Science Forum: Best practices to promote rigor and reproducibility in the era of sex-inclusive research
- Article
- Figures and data
- Abstract
- Introduction
- Exploratory vs. confirmatory research: An important distinction
- Consideration phase: Consider sex and commit to an exploratory or confirmatory approach to sex differences
- Collection phase: Collection of sex-based data
- Characterization phase: Analysis of sex-based data
- Communication phase: Presenting and interpreting sex differences
- Conclusion
- Data availability
- References
- Decision letter
- Author response
- Article and author information
- Metrics
Abstract
To enhance inclusivity and rigor, many funding agencies and journals now mandate the inclusion of females as well as males in biomedical studies. These mandates have enhanced generalizability and created unprecedented opportunities to discover sex differences. However, education in sound methods to consider sex as a subgroup category has lagged behind, resulting in a problematic literature in which study designs, analyses, and interpretations of results are often flawed. Here, we outline best practices for complying with sex-inclusive mandates, both for studies in which sex differences are a primary focus and for those in which they are not. Our recommendations are organized within the “4 Cs of Studying Sex to Strengthen Science: Consideration, Collection, Characterization and Communication,” a framework developed by the Office of Research on Women’s Health at the National Institutes of Health in the United States. Following these guidelines should help researchers include females and males in their studies while at the same time upholding high standards of rigor.
Introduction
Effective medical treatments and public health rely on rigorous, inclusive research that takes into account variation across and within populations. In response to an historical exclusion of women from biomedical studies and trials, the US government has mandated the inclusion of women in clinical investigations since 1993 (Epstein, 2007). Similarly, researchers funded by the National Institutes of Health (NIH) in the US have been required to include females and males in preclinical non-human animal studies since 2016 (Clayton and Collins, 2014). Similar sex-inclusive policies have been implemented in Canada and Europe, and many journals now require the inclusion of females and males unless otherwise justifiable (Health Canada, 2023; Heidari et al., 2016; NIH, 2015a; White et al., 2021).
The rationale for such policies is founded on observations that, on average, men and women differ in the incidence and presentation of disease, risk factors for disease, treatment response and adverse drug reactions, as well as on the belief that similar, undiscovered differences may be widespread (van Anders, 2022; Clayton, 2018; Karp et al., 2017). Indeed, differences between men and women have been reported in the incidence and average age of onset of many diseases, notably auto-immune diseases such as lupus and multiple sclerosis (Voskuhl, 2011), mental health conditions such as depression (SAMHSA, 2012) and schizophrenia (Abel et al., 2010), substance use disorders (McHugh et al., 2018; Becker and Koob, 2016), and cardiovascular disease (Oneglia et al., 2020; Mosca et al., 2011; Ji et al., 2022). Diabetes and cigarette smoking are more potent risk factors for cardiovascular disease among women (Humphries et al., 2017), and women are more likely to present with atypical symptoms of coronary heart disease than are men (Canto et al., 2012; Mieres et al., 2011; Eastwood et al., 2013). Observations of such differences have, in rare cases, prompted different treatment recommendations for men and women. In 2013, for example, the US Food and Drug Administration (FDA) recommended lower dosing of the prescription drug zolpidem for women than for men, citing higher blood levels of the drug in women eight hours after it was taken (FDA, 2018). Overall, widespread findings of statistically significant group differences between men and women have led some to conclude that there are important differences between women and men yet to be discovered and that these differences will ultimately necessitate tailored treatments for each sex (Clayton, 2018).
As a result of the policies to increase inclusion, women are now represented in most clinical trials (Bierer and Meloney, 2022; Sosinsky et al., 2022; Feldman et al., 2019) and preclinical research increasingly includes female animals (Beery and Zucker, 2011; Woitowich et al., 2020). This change is a welcome and needed corrective; ignoring an entire sex or gender in research design, data analysis, and reporting, without strong justification, is arguably incompatible with rigorous or generalizable science. As we strive to right historical wrongs, we must maintain the high standards required of all scientific endeavors. This is easier said than done.
Sex-based research policies do not universally mandate direct comparisons between females and males. The NIH policy on sex as a biological variable (SABV), for example, explicitly states that although data must be disaggregated by sex, statistical comparisons are not required (NIH, 2020). Nonetheless, most researchers who comply with SABV do compare the sexes (Garcia-Sifuentes and Maney, 2021), and this practice appears to have led to a large increase in the number of differences reported (Maney and Rich-Edwards, 2023). For many researchers, including females and males represents a novel approach for which training could be lacking (Maney et al., 2023). Indeed, it is becoming more apparent that many claims of sex differences are based on flawed study designs, analyses or interpretations of results (Patsopoulos et al., 2007; Zell et al., 2015; Wallach et al., 2016; Kaul, 2017; David et al., 2018; Eliot et al., 2021; Garcia-Sifuentes and Maney, 2021; Maney et al., 2023; Maney and Rich-Edwards, 2023). The widespread use of suboptimal approaches suggests that training in methods to detect sex differences has not kept pace with the unprecedented opportunity to investigate them. Here, we seek to highlight and help address this methodological barrier by proposing best practices in study design, data analysis, and presentation of findings, emphasizing principles common to preclinical, clinical, and population science.
Exploratory vs. confirmatory research: An important distinction
The main elaboration we propose is to remind ourselves of the fundamental difference between exploratory (hypothesis-generating) research and confirmatory (hypothesis-testing) research. In its guide to reviewers, NIH distinguishes studies “intended to test for sex differences” from those that are not, seemingly marshalling two different approaches (NIH, 2015b). The NIH requires only studies intended to test for sex differences to have the statistical power and analytic methods adequate to the task; others are not held to such expectations – that is, their approach to sex as a variable may be more exploratory in nature (NIH, 2015b; NIH, 2020). In differentiating exploratory studies from those designed to interrogate a sex difference, the NIH effectively proposes two standards to which NIH-funded studies can be held. This is not inherently problematic; exploratory research, by definition, operates under a different standard.
The distinction between exploratory and confirmatory research has a long history that is useful to consider in the context of recent mandates (Forstmeier et al., 2017; Wagenmakers et al., 2012). In general, exploratory studies typically include neither a priori hypotheses nor sample sizes large enough to test for effects within or between subgroups (Schwab and Held, 2020). The strength of exploratory studies is that they allow for and acknowledge unexpected findings that can in turn be used to generate novel hypotheses; whether such findings are happy or unfortunate accidents must be left to future studies. In contrast, authors of confirmatory studies are motivated by preliminary data or prior literature to specify clear testable hypotheses, prespecify subgroup contrasts, and size their studies to formally test for subgroup differences. Exploratory research fertilizes the farm; confirmatory research separates the wheat from the weeds. By embracing both exploratory and confirmatory research – and not muddying the distinction – we can reap the strengths of both as we seek to make our work generalizable and inclusive.
