the ionic strength, we applied 800 mM of the ions. The FRET efficiencies at saturating Ni2+ concentrations reflect the average distance between the FRET probes and showed that K+ uniquely stabilizes a high FRET state, suggesting a shift in the conformational equilibrium of the transporter toward an outward-closed state by K+ (Figure 2—figure supplement 1A and B). The Ni2+ affinity for the His-X3-His site is increased ~3-fold when Na+ or K+ is added to LeuT relative to NMDG+. This indicates that both Na+ and K+ bind and stabilize LeuT, including the His-X3-His motif, whereas NMDG+ does not.
We proceeded by measuring the FRET efficiency only at saturating Ni2+ concentrations (10 mM) to obtain FRET efficiencies independent of potential differences in Ni2+ affinities. In addition to the conditions above, we measured FRET efficiencies for LeuT incubated in Na+ with alanine and in Rb+ (Figure 2B). Interestingly, applying Rb+, a cation often seen to be able to substitute for K+ in biological systems, also stabilized a more outward-closed state relative to that in NMDG+. The FRET efficiency in the Na+/leucine-bound state was decreased relative to that in the Na+-bound state, suggesting a, on average, more open-to-out state when adding leucine. We speculate that LeuT adopts a more conformationally restricted equilibrium upon binding of leucine relative to that with Na+ alone, and that this is reflected in a lower FRET efficiency (Figure 2—figure supplement 1A). This is also in line with the observation that alanine gives rise to a higher FRET efficiency than leucine (Figure 2B) as alanine binding allows a higher degree of conformational freedom in the transporter (Zhao et al., 2011; Calugareanu et al., 2022).
The large difference in tmFRET efficiencies between the apo state with NMDG+ and the K+-bound state of LeuTtmFRET allowed us to use the K+-induced change in conformational dynamics as a proxy for K+ binding. To estimate the affinity for K+ to apo state LeuT, we recorded the FRET efficiencies for LeuTtmFRET incubated with Ni2+ in increasing concentrations of K+ (Figure 2C). We observed an increase in FRET as a function of K+ yielding an EC50 of 183 [176; 189] mM, in line with the affinity determined by K+-dependent displacement of Na+ and [3H]leucine (Figure 1B). This EC50 is also in line with the affinity from the Schild analysis performed previously (Billesbølle et al., 2016). While Na+ did not impose major conformational changes, we found that also Rb+ induced a conformational response resembling that of K+, suggesting that Rb+ can indeed substitute for K+ although with a lower apparent affinity (Figure 2—figure supplement 1C). Of note, the high salt concentrations did not affect the intrinsic fluorescence properties of the fluorophore, validating that the responses to titration of the ions were direct results of conformational changes (Figure 2—figure supplement 1D). Along with the effect of K+ on Na+-dependent [3H]leucine binding, this finding supports the existence of a specific K+ binding site in LeuT, and that K+ binding to this site induces an outward-closed conformation.
K+ increases the rate of substrate uptake by LeuT
We have previously shown for LeuT reconstituted into liposomes that intra-vesicular K+ increases the concentrative capacity of [3H]alanine, probably by decreasing its efflux (Billesbølle et al., 2016). To expand on these findings and to characterize how substrate transport was affected by the cations in the intra-vesicular buffer, we reconstituted purified LeuT into liposomes containing either Na+, NMDG+, Cs+, Rb+, or K+ (Figure 3A). Under each of these conditions, we measured time resolved [3H]alanine uptake driven by a Na+ gradient (Figure 3B). With equimolar intra- and extra-vesicular Na+ concentrations, no [3H]alanine transport was observed, indicating that the established Na+ gradient did drive [3H]alanine uptake (Figure 3B). Interestingly, proteoliposomes containing K+ displayed a 2.5-fold increase in concentrative capacity compared to those containing Cs+ or NMDG+ (Figure 3—figure supplement 1; Table 1). To ensure that variations in the amount of active LeuT in the proteoliposomes did not affect the uptake capacity, we correlated it to the relative number of active LeuT under each condition (Figure 3—figure supplement 1A). The concentrative capacity with Rb+ was similar to that with K+, indicating that Rb+ can functionally substitute for K+. These data suggest that K+, and Rb+, are not obligate for LeuT transport, but add a concentrative potential for accumulation of alanine in addition to that obtained solely by the Na+ gradient.
LeuT mutant affinities for leucine and alanine.
| Leucine affinityKd (nM) | n | Alanine affinityKi (µM) | n | |
|---|---|---|---|---|
| WT | 6.9 [6.7; 7.2]A | 4 | 1.61 [1.58; 1.65] | 4 |
| A22V | 5.4 [4.9; 5.9]A | 3 | 1.02 [0.97; 1.08] | 4 |
| A22S | 8.4 [7.7; 9.1]A | 3 | 2.11 [2.05; 2.17] | 4 |
| N27Q | 1590 [1530; 1650] | 3 | 255 [248; 263] | 3 |
| T254S | 5.6 [5.4; 5.8]A | 3 | 1.17 [1.12; 1.21] | 3 |
| N286Q | 1460 [1390; 1540] | 3 | 287 [277; 297] | 3 |
| E290Q | 193 [169; 221] | 4 | 30.0 [28.9; 31.2] | 3 |
-
Table 1—source data 1
Pdf file with affinity curves for leucine and alanine binding to LeuT and mutants.
- https://cdn.elifesciences.org/articles/87985/elife-87985-table1-data1-v1.docx
-
Table 1—source data 2
Excel file containing data for leucine and alanine affinities for LeuT WT and all investigated mutants.
- https://cdn.elifesciences.org/articles/87985/elife-87985-table1-data2-v1.xlsx
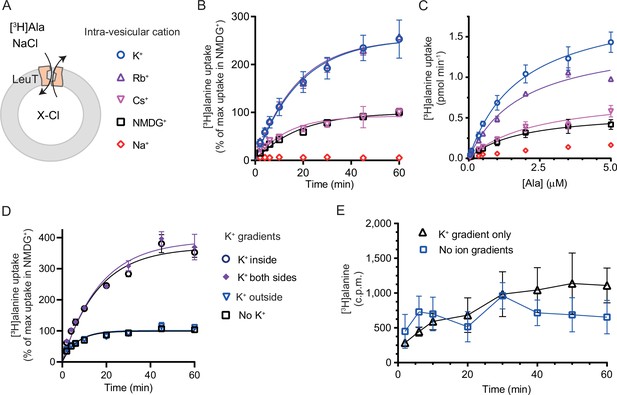
K+ modulates LeuT-mediated [3H]alanine transport.
(A) Schematic of LeuT reconstituted into liposome containing buffer with various cations (X) and Cl- as corresponding anion. (B) Time-dependent [3H]alanine (2 µM) uptake in the presence of 200 mM Na+ into liposomes containing 200 mM of the various cations (colored as in (A)). Data are fitted to a non-linear regression fit. The maximum uptake predicted with N-methyl-D-glucamine (NMDG+) is defined as 100% (Figure 3—source data 1). (C) Concentration-dependent [3H]alanine uptake for 5 min (colored as in (A)). Lines are fit to Michaelis-Menten kinetics (Figure 3—source data 2). None of the Km values were significantly different (Tukey multiple comparison-corrected one-way analysis of variance [ANOVA], p>0.05). The Vmax value in Cs+ was not significantly different from NMDG+ (Tukey multiple comparison-corrected repeated-measures one-way ANOVA, p=0.5780), but K+ and Rb+ were significantly different from NMDG+ (p=0.0011 and 0.0132, respectively). (D) Time-dependent [3H]alanine uptake in the presence of 50 mM Na+ and absence of K+ (black squares) was defined as 100%. Addition of 150 mM K+ (blue triangles) did not significantly change the maximal uptake (unpaired t-test, p=0.92). With 150 mM intra-vesicular K+ (blue circles) the maximum uptake increased to 368±15% (mean ± s.e.m). This increase was not significantly (unpaired t-test, p=0.43) affected further when 150 mM K+ was also added to the extra-vesicular side (purple diamonds). The ionic strength was kept constant by substitution with NMDG+. (E) Time-dependent [3H]alanine uptake into liposomes containing 25 mM Na+ and 200 mM K+ in the presence of either 25 mM Na+ and 200 mM K+ (black line, no ion gradients) or 25 mM Na+ and 200 mM NMDG+ (blue line, outward-directed K+ gradient). The data points from the two conditions are not significantly different (unpaired Mann-Whitney test, p=0.645). All data points are shown as mean ± s.e.m (error bars), n=3 performed in duplicates (B) or triplicates (C–E). All data is provided in the source data file.