The trouble comes in the expectation that within-sex analyses should be conducted in all studies – even those not adequately powered or designed to examine how treatment effects vary by sex (NIH, 2020; CIHR, 2023). As we explain below, an underpowered, within-subgroup analysis does not meet basic standards of analytical rigor, even when taking an exploratory approach to sex differences, and therefore threatens reproducibility (Rich Edwards et al., 2018). Furthermore, studies not designed to interrogate sex differences are often poorly equipped to test alternative explanations for sex effects, such as confounding by gender (Ritz and Greaves, 2022). Finally, while an explosion of exploratory analyses will undoubtedly promote discovery of sex differences, they will also maximize false positive discoveries (Maney and Rich-Edwards, 2023).
Below, we offer some tips to researchers who strive to comply with new guidelines while maintaining standards of rigor. Our approach is based on the “4 Cs” framework for studying sex, developed by the Office of Research on Women’s Health at the NIH (https://orwh.od.nih.gov/sex-gender/nih-policy-sex-biological-variable). This framework calls upon researchers to: Consider sex in study design; Collect sex-based data; Characterize sex-based data in analysis; and Communicate sex-based data through publication. We will elaborate upon and critique elements of the 4 Cs framework, naming ongoing challenges presented by SABV and similar policies, and proposing what we hope are practical solutions. Our recommendations are outlined in Figure 1, which depicts largely separate pathways for exploratory and confirmatory approaches to sex differences.

Exploratory and confirmatory research in the “4 Cs” framework for studying sex.
Decision tree depicting how the “4 Cs” framework can be applied to either an exploratory or confirmatory approach to testing for sex differences. Best practices are shown for testing for a sex difference in a treatment effect (or exposure-outcome association). For observational studies, “exposure” should be substituted for “treatment”.
Two prefatory notes before we continue. First, the principles outlined here apply to observational studies as well as experiments and clinical trials, and apply equally to pre-clinical, clinical and population science. Throughout, where we use “treatment” or “treatment effect”, observational researchers can substitute “exposure” or “exposure-outcome association” respectively. Second, we note that gender – which encompasses identity, expression, and sociocultural expectations – also affects human health and is already the subject of directives in some countries (Health Canada, 2023; NASEM, 2022). While this article highlights sex as the focus of current federal mandates in the US, the same research principles outlined below apply to the study of gender or any other subgroup.
Consideration phase: Consider sex and commit to an exploratory or confirmatory approach to sex differences
In this phase of study design, investigators should think about the potential impact of sex on the phenomenon under study and decide whether their hypotheses will include sex differences. This step includes consideration of the relevant literature to identify evidence for plausible effects of sex or sex-related factors on the variables of interest. It is at this stage, early in the research process, that researchers commit to either an exploratory or confirmatory approach to sex differences.
We recommend the following steps for the Consideration phase of all investigations (Figure 1):
Explicitly operationalize sex. The NIH defines sex as “a multidimensional construct based on a cluster of anatomical and physiological traits that include external genitalia, secondary sex characteristics, gonads, chromosomes, and hormones” (Barr and Temkin, 2022). Taken individually, none of the traits in the cluster can by themselves define a body as “male” or “female.” Thus, the term “sex” has unstable meaning (Richardson, 2022; Bhargava et al., 2021; Massa et al., 2023). For any particular study, the definition of sex can depend on the nature and goals of that study; in preclinical studies, for example, the sex of the research animals might be defined by anogenital distance or genotype. In most clinical and population studies, sex is defined by participant self-report (although best practices for the use of categories and checkboxes are without consensus and are rapidly changing; NASEM, 2022; Suen et al., 2020; Kronk et al., 2022; Garrett-Walker and Montagno, 2023). Depending on study aims, variables such as pubertal or menopausal status may be more useful than binary sex. Regardless, within a study, the definition should remain consistent throughout each phase, including the interpretation of results. Note that sex should be clearly operationalized even for single-sex studies.
Unless otherwise justified, include more than one sex (e.g., females and males) to improve generalizability from a sample to the general population.
Although sex inclusion policies typically endorse a binary approach to sex as a variable, consider whether sex must be binarized for the particular study at hand. For example, some sex-related variables that might be used to operationalize sex (e.g., hormone levels, anogenital distance, some sex-associated behaviors) are not categorical. Other authors have provided excellent additional guidance for authors seeking to avoid binary operationalizations or to take more nuanced approaches (van Anders, 2022; Ritz and Greaves, 2022; Richardson, 2022; Joel and Fausto-Sterling, 2016; Hyde et al., 2019; Massa et al., 2023).
In addition to the above, studies taking a confirmatory approach to sex differences require researchers to:
State and justify hypotheses about sex differences a priori.
Prespecify in the study protocol an analytic approach to test for sex differences. Most researchers conducting experiments will plan to test for sex-by-treatment interactions in a factorial design, as described below.
Specify the expected direction and magnitude of hypothesized sex differences. Predicting the size of sex differences is essential to estimating the sample size necessary to detect them; it is often helpful to anticipate a range of magnitudes.
Plan for a sample size that provides enough statistical power to detect sex differences of the expected size.
As elaborated below, consider other variables that often co-vary with sex and whether steps can be taken to control for them. In human studies, for example, gendered occupations could result in different exposures; in non-human animal studies, housing only the males singly (to prevent fighting) can be similarly confounding.
Studies taking an exploratory approach to sex differences require neither a hypothesis about sex differences nor a sample size adequate to test for them; however, it is at this design point that an exploratory study commits itself to being transparent in reporting its exploratory nature – even if it does reveal an incidental sex difference.
Collection phase: Collection of sex-based data
In the Consideration phase described above, we will have made a careful decision about what “sex” means in the context of our study. Sex-informed data collection, described in this section, requires equally careful consideration of what sex is not. The issue is one of precision. Sex is not itself a tangible, single variable; it is simply a proxy for many factors that covary imperfectly with each other (Richardson, 2022; Maney, 2016; Massa et al., 2023). This fact can lead to a scientific sleight-of-hand that goes unnoticed when we use sex as shorthand for a grab bag of factors correlated with sex. When possible, it is better to measure sex-related variables, such as hormones or gene expression, than to reason backwards from sex to speculative biologic mechanisms. When collecting such data is not possible, extra care must be taken in the Characterization and Communication phases to govern reflexive assumptions about sex and its mechanisms.
Sex is related not only to physiological variables such as gene expression but also to gendered behaviors and social environments, which in turn affect physiology (Ritz and Greaves, 2022). Sex and gender thus nearly always confound each other in human studies. The effects of gender expression, gendered occupations, and disparities in access to health care can be challenging to disentangle from manifestations of ‘sex,’ not only because the construct of gender is complex but also because it is exquisitely sensitive to place and time (Nielsen et al., 2021). Even non-human animals, regardless of whether they experience something we could call gender, can experience sex-specific environmental factors such as housing density, aggression from conspecifics, access to resources, and so on Cortes et al., 2019. Thus, in human and non-human animal studies alike, it can be important to collect data on environmental factors that covary with and are easily conflated with sex.
Figure 1 acknowledges requirements in the Collection phase for exploratory and confirmatory studies. For studies taking a confirmatory approach to sex differences, we recommend the following steps:
Collect data on the variable used to operationalize sex. For example, if sex is operationalized by anogenital distance in newborn mice, measure and record that distance for each mouse.