-
Figure 3—source data 1
Word file containing data table for rate constants for time-dependent [3H]alanine transport by LeuT into PLs.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig3-data1-v1.docx
-
Figure 3—source data 2
Word file containing data table for Vmax and Km of [3H]alanine transport by LeuT into PLs.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig3-data2-v1.docx
-
Figure 3—source data 3
Word file containing data table for Vmax and Km of [3H]alanine transport as an effect by various K+ concentrations.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig3-data3-v1.docx
-
Figure 3—source data 4
Excel file containing data for [3H]alanine uptake by LeuT reconstituted into PLs.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig3-data4-v1.xlsx
Next, we investigated how the identity of the intra-vesicular cation affected alanine uptake rate. The Km for [3H]alanine in vesicles containing K+ was 1.8±0.4 µM, and it was not significantly different upon substitution of intra-vesicular K+ with NMDG+, Cs+, or Rb+ (Figure 3C and Figure 3—source data 2). The Vmax for [3H]alanine uptake was not significantly different between NMDG+- and Cs+-containing proteoliposomes. In contrast, the Vmax increased 2.5- to 3-fold when NMDG+ was substituted with Rb+ or K+. The increased uptake rate could originate from increases in the rates of certain steps in the transport cycle, from a decrease in [3H]alanine efflux, or a combination of both.
To gain further insight into the relationship between substrate transport and intra-vesicular K+, we investigated how the K+ concentration affected Km and transport velocity for [3H]alanine. Accordingly, LeuT-containing proteoliposomes were prepared in a range of intra-vesicular K+ concentrations. We observed that the Vmax for [3H]alanine transport increased with increasing intra-vesicular K+ concentration, whereas the estimated Km values for alanine were not significantly different (Figure 3—figure supplement 1B and Figure 3—source data 3), suggesting that the effect of K+ on Vmax increases with increasing occupancy of the K+ binding site. To assess how the concentration of extra-vesicular Na+ affected the substrate uptake, we measured [3H]alanine uptake at initial velocity conditions in increasing concentrations of extra-vesicular Na+. We found that the half-saturating extra-vesicular Na+ concentration was ∼25 mM for vesicles containing 200 mM intra-vesicular K+, which is in line with the higher affinity for Na+ to LeuT compared to K+ (Figure 3—figure supplement 1C).
The increase in [3H]alanine transport rate by K+ could be due to an imposed driving force by the outward-directed gradient. If so, dissipation of the K+ gradient would decrease the [3H]alanine transport rate. To allow for the addition of K+ on the extracellular side also, we lowered the inward-directed Na+ gradient to 50 mM. The lowered Na+ gradient still resulted in an ~3.5-fold increased transport capacity with intra-vesicular K+ relative to NMDG+ (Figure 3D). However, dissipation of the K+ gradient by application of equal amounts of K+ on both sides did not change the transport capacity. Addition of K+ only on the outside did also not change the transport capacity relative to that with the Na+ gradient only (Figure 3D), and only having an outward-directed K+ gradient could not drive [3H]alanine transport (Figure 3E). These results suggest that it is the intra-vesicular K+ per se that increases the transport rate of alanine and not a K+ gradient.
It is difficult to control the directionality of proteins when they are reconstituted into lipid vesicles. They will be inserted in both orientations. Outside-out and inside-out. In the case of LeuT, it is the imposed Na+ gradient which determines the directionality of transport. Uptake through the inside-out transporters will probably also happen. Note that the inside-out LeuT will not have the K+ binding site exposed to the intra-vesicular environment. Accordingly, a propensity of transporters will likely not be influenced by the added K+ and will tend to mask the contribution of K+ on the transport mode from the right-side out LeuT. To investigate LeuT directionality in our reconstituted samples, we performed thrombin cleavage of accessible C-terminals on intact and perforated vesicles, respectively. The result suggests that the proportion of LeuT inserted as outside-out is larger than the proportion with an inside-out directionality (Figure 3—figure supplement 1D).
Mutations in the Na1 site change the affinity for K+
With the elucidation of the impact of K+ on LeuT substrate transport, we next sought to identify the binding site for K+ in LeuT. Since K+ and Na+ binding are competitive and K+ excludes substrate binding, we chose to focus on the Na1 site (Figure 4). Accordingly, we introduced the following conservative mutations of the amino acid residues in the Na1 site: A22S, A22V, N27Q, T254S, and N286Q. The aim was to keep LeuT functional but perturb K+ binding. Since it has been shown that H+ can substitute for the K+ antiport in SERT (Keyes and Rudnick, 1982), we also mutated the adjacent Glu290, which has been proposed to facilitate H+ antiport in LeuT (Zomot et al., 2007; Forrest et al., 2007; Kantcheva et al., 2013; Malinauskaite et al., 2016). Thus, by substituting it with glutamine (LeuT E290Q), we aimed to replicate the protonated state of Glu290. If the mechanism was similar to that in SERT but facilitated through this non-conserved residue, it should prevent K+ binding.
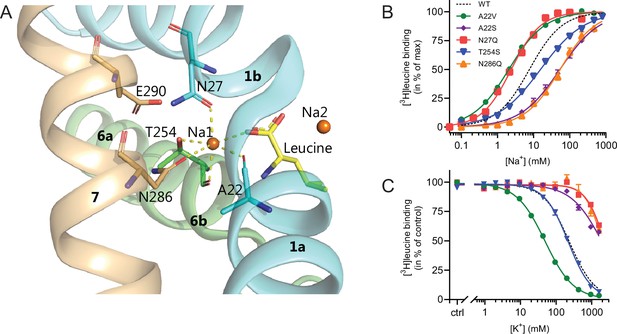
Na1 site mutants of LeuT display altered ion selectivity.
(A) Cartoon representation of the Na1 site from the outward-occluded LeuT structure (PDB ID: 2A65) with the endogenous Na1 site coordinating residues along with the substrate leucine (yellow) and Glu290 labeled and shown as sticks. The coordination of Na+ is depicted with dashed lines. Structural elements not involved in Na1 site are omitted for clarity. (B) Na+-mediated [3H]leucine (10× Kd) binding for Na1 site mutations, A22V (green circles), A22S (purple rhombi), N27Q (red squares), T254S (blue triangles), and N286Q (orange triangles), with WT shown for reference (black dashed line). Data are normalized to Bmax and fitted to a Hill equation. (C) K+-dependent displacement of Na+-mediated [3H]leucine binding for the Na1 site mutants (colored as in (B)). Na+ concentrations equivalent to the EC50 values (determined in (B)) and 10× Kd of [3H]leucine for each mutant were used. Data points are normalized to a control without K+ and modeled by the Hill equation. All data points are mean ± s.e.m., n=3–6. The ionic strength was maintained upon substitution with Ch+. EC50 and IC50 values for (B) and (C) are summarized in Table 2. All data is provided in the source data file.
-
Figure 4—source data 1
Excel file containing data for Na+-mediated [3H]leucine binding for Na1 site mutations and how that is inhibited by K+.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig4-data1-v1.zip
All mutants were expressed in E. coli and purified in DDM. They all bound leucine and alanine (Table 1). The LeuT mutants A22V, A22S, and T254S retained WT-like substrate affinities whereas the mutation E290Q decreased the affinity one order of magnitude and the mutations N27Q and N286Q decreased the affinities about two orders of magnitude. To estimate their Na+ affinities, we measured the Na+-dependent [3H]leucine binding in a next to saturating [3H]leucine concentration (10× Kd), thereby taking the differences in substrate affinity for the individual mutants into account (Figure 4B and Table 2). Mutation of A22V and N27Q increased the Na+ affinity by ~3-fold relative to WT. The T254S mutant caused a 2-fold decrease whereas the A22S and N286Q mutants both decreased the apparent Na+ affinity by ~7-fold. The E290Q mutant retained close to WT Na+ affinity.