If the hypotheses specify potential mechanisms underlying sex differences, collect data that can provide information about those mechanisms. For example, if sex (operationalized using genotype) is hypothesized to affect the outcome variable via an association with estradiol, collect data on both genotype and estradiol. These data will lead to more informative conclusions.
As noted above, many physiological and environmental factors covary with sex no matter how it is defined or operationalized. These factors include those that are not part of the hypothesis and therefore could potentially confound any test for sex differences, for example gendered occupational exposures or, in non-human animal studies, housing conditions. When possible, collect data on the most relevant correlates of sex (those that may affect the endpoint) to allow accounting for or adjusting for factors that could masquerade as sex.
In clinical studies, consider allocating participants first to sex strata, then randomizing participants to treatment groups in a systematic way to balance numbers of each sex proportionally across treatments. This strategy, called sex-stratified randomization, distributes sex and sex-related factors equally across treatment arms (Piantadosi, 2005). Sex-stratified randomization enhances the ability to examine interactions between treatment and sex, mitigates potential confounding bias and noise, and can increase statistical power (Piantadosi, 2005).
Studies taking an exploratory approach to sex differences need not take the above steps. For such studies, we recommend the following:
Collect data on important potential confounders to avoid generating post hoc hypotheses about sex differences that would be better explained by other factors.
In an exploratory approach to sex differences, sex may not be as high a priority for stratification as factors known to be strongly associated with outcome or treatment response (e.g., pre-existing health conditions, age, study site, etc.). Therefore, to maximize statistical and logistical efficiency, trialists may choose to stratify randomization on other factors. For most such studies, sex can nonetheless be included as a variable of interest in the analysis, keeping in mind that in the absence of sex-stratified randomization, within-sex analyses forfeit protection against confounding by prognostic factors that co-vary with sex (Piantadosi, 2005; Cui et al., 2002).
Characterization phase: Analysis of sex-based data
In this section, we will address issues related to testing for sex differences. First, a note about the two main categories of sex differences: traits and effects. We may be interested in sex-based variation in certain traits, such as behaviors or the incidence of disease; these contrasts involve two variables, the trait and sex. For example, the question of whether women are more likely to get autoimmune disease than men is a simple two-variable question (the extent to which sex predicts disease status).
More often, though, when we speak of a sex difference in research findings, we are referring to the impact of sex on the association between two other variables. For example, we may be interested in whether sex interacts with, or modifies, the relationship between treatment and disease (in an experiment) or exposure and outcome (in an observational study). In a clinical trial, researchers may examine the influence of sex on the efficacy of aspirin to prevent heart disease; this is a three-variable problem with sex, treatment, and outcome. An observational study may query whether the association between diabetes and future cardiovascular disease differs between men and women, a question involving sex, exposure (diabetes), and outcome. It is these three-variable questions that researchers typically face when, to comply with a new policy, they add another sex to their study. For these, comparing the sexes means testing whether the effect is stronger, or perhaps goes in a different direction, in one sex vs. another. These are important questions to answer as accurately as possible; when translated, sex interactions can influence the treatments and doses that are offered to women and men (FDA, 2018; Zhao et al., 2023).
Unfortunately, many studies fumble the three-variable question (sex/treatment/outcome) by turning it into a pair of two-variable problems (treatment/outcome for males and treatment/outcome for females); this practice of qualitatively comparing the within-sex p-values fails to actually contrast the sexes. For example, if we observe a statistically significant treatment effect among males but not females (or vice versa; left panel of Figure 2A), we may prematurely claim a “sex difference” or a “sex-specific effect.” Similarly, we might mistakenly declare the response to treatment as the “same” if the within-sex tests were either both significant or both not significant (left panel of Figure 2B). In fact, we have not directly compared the treatment effect between the sexes at all. We have instead committed a logical error so widespread among subgroup analyses that it has been called out as one of the most common statistical mistakes in science (Makin and Orban de Xivry, 2019; Allison et al., 2016). The error is easy to commit (and both authors of this article have unwittingly made it in their earlier work). In the context of sex differences, where it is rampant, we have proposed calling it the difference in sex-specific significance (DISS) error (Maney and Rich-Edwards, 2023).
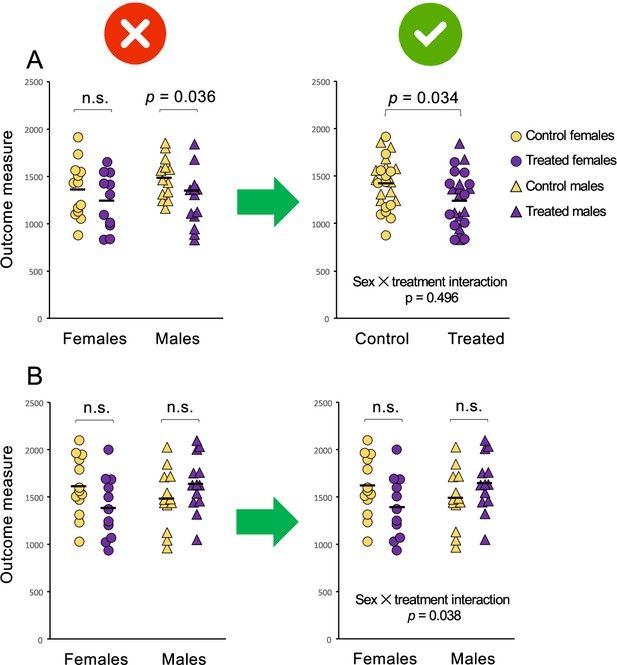
Analysis of data separately by sex can lead to erroneous conclusions.
The graphs above show the response to a control (yellow) or experimental (purple) treatment in females (circles) and males (triangles). Solid black lines represent the mean within each group. The top two graphs (A) depict two different approaches to the same dataset, in which there is an effect of treatment irrespective of sex and no sex difference in the response to treatment. The left panel shows how analyzing the data separately by sex, that is, making the difference in sex-specific significance (DISS) error (Maney and Rich-Edwards, 2023) leads to a false positive finding of a sex difference. The effect of treatment is statistically significant for males (*P=0.036) but not for females (P=0.342) but concluding a sex difference in the response to treatment constitutes an error – the sexes have not been compared statistically. To compare the responses between males and females, we can test for an interaction between sex and treatment, for example in a two-way analysis of variance (ANOVA). The sex × treatment interaction is not statistically significant (P=0.496), meaning there is no evidence that males and females responded differently and no justification to test for effects of treatment separately within each sex. The main effect of treatment, which is estimated from all subjects combined, is significant (P=0.034) suggesting that the treatment altered the outcome measure irrespective of sex. To communicate the main effect of treatment, which is the important finding in this case, the sexes are combined for presentation (right panel). The bottom two graphs (B) depict two approaches to a different dataset, in which there is no main effect of treatment but the sexes did respond differently. The effect of treatment is not statistically significant within females (P=0.115) or within males (P=0.185), so separate analysis (DISS error) could lead to a false negative, i.e. concluding that the sexes responded similarly when they responded differently. The sex × treatment interaction, which is statistically significant (P=0.038), indicates the difference in the responses of females and males. Because of this significant interaction, the data from males and females are presented separately (right panel) and post hoc tests of the effect of treatment are presented for each sex. See Figure 2—source data 1 for the data depicted in A and B.