Na+-mediated [3H]leucine binding and the effect of K+ addition, and K+-dependent Na+/[3H]leucine displacement in Na1 site mutants.
For LeuT Na1 site mutants (and K398C), Na+-mediated [3H]leucine binding was assayed using 10× Kd of [3H]leucine in the presence of 0, 200, and 800 mM K+. For K+-dependent displacement of Na+-mediated [3H]leucine binding, 10× Kd of [3H]leucine and Na+ equivalent to the EC50 value for each of the mutants were used. Na+ and K+ were substituted for Ch+ to preserve the ionic strength. Data were fitted to a Hill equation, yielding EC50 and IC50 values reported here as mean [s.e.m. interval], n=3–6 determined in triplicates. Comparing the Na+ EC50 obtained for the mutants with that of WT showed significant difference for all (p<0.0001) except for E290Q (p>0.05), and for the K+ IC50 all showed significant differences from WT (p<0.0001) expect for T254S (p>0.05) (Dunnett’s multiple comparison-corrected one-way analysis of variance [ANOVA]). N.d. indicates that the value is not determined. WT data are also reported in Figure 1.
| EC50 Na+ (mM) | IC50 K+ (mM) | EC50 Na++200 mM K+ (mM) | EC50 Na++800 mM K+ (mM) | EC50800/EC50 | |
|---|---|---|---|---|---|
| WT | 7.7 [7.3; 8.1] | 235 [228; 241] | 19.4 [19.1; 19.8] | 48.5 [47.5; 49.5] | ~6 |
| A22V | 2.0 [2.0; 2.1] | 49.0 [47.7; 50.4] | 23.0 [21.7; 24.5] | 70.7 [69.2; 72.2] | ~35 |
| A22S | 53.3 [45.5; 62.5] | >1900 | 72.4 [62.3; 84.2] | 117 [100; 137] | ~2 |
| N27Q | 2.4 [2.3; 2.5] | >2,300 | n.d. | 4.4 [4.0; 4.8] | ~2 |
| T254S | 15.1 [14.2; 16.0] | 219 [209; 229] | 43.5 [37.4; 50.5] | 90.2 [73.8; 110] | ~6 |
| N286Q | 54.6 [47.5; 62.6] | >2000 | n.d. | 121 [111; 133] | ~2 |
| E290Q | 9.6 [8.8; 10.4] | n.d. | n.d. | 144 [132; 157] | ~15 |
| K398C | 7.7 [6.8; 8.8] | n.d | n.d. | 51.5 [48.1; 55.1] | ~7 |
To assess if the mutations in the Na1 site affected the ability to bind K+ and test if the competitive mechanism of inhibition was preserved, we repeated the Na+-dependent [3H]leucine binding experiments in the presence of 800 mM K+. For LeuT WT, the added K+ caused an ~6-fold increase in the EC50 for Na+-dependent [3H]leucine binding. Interestingly, mutation E290Q and A22V resulted in increased antagonism by K+ relative to WT, causing a 15-fold and 35-fold change, respectively (Figure 5 and Table 2). For the remaining mutants, the antagonism by K+ was either less (A22S, N27Q, and N286Q) or similar (T254S) (Figure 5 and Table 2). The observation that the inhibition of Na+-dependent ligand binding by K+ is retained for LeuT E290Q suggests that binding of K+ is not dependent on a negative charge at Glu290 and can occur in parallel with H+ antiport via Glu290 in LeuT. Also, the fact that the effect of K+ on the T254S mutation was indifferent to WT suggests that the serine residue can completely substitute for threonine in this position.
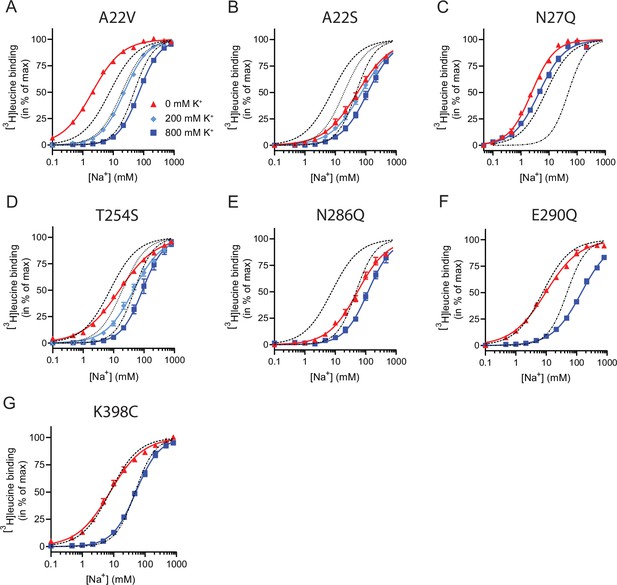
Na+-mediated [3H]leucine binding and inhibition by K+ by the LeuT mutants.
(A–F) Na+-mediated [3H]leucine (10× Kd) binding for the Na1 site mutants performed in the absence (red triangles) and presence of 200 mM (light blue diamond) and 800 (blue squares) mM K+. (A) A22V, (B) A22S, (C) N27Q, (D) T254S, (E) N286Q, (F) E290Q, and (G) K398C. LeuT K398C was included as negative control. WT is shown for reference for 0 mM (dashed line), 200 mM (dotted line), and 800 mM (dash-dotted line) of K+. Ionic strengths were maintained using Ch+. All data points are shown as mean ± s.e.m. normalized to Bmax and fitted to a Hill model. EC50 values are summarized in Table 2, n=3–4. All data is provided in the source data file.
-
Figure 5—source data 1
Excel file containing data for Na+-mediated [3H]leucine binding for the Na1 site mutants and in the presence of K+.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig5-data1-v1.zip
Next, we assessed the impact of the mutations in the Na1 site on K+ affinity by measuring the potency by which K+ inhibits [3H]leucine binding. Again, [3H]leucine was added in a near-saturating concentration (10× Kd) and Na+ at its determined EC50. We observed a marked (~10-fold) decrease in K+ sensitivity for A22S, N27Q, and N286Q, suggesting perturbed K+ binding by these mutants (Figure 4C, Figure 5). Note that the same N27Q mutant had an increased EC50 for Na+. In contrast, the potency for K+ in inhibiting [3H]leucine binding was almost 5-fold increased by the A22V mutation (Figure 4C, Figure 5, and Table 2). The T254S mutation did not alter the K+ sensitivity relative to LeuT WT (Figure 4C, Figure 5, and Table 2). The cognate position to Thr254 in SERT is also a serine residue, which could indicate that the threonine to serine substitution is tolerated in terms of K+ sensitivity between NSSs from different species.
Taken together, the Na1 site mutations showed a marked and differentiated response to the effects of both Na+ and K+. For some mutants, the affinities for both Na+ and K+ were decreased (A22S and N286Q) or increased (A22V). For one, the effects were differentiated so that the Na+ affinity was increased, while the K+ affinity was decreased (N27Q).
The conformational responsiveness is altered in the Na1 site mutants
With coordinates from TM1, -7 and the bound substrate, the Na1 site is likely a central mediator of conformational changes. To investigate how the conformational equilibria in the transporters were affected by the Na1 site mutations, we introduced them into the LeuTtmFRET background and probed their response to Na+, K+, and substrate binding with respect to changes in tmFRET. We first investigated the conformational equilibria for N286QtmFRET in NMDG+ (apo state), in Na+ with and without leucine, and in K+. Surprisingly, even though the EC50 and IC50 values for Na+ and K+, respectively, were markedly increased (decreased affinities), the conformational equilibria of N286Q resembled those of LeuT WT (Figure 6A). This suggests that the substantial decreases in ion affinities and selectivity, imposed by the asparagine to glutamine substitution, do not result from conformational biases, but likely from a direct mutual modulation of their binding site. However, we failed to observe any [3H]alanine transport activity by reconstituted LeuT N286Q, likely as a result of its low substrate affinity.