-
Figure 2—source data 1
Source data for Figure 2, showing the response of females and males to a control or experimental treatment.
- https://cdn.elifesciences.org/articles/90623/elife-90623-fig2-data1-v1.xlsx
DISS is an error for several reasons. First and foremost, within-group analyses tell us nothing about between-group differences; when we perform the ‘parallel play’ of separate analyses by sex, we have neither quantified a sex difference in treatment effect nor considered the role of chance in its appearance (Maney and Rich-Edwards, 2023). Second, p-values are driven by factors (such as subgroup sample size) that have no bearing on the treatment effects researchers intend to compare. Third, contrasting p-values above and below an arbitrary threshold such as 0.05 tempts researchers to categorically declare an effect in one sex and a lack of effect in the other, forgetting that a statistical test can be used only to reject a null hypothesis, not support one (Maney, 2015).
To provide real evidence of sex differences in the response to treatment, we must directly compare the treatment effects between males and females (Nieuwenhuis et al., 2011). In the biomedical sciences, this test usually consists of an interaction term (sex-by-treatment) that is included in the statistical model, along with the main effects of the factors sex and treatment. In preclinical research, if the main effects in the model are categorical, the design is typically called ‘factorial,’ meaning there are multiple factors of interest, such as sex and treatment. In clinical and population science, the main effect of sex and its interactions with treatment are typically captured by terms included in a regression model. The principle, however, is the same no matter the method: the sex-by-treatment interaction term represents the difference in treatment effect between males and females. If this interaction is statistically significant, only then do we have evidence that the sexes responded differently to the treatment. In that case, post hoc tests of the treatment effect could be done within each sex but the resulting sex-specific p-values would not provide more information about the size or statistical significance of the sex difference itself.
Despite the invalidity of the DISS approach, it is widespread in sex differences research (Garcia-Sifuentes and Maney, 2021). Its adoption may be attributable to several factors. First, statistical interactions can require notoriously large sample sizes to detect (Galea et al., 2020); authors of underpowered exploratory studies sometimes decide to skip testing for them altogether and resort instead to a DISS approach. But underpowered studies are particularly vulnerable to DISS errors; as power decreases, the odds of discordant p values in the female and male subgroups increase rapidly. Consider, for example, an experiment with equal numbers of males and females designed to have 80% statistical power to detect a non-null (P<0.05) treatment effect in the entire sample. Such a study typically has only 50% power to detect a within-sex treatment effect. Under this common scenario, when the treatment effect is non-null and does not, in fact, vary by sex, the likelihood of one group yielding P<0.05 and the other P>0.05 is 50% (Bland and Altman, 2011; George et al., 2016; Brookes et al., 2004). In other words, half the time, by chance alone, the treatment effect within males will be statistically significant while that for females is not, or vice versa. One might as well flip a coin to declare a sex difference – obviously not a strategy that promotes rigor and reproducibility. A rigorous strategy would be to test for a sex-by-treatment interaction regardless of whether we are powered to detect one and to accept the limitations of our exploratory approach.
Second, perhaps inspired by calls to find sex differences (Shansky and Murphy, 2021; Rechlin et al., 2021; Tannenbaum et al., 2019), authors may be concerned about missing them and therefore choose a less conservative approach. But, although the DISS approach is biased toward false positive findings (Bland and Altman, 2011; George et al., 2016), it can also produce false negatives – that is, it can cause investigators to miss real sex differences (left panel of Figure 2B). For example, in a recent study of abdominal obesity in children (AO), the authors missed a large sex difference in the association between AO and a measure of lipoprotein particle number because their within-sex p-values showed non-significant associations in both girls and boys (Akiyama et al., 2022). In fact, the interaction between sex and AO was highly significant (P=0.001); the association in girls was positive and among boys, negative (Vorland et al., 2023). The significant interaction serves as strong evidence that the association between AO and this measure of lipoprotein does depend on sex – a potentially important finding that was masked by a DISS approach.
Finally, some investigators may adopt a DISS approach because they believe it accounts for baseline sex variability (i.e., noise) that could mask effects of the other variables of interest. They could also be concerned that effects of treatment could vary so much between the sexes that a main effect is cancelled out. But in the case of a baseline sex difference, the better strategy is still to include sex as a variable of interest; the main effect of sex captures any baseline variation due to sex (Phillips et al., 2023). Including sex in the statistical model can have the added advantage of unmasking effects of treatment when the effect is much larger in one sex than another or goes in different directions in each sex (Phillips et al., 2023; Buch et al., 2019). Similarly, clinical and population researchers can include sex as a covariate in their regression models to reduce extraneous variation and control for confounding by sex; this practice is particularly important in observational studies or in randomized trials that were not stratified by sex.
We should point out here that the main effect of sex is often confused with the sex-by-treatment interaction term; that is, a significant main effect of sex is misinterpreted as a sex difference in the response to treatment (Eliot et al., 2023). In the example above regarding the effect of aspirin on heart disease, a significant sex-by-treatment interaction would provide evidence that the effect of aspirin on heart disease differed between men and women; in contrast, a significant main effect of sex simply means that there is a sex difference in the incidence of heart disease. It does not indicate a sex difference in the efficacy of aspirin. This mistaken interpretation of the outputs of analyses of variance (ANOVAs) is common (Garcia-Sifuentes and Maney, 2021) and, notably, has been endorsed even in NIH training materials about how to incorporate sex as a variable into research designs (NIH, 2020).
Our recommendations for the Characterization (analysis) phase are provided in Figure 1. For all studies, our recommendations are the following:
Test for a statistical interaction between sex and treatment. This test should be conducted regardless of statistical power.
Note the statistical significance and magnitude (effect size) of the interaction and avoid the trap of conflating the p-value and the effect size. For example, it is possible for a large sex difference to fall short of statistical significance in a very small study (and conversely, for a trivial sex difference to be highly statistically significant in a very large study). The magnitude of an interaction is captured by the eta squared from an ANOVA or the beta coefficient for the sex-by-treatment interaction term in a regression.
Interpret a significant effect of sex (e.g., the “main effect of sex” in an ANOVA) as a sex difference that does not depend on the treatment.
If the interaction between sex and treatment is statistically significant:
The interaction alone is sufficient evidence for a sex difference in the response to treatment.
Be aware that post hoc tests for a significant effect of treatment within each sex do not provide information about the sex difference, since the practice of contrasting subgroup p-values remains an illogical DISS error even in the presence of a statistically significant interaction.
Instead of comparing p-values, consider the magnitude (effect sizes) and directions of the within-sex effects.
If the interaction is not statistically significant, do not test for effects of treatment/exposure within sex.
Not all studies fall into the category of three-variable, meaning that there may be only two or more than three variables of interest. Studies focused on the effect of sex on the endpoint, for example (the two variable trait question outlined above), will not be testing the impact of sex on a treatment effect; in these cases, the statistical comparison of the sexes might be as simple as a t-test or chi-square test. Potential confounders of sex, such as gender-related exposures, should be considered and accounted for, usually by adding such potential confounders as covariates in a regression model.
For complex designs with multiple variables of interest (e.g., treatment, sex, time, knockout genotype, etc.), it is good practice to include all interaction terms in the model to examine interactions between sex and each other factor, three-way interactions with sex, etc. as appropriate for the hypotheses. Doing so is important if you wish to draw conclusions about the extent to which interactions among the other variables depended on or differed by sex. For clinical or population studies in which many variables are measured, including all interaction terms may not be feasible, however.
Communication phase: Presenting and interpreting sex differences
Findings of sex differences are often used by non-scientists and scientists alike to shape public policy (Maney, 2015; Maney, 2016). It is therefore critical that such findings be presented in a transparent way that is neither misleading nor dogmatic. In this section, we first consider how to present sex-based data in both exploratory and confirmatory contexts. Then, we discuss pitfalls related to interpretation of data and communication of conclusions.
Presentation of sex-based data
Above, we made the argument that statistical approaches to sex-based data should be similar for both exploratory and confirmatory studies; namely, we should test for statistical interactions between sex and other factors, such as treatment or exposure, no matter the statistical power. The steps of the Communication phase are dictated both by the outcome of that statistical interaction test and whether the study is taking an exploratory or confirmatory approach to sex differences (Figure 1).
In any study, the presentation of data is informed by the hypotheses. The figures and tables highlight the planned comparisons and depict the extent to which the a priori predictions were borne out in the data. Exploratory studies typically begin without a priori hypotheses and predictions about sex; thus there is little reason for a reader to expect results to be presented separately by sex, particularly if the findings regarding sex differences are null. Whether or not a sex-by-treatment interaction is statistically significant, it is critical to report both the magnitude (effect size) of the interaction as well as its standard error, p-value and/or confidence interval. Publishing these statistics will help future researchers decide whether to test formally for sex differences, and if they do, to calculate the sample size required to detect them.
Our recommendation for presenting findings of exploratory studies are as follows:
If the sex-by-treatment interaction is not statistically significant:
Present means, errors, and graphs for the entire sample (not separated out by sex) in the main body of the paper. If the data are being graphed in a way that shows individual data points (e.g., scatterplots, dotplots), it can be informative to use different symbols for males and females (see right panel of Figure 2A). Note that this recommendation does not mean that sex should not be included in the statistical model; on the contrary, we strongly recommend including sex as a variable of interest in all statistical models and publishing those results.
If the journal requires results to be presented separately for females and males, do so in Supplementary Material without within-sex statistical tests of treatment effects (which beg the reader to commit a DISS error even when the authors have not done so).
In the Discussion, acknowledge that the study was underpowered to detect sex-specific treatment effects of this size.
If the interaction is statistically significant:
It is usually appropriate to present tables and graphs with data separated by sex, with the caveat that the approach was exploratory and that the sex difference needs replication.
Occasionally, researchers may judge a statistically significant sex-by-treatment interaction found using an exploratory approach to be not particularly important to the research question. For example, the effect could be quite small (e.g., in the case of very large sample sizes) or there could be reason to believe it is spurious (e.g., in the case of obvious potential confounders). In these rare cases, it may be more appropriate to present tables and graphs showing the data for the entire sample, not separated by sex. As noted above, supplementary material can be used to present findings for females and males separately, depending on journal policy. In addition, as stated previously, no matter the result, it is imperative to publish the magnitude of the interaction (the eta squared from an ANOVA or the beta coefficient for the interaction from a regression) in addition to the F and p values for the interactions with sex.
Authors should acknowledge the exploratory approach and may wish to call for a larger study to confirm the finding of a sex difference.
In all cases, whenever possible, publish or make available raw data with sex indicated for each sample.
Confirmatory studies differ from exploratory ones in that the authors are keenly interested in sex differences—they have powered their study to detect them and have clear a priori hypotheses and predictions about how their results are expected to differ by sex. Thus, as a matter of course the data will be presented in a way that depicts the sex comparison regardless of whether a difference is found. As is the case for an exploratory study, a positive finding of a sex-specific effect in a confirmatory study is evidenced by a significant interaction between sex and another factor, not by within-sex tests. For researchers wishing to indicate the sex difference on a graph, the p-value for the sex-by-treatment interaction can be depicted either on the graph itself or in a caption (see right panels in Figure 2).
We caution that not all sex differences, even statistically significant ones, are large enough to be important. Big data analyses in particular often have enough statistical power to unearth truly trivial sex differences. Whether a sex difference is clinically meaningful or actionable depends very much on context and must be judged on the size of the difference, not the size of the p-value (Klein et al., 2015).
Drawing conclusions from sex-based data
As we interpret findings of sex differences and place them into context, we should consider a number of common pitfalls. The first is the failure to be transparent about unexpected findings of sex differences from exploratory research – including statistically significant interactions. We have all had unexpected findings jump from our data; conservative treatment requires us to indicate when these are surprises. We must avoid the temptation of HARKing (Hypothesizing After Results are Known; Kerr, 1998); such a posteriori justification of an unplanned result is hard for readers to detect unless study protocols are pre-registered (as is required for clinical trials). Conservative communication of exploratory findings relies upon researchers’ honor; indeed, the reputation of the field rests on such transparency.
Transparency is especially important in exploratory research. If looking for a sex difference is justified neither by biology nor by prior data, the probability of chancing upon a true positive is lower than for confirmatory research. Kent and colleagues estimated that only one in four subgroup interactions observed in exploratory analyses are true positives (Kent et al., 2018). In contrast, when tests are justified by strong prior hypotheses – i.e., in confirmatory studies – subgroup interactions are more likely to be true as they start from a higher pre-test probability. In other words, for exploratory studies, even statistically significant findings of sex differences should be treated with caution and caveat.
Second, for both two-variable ‘trait’ questions and multiple-variable ‘interaction’ questions, some researchers may choose to speculate about the potential causes of the sex differences they find. In these cases, it is good practice to discuss both specific and alternative explanations. In terms of specificity, it is important not to fall into the trap of attributing all sex differences to unmeasured covariates. For example, there is a particular inaccuracy – verging on laziness – in the reflexive assumption that phenomena observed in females must be driven by estrogens. In terms of alternative explanations, observational studies must at least consider the role that gendered exposures and experiences might play; not every putative sex difference can be explained by the usual “biological” suspects (Ritz and Greaves, 2022).
Finally, we must be wary of sex essentialism and reification. Most human societies (including some study sections and promotions committees) can be fascinated by sex differences, no matter how small. The failure to consider large overlap between sexes can lead us to inflate the importance of sex differences and reify notions of sex that our investigations should seek to query. The unfortunate result of an over-emphasis on sex, beyond what is warranted by the data, will be a “precision medicine” plagued by imprecision – separate treatments for women and men that do not consider individual physiologies or the myriad traits that predict treatment efficacy better than sex. At this time, at least one treatment has been made less available to women in the US on the basis of “sex differences” that are relatively small and of controversial importance (Zhao et al., 2023). Availability of other treatments and preventatives are in danger of being similarly curtailed for women without sufficient evidence (Denly, 2021; Tadount et al., 2020). It is incumbent upon researchers to consider our own biases and to anticipate the ways in which our findings will be used by clinicians and spun by the media.