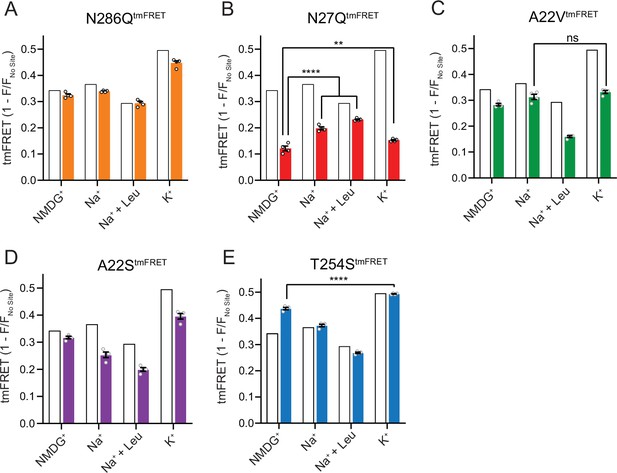
The Na1 site LeuT mutants exhibit different conformational equilibria.
(A–E) Transition metal ion FRET (tmFRET) efficiencies (1 – F/Fno site) for N286QtmFRET (orange, A), N27QtmFRET (red, B), A22VtmFRET (green, C), A22StmFRET (purple, D), and T254StmFRET (blue, E) incubated in 800 mM of the indicated ions and 50 µM for leucine. The His-X3-His site was saturated with 10 mM Ni2+. The corresponding tmFRET efficiencies for LeuTtmFRET (white bars) are shown for reference. All data points are mean ± s.e.m., n=4, performed in triplicates. In (B), **p<0.001; ****p<0.0001 represent the significance levels from a Tukey multiple comparison-corrected one-way analysis of variance (ANOVA), comparing the mean obtained in K+ with that in N-methyl-D-glucamine (NMDG+), and the mean in Na+ and Na+±leucine with that in NMDG+. In (C) and (E), ns, not significant and ****p<0.0001 using a one-way ANOVA with Bonferroni multiple comparison correction. All data is provided in the source data file.
-
Figure 6—source data 1
Excel file containing data for transition metal ion FRET (tmFRET) efficiencies for all mutants in N-methyl-D-glucamine (NMDG+), Na+, leucine, or K+.
- https://cdn.elifesciences.org/articles/87985/elife-87985-fig6-data1-v1.zip
The N27QtmFRET showed a markedly lower tmFRET efficiency in the apo state compared to LeuTtmFRET (Figure 6B), suggesting that the mutation biases the conformational equilibrium toward a more outward-open conformation. Addition of Na+ and leucine restored the conformational equilibrium toward significantly more outward-closed states, but not to the same extent as found for the LeuTtmFRET construct. However, we hardly observed any change in the tmFRET efficiency with K+ in the N27Q mutant. This is in line with its marked decrease in K+ affinity. The result could either suggest that Asn27 is important for the binding of K+, or that the mutation causes a conformational bias which makes this mutant rarely visit the K+-selective state. We were unable to measure any [3H]alanine transport activity by the LeuT N27Q mutant when reconstituted into K+-containing vesicles.
Mutant A22VtmFRET displayed similar tmFRET efficiency as LeuTtmFRET in NMDG+ and Na+, but the tmFRET efficiency induced by K+ was not significantly different from that induced by Na+ (Figure 6C). This indicates that the conformational equilibrium of A22VtmFRET apo-form is not changed by the mutation. In addition, although both Na+ and K+ ions bind with a higher affinity to LeuT A22V than to WT (Figure 4), the tmFRET data suggest that K+ no longer promotes the conformational shift toward the outward-closed conformation. To further evaluate this, we reconstituted the mutant into liposomes and measured its ability to transport alanine (Figure 6—figure supplement 1A). With equimolar Na+ on each side of the lipid bilayer, we observed a specific [3H]alanine signal, which could either reflect transport driven by the [3H]alanine gradient alone or simply binding of [3H]alanine to LeuT. In the presence of a Na+ gradient, we observed a minor, but significant increase in specific [3H]alanine counts. However, the substitution to intra-vesicular K+ did not affect the [3H]alanine activity. Further investigations must clarify whether the changes in observed [3H]alanine activity constitute a transport or a binding event.
The LeuT A22S mutant displayed a decrease in both Na+ and K+ affinity (Figure 4). When inserting it into the LeuTtmFRET background (A22StmFRET), the pattern in tmFRET efficiencies were largely unaltered from LeuTtmFRET, although with a minor reduction in responses upon addition of ligands (Figure 6D). This suggests that the mutation only has minor effect on the overall conformational equilibrium independent of the added ligands. When inserted into liposomes, LeuT A22S retained the ability to transport alanine in the presence of a Na+ gradient. As for LeuT WT, intra-vesicular K+ increased the concentrative capacity for [3H]alanine transport. However, the increase was 3-fold higher than what we observed for LeuT WT (Figure 6—figure supplement 1B). The WT-like conformational equilibrium could suggest that the decreased Na+ and K+ affinities are due to a direct perturbation of the ion binding site by the A22S mutation.
Finally, we characterized the tmFRET response for T254StmFRET. The mutant exhibited a slightly higher tmFRET efficiency in the apo state (NMDG+), but its conformational response to Na+, substrate, and K+ binding was similar to LeuTtmFRET (Figure 6E). Reconstituted into liposomes, LeuT T254S transported [3H]alanine and retained a WT-like increase in concentrative capacity with intra-vesicular K+ (Figure 6—figure supplement 1C). As the substitution is the only apparent difference between the Na1 site in LeuT relative to the human SERT, DAT, and NET, it could suggest a similar functionality of the Na1 site by the four transporters.
In all, although we are unable to discern between direct and indirect effects imposed by the mutants, our results do reflect both concerted and opposed consequences of Na+ and K+ binding, conformation, and substrate transport.
Discussion
In this study, we have examined the binding of K+ ions to purified LeuT stabilized in detergent micelles. We have determined the binding potency through competition binding with Na+ and radiolabeled ligand, and by changes in the conformational equilibrium of the transporter induced directly by K+, as well as explored the role of K+ in the transport process with LeuT reconstituted into liposomes. Additionally, we have investigated the binding site for K+ by a mutational screen of the residues contributing to the already known sodium binding site, Na1, which is conserved among NSSs.
To define an interaction between a ligand and a protein as being the result of a binding site, it must be saturable. Here, we show that K+ binding is saturable both when assessing its inhibition of Na+-dependent [3H]leucine binding and when applying tmFRET as a direct conformational readout. The affinity is around 180 mM measured with tmFRET and by the Cheng-Prusoff equation, applied for competitive inhibitors, the IC50 from the Na+-dependent [3H]leucine binding converts to a Ki of 124 [122; 127] mM. This is in the same range as reported previously (Billesbølle et al., 2016). Even though ions are quite abundant in many biological systems, an affinity above 100 mM is rather low. We can only speculate whether this is within a physiological range for A. aeolicus. However, even at low occupancy, e.g., at its Ki when occupancy is 50%, the bound K+ would regulate the transport velocity.
TmFRET provides a means for a direct measurement of K+ binding to LeuT based on changes in the conformational equilibrium of the ensemble of transporters. The tmFRET efficiencies in this study reflects intramolecular distance changes between the extracellular side of TM10 and EL4. According to the solved LeuT crystal structures, it predicts that this distance will gradually decline upon transition from the outward-open, through the occluded, to the inward-open conformation (Yamashita et al., 2005; Krishnamurthy and Gouaux, 2012). K+ specifically induces a high FRET efficiency relative to those of the other ions and ligands tested, and titration with K+ fits a Hill model with a slope of 1.15±0.03 (mean ± s.e.m.), which is in accordance with the existence of one K+ binding site in LeuT that – when occupied – biases the conformational equilibrium towards an outward-closed state. Interestingly, Rb+ induced a response similar to that of K+, which correlates with previous observations that Rb+ can substitute for K+ binding due to similarities in size and preferred coordination (Billesbølle et al., 2016). The decreased FRET efficiency in the Na+/leucine-bound state, relative to that in the Na+-bound state, could suggest that the binding of leucine on average favors a more open-to-out state, although this contradicts with the known crystal structures. However, we speculate that instead this result could be a consequence of the principle by which steady-state FRET efficiencies for heterogeneous dynamic ensembles are biased toward shorter distances (Taraska et al., 2009a; Beechem and Haas, 1989; Lakowicz, 2006). If LeuT adopts a more conformationally restricted equilibrium upon binding of Na+ and leucine relative to that with Na+ alone, thereby lowering the frequency by which the transporter visits the outward-closed state, this could reflect the lower FRET efficiency observed for the Na+/leucine-bound state.