Our recommendations for reporting interpretations of sex comparisons are as follows:
Indicate whether tests for sex differences were exploratory or confirmatory.
Refrain from manufacturing ‘prior’ hypotheses about sex differences after the results are known.
Contextualize the magnitude of any sex difference and, where relevant, comment on its clinical or biological importance (or lack thereof).
Discuss the degree of overlap in traits (or treatment effects, when possible) across sex.
Avoid over-interpreting findings of sex difference in written and oral presentations, including media interviews.
Conclusion
Advocates of sex-inclusive research policies have inspired a large and growing community of scientists eager to correct decades of neglect of women and females in biomedical research. The success of this movement relies on the rigor and quality of our science. In mandating sex-inclusive research without requiring sound methodology, we are attempting to reap the benefits of exploratory research without the discipline of confirmatory research. In no other arena has one particular subgroup analysis been singled out as appropriate for every study, whether or not the investigators hypothesized differences to begin with. We will find ourselves shouting sex differences from every mountaintop, simply because we searched for them under every rock. Thus, although the recent dramatic increase in reports of sex differences (Maney and Rich-Edwards, 2023) is encouraging, it is at the same time concerning. While some of these discoveries will prove to be real and in fact meaningful, many will not. We advocate a return to the fundamental principles of exploratory and confirmatory research to promote rigorous, inclusive science. By adhering to best practices, including appropriate presentation and sharing of data, the research community will be better equipped to move quickly from exploratory to confirmatory studies without incurring the unnecessary and dangerous costs of false positives.
The science of sex differences has an interesting future that will almost certainly move us away from sex as a binary category. As a variable in scientific research, “sex” is problematic, an imperfect proxy for myriad traits that can covary tightly with each other (but often do not). Many authors have called for new ways of operationalizing and conceptualizing sex, not as a way to deny or minimize sex differences but rather to increase precision in our science (Ritz and Greaves, 2022; Richardson, 2022; Joel and Fausto-Sterling, 2016; Hyde et al., 2019; Massa et al., 2023). As we move into that future, the statistical treatment of sex as a factor will also evolve. Nonetheless, the difference between exploratory and confirmatory studies will remain, as will our conviction that claims of sex differences should be grounded in solid evidence.
Data availability
The example dataset presented in Figure 2 is included as Figure 2—source data 1.
References
-
Sex differences in schizophreniaInternational Review of Psychiatry 22:417–428.https://doi.org/10.3109/09540261.2010.515205
-
Lipoprotein-subclass particle numbers in children with abdominal obesityPediatrics International 64:e15045.https://doi.org/10.1111/ped.15045
-
Sex differences in animal models: Focus on addictionPharmacological Reviews 68:242–263.https://doi.org/10.1124/pr.115.011163
-
Sex bias in neuroscience and biomedical researchNeuroscience and Biobehavioral Reviews 35:565–572.https://doi.org/10.1016/j.neubiorev.2010.07.002
-
Strategies to optimize inclusion of women in multi-national clinical trialsContemporary Clinical Trials 117:106770.https://doi.org/10.1016/j.cct.2022.106770
-
Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction testJournal of Clinical Epidemiology 57:229–236.https://doi.org/10.1016/j.jclinepi.2003.08.009
-
Benefits of a factorial design focusing on inclusion of female and male animals in one experimentJournal of Molecular Medicine 97:871–877.https://doi.org/10.1007/s00109-019-01774-0
-
WebsiteOnline training modules: Integrating sex & gender in health researchAccessed March 28, 2023.
-
Does gender leave an epigenetic imprint on the brain?Frontiers in Neuroscience 13:173.https://doi.org/10.3389/fnins.2019.00173
-
Issues related to subgroup analysis in clinical trialsJournal of Biopharmaceutical Statistics 12:347–358.https://doi.org/10.1081/bip-120014565
-
The effect of sex on responses to influenza vaccinesHuman Vaccines & Immunotherapeutics 17:1396–1402.https://doi.org/10.1080/21645515.2020.1830685
-
Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond sizeNeuroscience & Biobehavioral Reviews 125:667–697.https://doi.org/10.1016/j.neubiorev.2021.02.026
-
Why and how to account for sex and gender in brain and behavioral researchJournal of Neuroscience 43:6344–6356.https://doi.org/10.1523/JNEUROSCI.0020-23.2023
-
BookInclusion: The Politics of Difference in Medical ResearchChicago: University of Chicago Press.https://doi.org/10.7208/chicago/9780226213118.001.0001
-
Detecting and avoiding likely false-positive findings - a practical guideBiological Reviews of the Cambridge Philosophical Society 92:1941–1968.https://doi.org/10.1111/brv.12315
-
The promises and pitfalls of sex difference researchFrontiers in Neuroendocrinology 56:100817.https://doi.org/10.1016/j.yfrne.2019.100817
-
Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended useResearch Integrity and Peer Review 1:2.https://doi.org/10.1186/s41073-016-0007-6
-
Sex differences in cardiovascular disease - Impact on care and outcomesFrontiers in Neuroendocrinology 46:46–70.https://doi.org/10.1016/j.yfrne.2017.04.001
-
The future of sex and gender in psychology: Five challenges to the gender binaryThe American Psychologist 74:171–193.https://doi.org/10.1037/amp0000307
-
Sex differences in myocardial and vascular agingCirculation Research 130:566–577.https://doi.org/10.1161/CIRCRESAHA.121.319902
-
Beyond sex differences: New approaches for thinking about variation in brain structure and functionPhilosophical Transactions of the Royal Society B 371:20150451.https://doi.org/10.1098/rstb.2015.0451
-
Prevalence of sexual dimorphism in mammalian phenotypic traitsNature Communications 8:15475.https://doi.org/10.1038/ncomms15475
-
Do women really respond differently to antiplatelet therapies?: The evidence just doesn’t add upJournal of the American College of Cardiology 69:1560–1563.https://doi.org/10.1016/j.jacc.2017.01.029
-
HARKing: hypothesizing after the results are knownPersonality and Social Psychology Review 2:196–217.https://doi.org/10.1207/s15327957pspr0203_4
-
Transgender data collection in the electronic health record: Current concepts and issuesJournal of the American Medical Informatics Association 29:271–284.https://doi.org/10.1093/jamia/ocab136
-
Just like a circus: the public consumption of sex differencesCurrent Topics in Behavioral Neurosciences 19:279–296.https://doi.org/10.1007/7854_2014_339
-
Perils and pitfalls of reporting sex differencesPhilosophical Transactions of the Royal Society B 371:20150119.https://doi.org/10.1098/rstb.2015.0119
-
Considering sex as a variable at a research university: Knowledge, attitudes, and practicesJournal of Women’s Health 32:843–851.https://doi.org/10.1089/jwh.2022.0522
-
Sex-inclusive biomedicine: Are new policies increasing rigor and reproducibility?Women’s Health Issues 33:461–464.https://doi.org/10.1016/j.whi.2023.03.004
-
Deconstructing sex: Strategies for undoing binary thinking in neuroendocrinology and behaviorHormones and Behavior 156:105441.
-
Sex and gender differences in substance use disordersClinical Psychology Review 66:12–23.https://doi.org/10.1016/j.cpr.2017.10.012
-
BookMeasuring Sex, Gender Identity, and Sexual OrientationNational Academies Press.https://doi.org/10.17226/26424
-
Gender-related variables for health researchBiology of Sex Differences 12:23.https://doi.org/10.1186/s13293-021-00366-3
-
Erroneous analyses of interactions in neuroscience: A problem of significanceNature Neuroscience 14:1105–1107.https://doi.org/10.1038/nn.2886
-
WebsiteConsideration of sex as a biological variable in NIH-funded researchAccessed September 19, 2023.
-
Sex differences in cardiovascular aging and heart failureCurrent Heart Failure Reports 17:409–423.https://doi.org/10.1007/s11897-020-00487-7
-
BookClinical Trials: A Methodologic PerspectiveWiley-Interscience.https://doi.org/10.1002/0471740136
-
Sex ContextualismPhilosophy, Theory, and Practice in Biology 14:2.https://doi.org/10.3998/ptpbio.2096
-
Transcending the male-female binary in biomedical research: Constellations, heterogeneity, and mechanism when considering sex and genderInternational Journal of Environmental Research and Public Health 19:4083.https://doi.org/10.3390/ijerph19074083
-
Enrollment of female participants in United States drug and device phase 1-3 clinical trials between 2016 and 2019Contemporary Clinical Trials 115:106718.https://doi.org/10.1016/j.cct.2022.106718
-
Gender/sex/ual diversity and biobehavioral researchPsychology of Sexual Orientation and Gender Diversity 1:sgd0000609.https://doi.org/10.1037/sgd0000609
-
Sex differences in autoimmune diseasesBiology of Sex Differences 2:1.https://doi.org/10.1186/2042-6410-2-1
-
An agenda for purely confirmatory researchPerspectives on Psychological Science 7:632–638.https://doi.org/10.1177/1745691612463078
-
The integration of sex and gender considerations into biomedical research: Lessons from international funding agenciesJournal of Clinical Endocrinology & Metabolism 106:3034–3048.https://doi.org/10.1210/clinem/dgab434
-
Evaluating gender similarities and differences using metasynthesisAmerican Psychologist 70:10–20.https://doi.org/10.1037/a0038208
Decision letter
-
Hazel WalkerReviewing Editor; eLife, United Kingdom
-
Peter RodgersSenior Editor; eLife, United Kingdom
In the interests of transparency, eLife publishes the most substantive revision requests and the accompanying author responses.
Decision letter after peer review:
Thank you for submitting your article "Best Practices in the Search for Sex Differences" to eLife for consideration as a Feature Article. Your article has been reviewed by three peer reviewers, and the evaluation has been overseen by two members of the eLife Features Team (Peter Rodgers and Hazel Walker). The following individuals involved in review of your submission have agreed to reveal their identity: Stacey Ritz.
The reviewers and editors have discussed the reviews and we have drafted this decision letter to help you prepare a revised submission.
Summary:
The implementation of the NIH's sex as a biological variable (SABV) policy, the Sex and Gender Equity in Research (SAGER) guidelines, the CIHR's requirement for researchers to address sex and gender considerations, and other journal and funder policies, mean that an increasing number of biomedical researchers are incorporating sex and gender into their research. However, many of these researchers don't have a deep grounding in understanding the complexities of sex and gender, and as a result, much of this work takes a relatively shallow view of how sex and gender might influence health and biology. This article provides advice to researchers on how to implement sex as a biological variable in both exploratory and confirmatory studies. However, there are a number of points that need to be addressed to make the article suitable for publication.
Essential revisions:
1. The title needs attention. Although it has a punchy appeal in its current form, I can't help but feel that the approach of *searching for* sex differences is, itself, one of the practices we actually want people to avoid? Certainly we want researchers to study how sex-related factors influence important biological and health phenomena, but a "search for sex differences" seems to presume the outcome of a male-female comparison, or to suggest that finding a male-female difference is a a more desirable outcome than not finding a difference.
2. Explanatory vs confirmatory studies. I don't agree with some of the recommendations, and question whether the differences between explanatory and confirmatory studies are significant enough to justify a different workflow (beyond that a confirmation needs a power calculation). Specifically:
a. Exploratory – why not use a sex stratified randomisation?
b. Both sexes could be included to estimate a generalisable effect. The experiment could still be described as confirmation or explanatory in the context of the intervention effect. Alternatively, it could be confirmatory or explanatory in terms of the exploration of the intervention effect depending on sex. This needs exploring in the manuscript and the diagram needs to give clearer context to which situation they are referring. In my conversations with both the NIH and the MRC, the sex inclusion policies are not to study sex differences but rather to deliver generalisable estimates with the scenario that if the intervention effect is very depend on sex this would be detected.
c. I question the proposal "acknowledge that discordant p values …" Wouldn't it be better practice to report the interaction p value?
d. LHS of Figure 1: if the interaction term is significant, "If desired, …" do you mean if the ES looks biological interesting?
e. LHS of Figure 1: when interaction is not significant "publish aggregated results". Why not present the statistical results of the main effect which is estimated across the two sexes? Pooling is bad practice.
f. LHS of Figure 1: interaction significant – why not comment on the importance of a sex differences of this size?
g. Is it clear, that you should be operating on the LHS when your goal is a generalisable estimate?
3. The following statements are judgemental and at odds with the research for the preclinical field.
"some loss of integrity … due to investigator … disinterest".
"After all, … sex differences.. nor even a passing interest".
4. The manuscript talks about power and the challenges in detecting sex differences but doesn't reference nor consider an important paper exploring this topic (Phillips et al., 2023). This paper shows that when the intervention effect is very different (opposite direction or only in one sex) the power does pass from the main effect to the interaction term providing evidence that it studying the two sexes separately. How does this finding intersect with their arguments?
Phillips B, Haschler TN, Karp NA (2023) Statistical simulations show that scientists need not increase overall sample size by default when including both sexes in in vivo studies. PLoS Biol 21(6): e3002129. https://doi.org/10.1371/journal.pbio.3002129
5. Page 4: "Here we seek to … address some of the methodological barriers " There have been discussions on the barriers in the preclinical space (eg Karp and Reavey 2019 British J Pharmacology 176:4107-4118) that are not considered.
It wasn't clear whether it was aimed at preclinical or clinical research to me. For example – most referencing come from clinical. Being confounded by gender is only relevant to clinical. I think it needs to be clear on scope – analytical and design barriers? It doesn't cover ethical (3Rs, reproductive safety concerns), welfare or recruitment challenges.
6. The authors make many statements that should be supported by published data – even and especially when it concerns a topic of current political interest and pressure (the authors explicitly discuss these dangers themselves). An example is the statement about the "historical exclusion of women in biomedical studies". Or the requirement to include both sexes in pre-clinical studies (which implies that it was not done before, that males were mostly used – which is not supported by the literature). Another example is that "similar policies have been implemented in Canada and Europe and many journals", which is only partially supported by the chosen literature.
Carefully check all statements throughout the manuscript as to whether they need literature support.
7. The terms sex and gender should be appropriately introduced at the very beginning (see e.g., Clayton and Tannebaum 2016). They are still mixed up frequently in literature, with using gender regularly for research animals.