When we reconstituted LeuT into liposomes, intra-vesicular K+ increased the capacity and Vmax for [3H]alanine uptake compared to intra-vesicular NMDG+ and Cs+. Rb+ displayed a similar effect, suggesting that the conformational effect of Rb+ measured by tmFRET translates to an effect on transport function. Dissipating the K+ gradient or adding K+ solely on the extra-vesicular side of the proteoliposomes did not affect uptake capacity. Neither were a K+ gradient alone able to drive uptake of [3H]alanine. This suggests that the effect of K+ on LeuT uptake is independent of a K+ gradient. Conservative mutations in the Na1 site and the adjacent residue Glu290 retained the ability of the transporter to bind ligands, but only A22S and T254S showed sustained [3H]alanine transport into proteoliposomes, and a persistent functional effect of K+. Interestingly, the LeuT N27Q possessed low affinity for K+ but increased Na+ affinity. Also, the tmFRET data suggested it irresponsive to K+, but also showed an altered conformational equilibrium. This could suggest that the N27Q mutation causes a bias toward an outward-open conformation. [3H]Alanine transport by LeuT A22V showed no effect by the addition of intra-vesicular K+. This correlates with the loss of apparent K+-induced conformational response as assessed in the tmFRET studies (Figure 6). However, even though the mutant showed Na+-dependent [3H]alanine transport, the rate constant was high (1.55 min–1) relative to LeuT WT (0.056 min–1). This could suggest that the A22V mutation influences additional functional properties, such as entering an exchange mode. Further investigations are needed to clarify this.
Despite the known role of K+ in the transport mechanism of SERT and other NSSs, only few studies have attempted to localize the K+ binding site (Billesbølle et al., 2016; Mari et al., 2004; Khelashvili et al., 2016). Mutations of Asn338 (corresponding to Asn286 in LeuT) in the Na+- and K+-coupled amino acid transporter KAAT1 from Manduca sexta suggest that this residue is important for cation selectivity and coupling (Mari et al., 2004). Molecular dynamics simulations of LeuT showed that the K+ ion could jump between the Na2 and Na1 sites (Khelashvili et al., 2016). Mutation in the Na2 site of LeuT (T354V) resulted in a transporter locked in the outward-closed state, and mutation in the Na1 site (T254V) resulted in a transporter which showed a decreased conformational response to K+ (Billesbølle et al., 2016).
To the best of our knowledge, this work represents the first systematical examination of K+ binding in the Na1 site. We found mutations that either increase or decrease the affinities for both Na+ and K+, but we also saw mutations with opposite effects with respect to the affinities, and thereby selectivity, for the two ions. These changes in ion affinities were independent of conformational biases as assessed with tmFRET. This decoupling in ion selectivity and conformational bias suggests that the alterations result from direct modulations of the binding site for K+. However, binding of the two Na+ ions and substrate in LeuT is synergistic, making it difficult to completely isolate effects of mutations to a specific site. Consequently, we cannot exclude the possibility that K+ binding could also involve the Na2 site or another unknown site. Nevertheless, we consider our mapping of the effect on K+ binding by mutations in Na1 a robust starting point for the quest to identify the K+ binding site in NSSs.
We propose that K+ binding either facilitates LeuT transition from inward- to outward-facing (the rate-limiting step of the transport cycle) or solely prevents the rebinding and possible efflux of Na+ and substrate. It could also be a combination of both. Either way, intracellular K+ will lead to an increase in Vmax and concentrative capacity. Note that our previous experiment showed an increased [3H]alanine efflux when LeuT transports alanine in the absence of intra-vesicular K+ (Billesbølle et al., 2016). Specifically, the mechanistic impact of K+ could be to catalyze LeuT away from the state that allows the rebinding of Na+ and substrate. This way, K+ binding would decrease the possible rebinding of intracellularly released Na+ and substrate, thereby rectifying the transport process and increase the concentrative capacity and Vmax (Figure 7). Our results suggest that K+ is not counter-transported but rather promotes LeuT to overcome an internal rate-limiting energy barrier. However, further investigations must be performed before any conclusive statement can be made here. If K+ is not counter-transported, LeuT might comply with the mechanism previously suggested for the human DAT (Bhat et al., 2021).
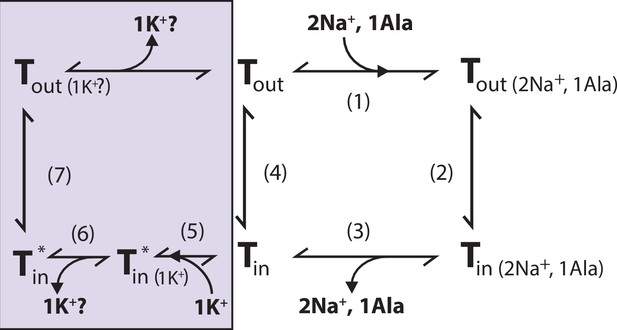
Proposed role of K+ in the translocation cycle.
Here, exemplified for alanine (Ala) as substrate. LeuT apo-form will likely reside in an equilibrium between its outward- (Tout) and inward-facing (Tin) conformation. After Na+ and Ala are bound (1) and released (3), LeuT can either (i) rebind Na+ and Ala, which will promote efflux (2); (ii) transition to the outward-open conformation in its apo-form (4), or (iii) bind K+ (5). K+ binding will promote an inward-facing LeuT state (T*) which is unable to bind Na+, either by competitive inhibition or by promoting a state that does not allow Na+ binding. From here, K+ could either be released again to the intracellular environment (6) or be counter-transported (7).
Taken together, K+ binding seems conserved between LeuT, SERT (Rudnick and Nelson, 1978; Schicker et al., 2012), dDAT (Schmidt et al., 2022), and potentially human DAT (Schmidt et al., 2022; Li and Reith, 2000). Also, the ability of K+ binding to induce a conformational change toward an outward-closed/inward-open state appears to be a common mechanism (Yang and Gouaux, 2021; Schmidt et al., 2022; Schicker et al., 2012; Möller et al., 2019). The conservation of K+ binding, but lack of K+ antiport, has also been observed in a bacterial member of the SLC1 carrier family (Ryan et al., 2009; Wang et al., 2019). Perhaps, regulation of Na+-dependent substrate transport by K+ is a more common mechanism than previously anticipated.
Materials and methods
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Aquifex aeolicus) | LeuT gene | https://doi.org/10.1038/ncomms12755 | ||
| Strain, strain background (Escherichia coli) | BL21(DE3) | Sigma-Aldrich | CMC0016 | Electrocompetent cells |
| Strain, strain protein expression (Escherichia coli) | C41(DE3) | Sigma-Aldrich | CMC0017 | Cells for protein expression |
| Recombinant DNA reagent | LeuT; LeuT WT (plasmid) | https://doi.org/10.1038/ncomms12755 | pET16b backbone | |
| Recombinant DNA reagent | LeuTtmFRET; LeuT A313H-A317H-K398C (plasmid) | https://doi.org/10.1038/ncomms12755 | pET16b backbone | |
| Recombinant DNA reagent | LeuTK398C; LeuT K398C (plasmid) | https://doi.org/10.1038/ncomms12755 | pET16b backbone | |
| Sequence-based reagent | Primers for generation of LeuT mutants | This paper | See list of primers in Appendix. Primers were synthesized by Eurofins Genomics | |
| Chemical compound, drug | fluorescein-5-maleimide; F5M | Thermo Fischer Scientific | F150 | |
| Chemical compound, drug | [3H]leucine | PerkinElmer | NET1166001 | |
| Chemical compound, drug | E. coli polar lipid extract | Avanti Polar Lipids | 100600 C | |
| Chemical compound, drug | [3H]alanine | Moravek Biochemicals | Made on request | |
| Chemical compound, drug | Gel stain InstantBlue | abcam | Ab119211 | |
| Software, algorithm | GraphPad Prism 9.0 | GraphPad Software, Boston, Massachusetts USA | ||
| Other | Yttrium Silicate Copper (YSi-Cu) His-tag SPA beads; YSi-Cu His-tag SPA beads | PerkinElmer | RPNQ0096 | SPA beads used for scintillation proximity assays - See: Pharmacological characterization of LeuT mutants. In Materials & Methods Section |
| Other | thrombin | Cytiva | 27084601 | Protease used to cleave the His-tag from LeuT |
| Other | Filtermat B – GF/B | PerkinElmer | 1450–521 | Filters used to trap proteoliposomes for scintillation counting of [3 H]ligand |
| Other | MeltiLex B/HS | PerkinElmer | 1450–442 | Scintillation plates to add on Filtermats for [3 H] counting |
| Other | Nuclepore Track-Etch Membrane polycarbonate filter of pore size 400 nm | Sigma-Aldrich | WHA10417104 | For extrusion of PLs. |
| Other | SM-2 Bio-Beads | Bio-Rad Laboratories | 1523920 | For detergent extraction from the specimen to promote reconstitution of LeuT into PLs |
Reagents
Unless otherwise stated, reagents were purchased from Merck (Life Sciences).
Cloning of LeuT mutants
Request a detailed protocolDNA encoding LeuT from A. aeolicus, fused C-terminally to a thrombin protease cleavage site and an octahistidine tag, was cloned into a pET16b vector. Single mutations in the Na1 site (A22V, A22S, N27Q, T254S, N286Q, E290Q) were inserted into LeuT constructs with pre-existing pairs of mutations (A313H-A317H-K398C; K398C) and without (WT). Full gene sequences were verified by DNA sequencing (Eurofins Genomics).
Expression and purification of LeuT
Request a detailed protocolExpression and purification of LeuT variants containing the tmFRET pair mutations were performed essentially as described previously (Billesbølle et al., 2016). In brief, E. coli C41(DE3) cells were transformed with pET16b plasmid encoding the desired LeuT Na1 site variants and single colonies were cultivated at 37°C in Lysogeny Broth. Expression was induced at OD600∼0.6 upon induction with β-D-1-thiogalactopyranoside (IPTG). Harvested cells were disrupted using a cell disruptor (CF1, ConstantSystems) and isolated crude membranes were solubilized with 1.5% (wt/vol) DDM (anagrade, Anatrace). Solubilized LeuT was incubated with Ni2+-NTA resin (Thermo Fisher Scientific) and 40 mM imidazole, batch-washed and labeled overnight with F5M (Thermo Fisher Scientific). Following wash of the resin with 15 successive column volumes of buffer (20 mM Tris-HCl [pH 7.4], 20% [vol/vol] glycerol, 200 mM KCl, 0.05% [wt/vol] DDM, 100 µM tris(2-carboxyethyl)phosphine [TCEP] [hydrochloride solution]) containing 90 mM imidazole, immobilized LeuT was eluted and frozen in buffer with 340 mM imidazole. Labeling efficiency and specificity were examined by absorbance measurements (280 and 490 nm) and sodium-dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis, respectively. For LeuT variants on WT background (devoid of tmFRET mutations), the F5M labeling step was skipped and the resin washing procedure was performed with 3 and 5 column volumes of buffer containing 60 and 90 mM imidazole, respectively. For reconstitution in liposomes, LeuT was dialyzed overnight in buffer (20 mM Tris-HCl [pH 7.4], 20% [vol/vol] glycerol, 200 mM KCl, 0.05% [wt/vol] DDM) to remove imidazole. The protein was subsequently concentrated to >2 mg/ml.
Pharmacological characterization of LeuT mutants
Request a detailed protocolPharmacological studies were conducted on purified LeuT variants by virtue of the SPA. Leucine affinities for WT LeuT and mutants displaying WT substrate affinities (A22V, A22S) were determined by saturation binding of [3H]leucine (25 Ci/mmol) (PerkinElmer), with unspecific binding corrected for by the addition of 100 µM unlabeled leucine. Alanine and leucine affinities for the remaining LeuT variants were determined by the ability of increasing concentrations of unlabeled alanine or leucine, respectively, to competitively displace a fixed concentration of [3H]leucine. Assayed in a 96-well plate (Corning), LeuT was mixed with Yttrium Silicate Copper (YSi-Cu) His-tag SPA beads (PerkinElmer) and [3H]leucine in binding buffer (200 mM NaCl, 20 mM Tris [pH 8], 20% [vol/vol] glycerol, 0.05% [wt/vol] DDM, and 100 µM TCEP). The following conditions were applied for the mutants tested: WT, K398C, A22V, A22S: 0.3 µg/ml protein, 1.25 mg/ml YSi-Cu His-tag SPA beads, 120 nM [3H]leucine (25 Ci/mmol); N27Q, N286Q: 3 µg/ml protein, 1.6 mg/ml YSi-Cu His-tag SPA beads, 1200 nM [3H]leucine (4 Ci/mmol); E290Q: 1.5 µg/ml protein, 1.6 mg/ml YSi-Cu His-tag SPA beads, 200 nM [3H]leucine (20 Ci/mmol). The Na+ dependency on [3H]leucine binding was determined by mixing LeuT, YSi-Cu His-tag SPA beads (in equivalent concentrations as above) and a fixed concentration of [3H]leucine (10× Kd, as determined in 200 mM NaCl) in binding buffer supplemented with increasing concentrations of Na+. The specific activity of [3H]leucine was kept approximately inversely proportional to the concentration of [3H]leucine and protein used. This experiment was repeated in the presence of 200 and 800 mM K+. The K+-dependent inhibition of Na+-mediated [3H]leucine binding was assayed by subjecting LeuT mutants (double the concentration as above) to increasing concentrations of K+ (0–1600 mM) in the presence of Na+ equivalent to the EC50 determined with 10× Kd of [3H]leucine. The unspecific binding of [3H]leucine was determined upon addition of 100 (WT, A22V, A22S) or 300 µM (N27Q, N286Q) of unlabeled leucine. Ionic strengths were preserved by substituting Na+ and K+ for Ch+. For competition binding and ion-dependent [3H]leucine binding experiments, ligand depletion was avoided by maintaining ≥20-fold molar excess of [3H]leucine relative to LeuT. Sealed plates were incubated for ~16 hr at 4°C with gentle agitation and counts per minute (c.p.m.) were recorded on a 2450 MicroBeta2 microplate counter (PerkinElmer) in ‘SPA’ mode.
Analysis of SPA data
Request a detailed protocolSaturation binding data were corrected for unspecific binding, normalized to Bmax and fitted to a one-site binding regression from which Kd values were obtained using GraphPad Prism 9.0. For competition binding, data points were normalized to a control (without competing unlabeled leucine or alanine) and fitted to a single-site log(inhibitor)-response model. Derived IC50 values (inhibitor concentration that reduces binding of radioligand by 50%) were converted to inhibition constants Ki by the Cheng-Prusoff equation:
where [S] and Kd refer to the concentration and affinity, respectively, of the radioligand. Data points for K+ competition binding were corrected for unspecific binding and normalized to a control (absent for K+), whereas data for Na+-dependent [3H]leucine binding were normalized to the maximum response predicted by the model. Both were fitted to the Hill equation from which IC50 and EC50 values, respectively, were extracted. All experiments were performed at least three independent times in triplicates for specific and triplicates/duplicates for unspecific binding. Data points and Hill slopes are reported as mean ± s.e.m. and Kd, Ki, and EC50 values are reported as [s.e.m. interval]. Statistical analysis was performed with a post hoc Dunnett’s multiple test in a one-way analysis of variance (ANOVA), comparing every mean with that obtained in the absence of competitor.
Transition metal ion FRET
Request a detailed protocolLeuT Na1 site mutants examined with tmFRET were encoded with a set of FRET pair mutations (A313H-A317H-K398C/K398C) designed and characterized previously (Billesbølle et al., 2016). Purified and fluorescein-labeled LeuT variants were centrifuged at 15,000 × g at 4°C for 10 min, and diluted to ~10 nM (adjusted for labeling efficiency) in fluorescence buffer (20 mM Tris-Cl [pH 8], 0.05% [wt/vol] DDM, 100 µM TCEP), supplemented with 800 mM chloride salts of NMDG+, Na+±50 µM leucine/200 µM alanine, K+ or Rb+ as specified. Samples were incubated for 30 min at room temperature in the dark. If not otherwise specified, parallel experiments on LeuT constructs with and without the His-X3-His motif were conducted, here referred to with the suffixes tmFRET and K398C, respectively. Fluorescence measurements using a single saturating concentration of Ni2+ were assayed in a Swartz cuvette (Hellma Analytics) inserted into a FluoroMax-4 spectrophotometer (HORIBA Scientific) temperature controlled to 25°C. Reference-corrected fluorescence intensities (0.1 s integration time) were recorded following the incubation with 10 mM Ni2+, using constant excitation and emission wavelengths of 492 and 512 nm, respectively, and 4 nm excitation and emission slit widths. For ion titration experiments, LeuTtmFRET was incubated in fluorescence buffer containing 750 µM Ni2+ and increasing concentrations of Na+, K+, or Rb+ (0–1584 mM), substituted with Ch+ to preserve ionic strength. Fluorescence intensities were obtained using a FluoroMax-4 spectrophotometer and the same technical specifications as described above. For Ni2+ titration experiments performed in 96-well plates (Corning), LeuT was subjected to increasing concentrations of Ni2+ (10–7 to 10–2 M) in buffer containing 800 mM of NMDG+, Na+±50 µM leucine, or K±50 µM leucine. Fluorescence intensities at 512 nm were recorded on a PolarStar Omega plate reader (NMG Labtech) upon excitation at 492 nm.
Analysis of tmFRET data
Request a detailed protocolFluorescence intensities for LeuTtmFRET variants (F) were normalized to their equivalents for LeuTK398C (Fno site) without the metal-chelating His-X3-His site, to correct for the contribution of collisional quenching from free Ni2+, dilution, as well as the primary inner filter effect (1 – F/Fno site). An increase in 1 – F/Fno site implies an enhanced energy transfer between FRET donor (F5M) and acceptor (Ni2+) that, when plotted as a function of increasing Ni2+, was fitted to a Hill equation from which EC50 and maximal tmFRET values were obtained. Maximal tmFRET efficiencies were converted to distances by the FRET equation:
with E, R, and R0 being the efficiency of energy transfer, distance between FRET probes, and Förster distance (R0=12 Å for Ni2+/fluorescein; Taraska et al., 2009b), respectively. For ion titration experiments, fluorescence intensities for LeuTtmFRET (not normalized to LeuTK398C) at each ion concentration (F) were normalized to fluorescence intensities obtained in the absence (F0) of Na+, K+, or Rb+ (F0/F). As for 1 – F/Fno site, F0/F is inversely dependent on the distance between the FRET probes and when plotted against the ion concentration, data points could be fitted to a Hill equation, yielding the Hill slope and EC50 value. Experiments were repeated at least three independent times in triplicates using protein from two separate purifications. Data points and Hill slopes are reported as mean ± s.e.m., whereas EC50 values are mean [s.e.m. interval]. When indicated, data points were subjected to statistical analysis using either post hoc Tukey or Bonferroni multiple comparison test as part of a one-way ANOVA.
Reconstitution of LeuT in liposomes
Request a detailed protocolE. coli polar lipid extract dissolved in chloroform (Avanti Polar Lipids) was dried under a steam of N2 for 2 hr. Lipids were re-suspended to 10 mg/ml in reconstitution buffer (200 mM NMDG-Cl, 20 mM Tris/HEPES [pH 7.5]) by vortexing and 2× 10 min bath sonication. The lipid solution was subjected to five freeze-thaw cycles in ethanol dry ice bath. Subsequently, the liposomes were extruded with a mini extruder (Avanti Polar Lipids) 11 times through a Nuclepore Track-Etch Membrane polycarbonate filter of pore size 400 nm (GE Healthcare Life Sciences) and diluted to 4 mg/ml in reconstitution buffer. Liposome destabilization was induced by stepwise addition of 10 µl aliquots of 10% (vol/vol) Triton X-100. Destabilization of liposomes was followed by measuring absorbance at 550 nm. Triton X-100 was added until absorbance of the sample had reached a maximum and started to decrease. LeuT solubilized in DDM was added in a protein to lipid ratio of 1:25 (wt/wt) and the protein-liposome solution was incubated under slow rotation for 30 min at 4°C. Semidry SM-2 Bio-Beads (Bio-Rad Laboratories), equilibrated in reconstitution buffer, were added in aliquots of 8.5 mg beads/mg lipid after 30 min, 60 min, 120 min and after overnight incubation at 4°C with gentle agitation. The beads were filtered out 2 hr after the last addition of bio-beads. The proteoliposome solution was diluted ~20 times in the indicated internal buffer (200 mM salt [NaCl, KCl, NMDG-Cl, CsCl, or RbCl], 20 mM Tris/HEPES [pH 7.5]) and centrifuged for 1 hr at 140,000 × g at 4°C. Pelleted proteoliposomes were re-suspended in the indicated internal buffer to a final concentration of 10 mg lipid/ml. Single-use aliquots of proteoliposomes were flash-frozen in liquid N2 and stored at –80°C until further use.
[3H]Alanine uptake into proteoliposomes
Request a detailed protocolProteoliposomes were thawed and extruded through a Nuclepore Track-Etch Membrane polycarbonate filter with 400 nm pore size (GE Healthcare Life Sciences). Uptake was assayed in a 96-well setup in ultra-low attachment, round bottom plate (Costar) at room temperature (21–23°C). Uptake buffer (20 mM Tris/HEPES [pH 7.5], 200–225 mM salt [NaCl, KCl, or NMDG-Cl]) supplemented with [3H]alanine (Moravek Biochemicals) was added to each well in a volume of 190 µl. Subsequently, 10 µl of proteoliposome solution was added to start the uptake reaction. For time-dependent uptake, the uptake buffer was supplemented with 2 µM [3H]alanine with a specific activity of 1.335 Ci/mmol and proteoliposomes were added at the indicated time points (2–60 min). For concentration-dependent uptake, uptake buffer was supplemented with [3H]alanine with a specific activity of 3.73 Ci/mmol (50–2000 nM) or 1.07 Ci/mmol (3.5–6 µM). The uptake reaction was terminated after 5 min. To assess nonspecific [3H]alanine uptake and binding, proteoliposomes were pre-incubated with 200 µM unlabeled leucine for 15 min, and 100 µM unlabeled leucine was added to the uptake buffer to saturate all transporters with leucine. The uptake reaction was terminated by filtering the samples through a 96-well glass fiber filter (Filtermat B – GF/B, Perkin Elmer) soaked in 1.5% poly(ethyleneimine) solution, using a cell harvester (Tomtec harvester 96 match II). The filter was washed with 1 ml ice-cold wash buffer (200 mM ChCl, 20 mM Tris/HEPES [pH 7.5]) for each of the 96 positions on the filter. Filter plates were dried at 96°C before scintillation sheet (MeltiLex B/HS, Perkin Elmer) was melted on to the filter. Filters were counted on a 2450 MicroBeta2 microplate counter (PerkinElmer) in ‘normal’ counting mode.
SDS-PAGE analysis of LeuT orientation
Request a detailed protocolAs for uptake experiments, proteoliposomes were extruded through a Nuclepore Track-Etch Membrane polycarbonate filter with 400 nm pore size (GE Healthcare Life Sciences). Reconstituted LeuT corresponding to 8 µg was treated with thrombin (0.5 unit; Cytiva) and the reaction was quenched upon the addition of 500 µM PMSF after 1, 5, 10, 30, 60, 120, and 180 min. Controls without thrombin and with 1.5% DDM (for 180 min) were included. Following SDS-PAGE, the gel was stained with InstantBlue (abcam) and images were obtained using an ImageQuant 800 (Cystiva).
Radioactive binding assay for proteoliposomes
Request a detailed protocolTo assess the amount of active protein in each reconstitution condition, a sample of proteoliposomes from each of the different intra-vesicular buffer conditions was solubilized in buffer (30% glycerol, 1% [wt/vol] DDM, 20 mM Tris/HEPES [pH 7.5], 200 mM NaCl). The samples were left for 3 hr with gentle agitation at 4°C to dissolve proteoliposomes and solubilize LeuT in DDM detergent micelles. The amount of re-solubilized active LeuT was assessed by binding in a saturating [3H]leucine concentration (1 µM) in buffer (200 mM NaCl, 20 mM Tris [pH 8], 0.05% [wt/vol] DDM) by SPA. Measures of maximum binding from each condition were used to normalize the c.p.m. obtained from uptake experiments to ensure that uptake was not affected by variations in protein content in the proteoliposome samples. The highest maximum binding was used as normalization standard.
Analysis of [3H]alanine uptake data
Request a detailed protocolFor time-dependent uptake, specific uptake data, normalized to the amount of active protein (see section above), was fitted to a one-phase association using GraphPad Prism 9.0.
where p is the maximal uptake (the amplitude), k is the rate constant in min–1, t is time in min, and y is uptake in c.p.m. after normalization. Data from each experiment was subsequently normalized to the value of p from the condition with NMDG+. The normalized data sets from each experiment were combined and re-fitted to a one-phase association.
For concentration-dependent uptake, normalized, specific uptake data was fitted to the Michaelis-Menten equation:
where Vmax is the maximal uptake rate, Km is the substrate concentration where half-maximum uptake rate is reached, y is the uptake in c.p.m. after normalization. [s] is the [3H]alanine concentration in µM. Subsequently data was either combined or (if shown in % on the y-axis) normalized to the Vmax value determined with intra-vesicular NMDG+. The normalized data sets from each experiment were combined and fitted to the Michaelis-Menten equation. All uptake experiments were done in triplicates or duplicates as indicated and repeated three to four times using protein obtained from at least two different purifications and reconstitutions.
For concentration-dependent uptake with different intra-vesicular cations, c.p.m. were converted to d.p.m., and then to pmol of [3H]alanine per min, using the specific activity of [3H]alanine and a counting efficiency of 40% for 2450 MicroBeta2 microplate counter (PerkinElmer) in ‘normal’ counting mode.
Materials availability statement
Request a detailed protocolAll newly created materials (e.g. LeuT mutants) are available upon request.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting file. Source data files have been provided for Figures 1—5 and for Tables 1 and 2.
References
-
Overview of Monoamine TransportersCurrent Protocols in Pharmacology 79:12.https://doi.org/10.1002/cpph.32
-
Monoaminergic and histaminergic strategies and treatments in brain diseasesFrontiers in Neuroscience 10:541.https://doi.org/10.3389/fnins.2016.00541
-
Electrogenic binding of intracellular cations defines a kinetic decision point in the transport cycle of the human serotonin transporterThe Journal of Biological Chemistry 291:25864–25876.https://doi.org/10.1074/jbc.M116.753319
-
Coupling of transmembrane proton gradients to platelet serotonin transportThe Journal of Biological Chemistry 257:1172–1176.
-
Conformational dynamics on the extracellular side of LeuT controlled by Na+ and K+ ions and the protonation state of Glu290The Journal of Biological Chemistry 291:19786–19799.https://doi.org/10.1074/jbc.M116.731455
-
SLC6 neurotransmitter transporters: structure, function, and regulationPharmacological Reviews 63:585–640.https://doi.org/10.1124/pr.108.000869
-
The use of LeuT as a model in elucidating binding sites for substrates and inhibitors in neurotransmitter transportersBiochimica et Biophysica Acta 1850:500–510.https://doi.org/10.1016/j.bbagen.2014.04.011
-
Aspartate 338 contributes to the cationic specificity and to driver-amino acid coupling in the insect cotransporter KAAT1Cellular and Molecular Life Sciences 61:243–256.https://doi.org/10.1007/s00018-003-3367-2
-
Coupling between platelet 5-hydroxytryptamine and potassium transportThe Journal of Biological Chemistry 254:10084–10089.
-
Functional characterization of a Na+-dependent aspartate transporter from Pyrococcus horikoshiiThe Journal of Biological Chemistry 284:17540–17548.https://doi.org/10.1074/jbc.M109.005926
-
Unifying concept of serotonin transporter-associated currentsThe Journal of Biological Chemistry 287:438–445.https://doi.org/10.1074/jbc.M111.304261
-
A K+/Na+ co-binding state: Simultaneous versus competitive binding of K+ and Na+ to glutamate transportersJournal of Biological Chemistry 294:12180–12190.https://doi.org/10.1074/jbc.RA119.009421
Article and author information
Author details
Funding
Danmarks Frie Forskningsfond (1030-00036B)
- Claus J Loland
Lundbeck Foundation (R344-2020-1020)
- Claus J Loland
Novo Nordisk Fonden (NNF19OC0058496)
- Claus J Loland
Carlsbergfondet (CF20-0345)
- Claus J Loland
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We would like to thank Patricia Curran for technical guidance. We also acknowledge the NINDS intramural program for support. Funding sources: Support for this research was provided by the Independent Research Fund Denmark (1030-00036B to CJL), the Lundbeck Foundation (R344-2020-1020 to CJL), the Novo Nordic Foundation (NNF19OC0058496 to CJL), and the Carlsberg Foundation (CF20-0345 to CJL).
Version history
- Preprint posted:
- Sent for peer review:
- Reviewed Preprint version 1:
- Reviewed Preprint version 2:
- Version of Record published:
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.87985. This DOI represents all versions, and will always resolve to the latest one.
Copyright
This is an open-access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Metrics
-
- 849
- views
-
- 75
- downloads
-
- 4
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
The entorhinal cortex (EC) plays a pivotal role in memory function and spatial navigation, connecting the hippocampus with the neocortex. The EC integrates a wide range of cortical and subcortical inputs, but its synaptic organization in the human brain is largely unknown. We used volume electron microscopy to perform a 3D analysis of the microanatomical features of synapses in all layers of the medial EC (MEC) from the human brain. Using this technology, 12,974 synapses were fully 3D reconstructed at the ultrastructural level. The MEC presented a distinct set of synaptic features, differentiating this region from other human cortical areas. Furthermore, ultrastructural synaptic characteristics within the MEC was predominantly similar, although layers I and VI exhibited several synaptic characteristics that were distinct from other layers. The present study constitutes an extensive description of the synaptic characteristics of the neuropil of all layers of the EC, a crucial step to better understand the connectivity of this cortical region, in both health and disease.
-
- Neuroscience
Dopamine can play opposing physiological roles depending on the receptor subtype. In the fruit fly Drosophila melanogaster, Dop1R1 and Dop2R encode the D1- and D2-like receptors, respectively, and are reported to oppositely regulate intracellular cAMP levels. Here, we profiled the expression and subcellular localization of endogenous Dop1R1 and Dop2R in specific cell types in the mushroom body circuit. For cell-type-specific visualization of endogenous proteins, we employed reconstitution of split-GFP tagged to the receptor proteins. We detected dopamine receptors at both presynaptic and postsynaptic sites in multiple cell types. Quantitative analysis revealed enrichment of both receptors at the presynaptic sites, with Dop2R showing a greater degree of localization than Dop1R1. The presynaptic localization of Dop1R1 and Dop2R in dopamine neurons suggests dual feedback regulation as autoreceptors. Furthermore, we discovered a starvation-dependent, bidirectional modulation of the presynaptic receptor expression in the protocerebral anterior medial (PAM) and posterior lateral 1 (PPL1) clusters, two distinct subsets of dopamine neurons, suggesting their roles in regulating appetitive behaviors. Our results highlight the significance of the co-expression of the two opposing dopamine receptors in the spatial and conditional regulation of dopamine responses in neurons.