8. This reviewer disagrees with the statement (page 19) that when authors consider sex differences (statistically significant) not interesting, they should present aggregated data. This approach deprives the readers of making their own judgments.
9. Some specific points:
a. "As a result of the policies to increase inclusion of both sexes, women are now represented in most clinical trials; preclinical research increasingly includes female animals" – References for animal studies are missing
b. The extremely high percentage of sex differences in gene-deficient animals (see Karp 2017 Nat Comm) points towards possible sex differences in a large number of human-centric studies as well.
c. Statistically sound (or field standards) for group sizes in exploratory research are available – would not now be the time to call for increases in group sizes (exploratory) to make SABV analysis more sound?
d. In the consideration phase: Does one not start with the decision of confirmatory versus exploratory?
e. While for preclinical research the operationalization of sex is well-explained, it would be helpful to similarly give guidance on clinical/observational research.
f. A clearer description of block and/versus factorial design may benefit the reader, as well as pointing to respective literature for animal, human, and epidemiological studies to help implement it.
10. The statement at the top of p6 reads "sex is the only subgroup analysis mandated by the federal government." I have not read every piece of guidance and policy from the ORWH or NIH on the SABV policy, but I have heard a number of advocates of the policy state unequivocally that the SABV policy does not *require* researchers to compare males and females, so this may need to be given more nuance. I think it could also be very valuable for the authors to expand more here on the dangers of such an emphasis on 'sex differences' and sex-specific interventions, as many researchers engaging in this type of approach fail to see the problems that almost inevitably arise from uncritically implementing findings based on a M-F binary.
11. It could be useful for the authors to expand and offer a few more examples for their bullet point on page 8 about considering whether sex must be binarized -- this will likely be a new possibility for many readers and they could probably benefit from a little more explanation and examples.
12. I'd like to see the authors take a stronger stand in the final bullet point about becoming familiar with the literature. People doing exploratory studies should be expected to examine whether relevant findings have already been published; and people doing confirmatory studies absolutely *should* know what the literature already says about findings and possible mechanisms. They've phrased it as "a good idea" but I'd like to see them take a stronger stand.
13. The point on page 9 about sex category as a proxy, and that it is better to measure sex-related variables where possible, is an absolutely crucial point, perhaps one of the most important points in the paper. I would encourage the authors to emphasize this further, and perhaps revisit it in the conclusions or abstract, to ensure it receives appropriate attention from readers.
14. The final bullet point on page 11, it is not clear to me what "factorial (blocked) sex-stratified randomization" means -- I would suggest offering a concrete example to illustrate.
15. In the last bullet point on page 16, the authors briefly suggest that researchers should consider effect sizes and directions of within-sex effects. This is a point that I think warrants greater emphasis and discussion, particularly because researchers focussed on sex differences tend to over-interpret the importance of a difference in means, and neglect to evaluate or discuss the extent of heterogeneity within the groups and overlap between them, leading to hyper-polarized calls for sex-specific intervention that are not justified by the data. It would be valuable to have a paragraph or two discussing the importance of effect size (and measures of it) and warning against the tendency to call for sex-specific intervention whenever there is a difference in means.
https://doi.org/10.7554/eLife.90623.sa1Author response
Essential revisions:
1. The title needs attention. Although it has a punchy appeal in its current form, I can't help but feel that the approach of *searching for* sex differences is, itself, one of the practices we actually want people to avoid? Certainly we want researchers to study how sex-related factors influence important biological and health phenomena, but a "search for sex differences" seems to presume the outcome of a male-female comparison, or to suggest that finding a male-female difference is a a more desirable outcome than not finding a difference.
We have changed the title to ‘Rigor and Reproducibility in the Era of Sex-inclusive Research: Best Practices for Investigators’
2. Explanatory vs confirmatory studies. I don't agree with some of the recommendations, and question whether the differences between explanatory and confirmatory studies are significant enough to justify a different workflow (beyond that a confirmation needs a power calculation). Specifically:
a. Exploratory – why not use a sex stratified randomisation?
As requested below, we have added to the text a more comprehensive treatment of sex stratified randomization, including why it may not be appropriate for all exploratory studies (when variables other than sex may have more influence on the outcome and be higher priority for stratification). As randomization is germane only to experimental studies, we have removed the bullet from the figure.
b. Both sexes could be included to estimate a generalisable effect. The experiment could still be described as confirmation or explanatory in the context of the intervention effect. Alternatively, it could be confirmatory or explanatory in terms of the exploration of the intervention effect depending on sex. This needs exploring in the manuscript and the diagram needs to give clearer context to which situation they are referring.
To clarify, the distinction we make between confirmatory and exploratory research is with respect to their different intention and preparation to examine sex differences, not whether they take a confirmatory or exploratory approach to testing the main effects of treatments. We have clarified in the text and added to the Figure that the terms “confirmatory” and “exploratory” refer to the discovery of sex differences, not the main effect under study.
In my conversations with both the NIH and the MRC, the sex inclusion policies are not to study sex differences but rather to deliver generalisable estimates with the scenario that if the intervention effect is very depend on sex this would be detected.
The NIH and MRC now require that males and females be included in studies unless there is strong justification. Explicit sex comparisons are not required. Nonetheless, most researchers who include males and females end up comparing them, often using inappropriate approaches. For example, NIH has promoted within-sex analyses which leads to the DISS approach; in their online training course (https://cihr-irsc.gc.ca/e/49347.html), CIHR explicitly directs researchers to take a DISS approach to “reveal” sex differences. Our goal is to provide guidance about how to compare the sexes when that is what researchers would like to do, and how to avoid seeming to compare the sexes when in fact no comparison was undertaken. The paragraph of the introduction in which we introduce the problem we wish to address (lack of training in appropriate methods to compare the sexes) has been rewritten to better articulate these goals.
c. I question the proposal "acknowledge that discordant p values …" Wouldn't it be better practice to report the interaction p value?
We have clarified the language by changing the figure to read: “Consider the interaction, not discordant within-sex p values, as evidence of a sex difference.” The interaction should always be reported: that point is included in the next (Communicate) bubble, which reads “Publish interaction effect size, error, and statistical significance.”
d. LHS of Figure 1: if the interaction term is significant, "If desired, …" do you mean if the ES looks biological interesting?
When the interaction is significant in an exploratory study, within-sex tests are justified but not always necessary. We have now elaborated on this point in the text. Whether to explore the finding further depends on the research question, the size of the interaction, the likelihood of confounding bias, and the researcher’s goals. Most researchers will, in fact, conduct the post-hoc tests, likely because they believe they are required to do so. Our recommendation here is to clarify that they are not, and that there are cases in which it is probably OK to not conduct them.
e. LHS of Figure 1: when interaction is not significant "publish aggregated results". Why not present the statistical results of the main effect which is estimated across the two sexes? Pooling is bad practice.
We are not advocating for pooling (ignoring sex) in the statistical model. As noted in the manuscript, sex should always be included as a variable of interest in the analysis. Here, we are arguing that in the absence of evidence that the effect of treatment depended on sex, data from males and females should be presented (in graphs, etc.) together. This does not preclude or undo the inclusion of sex as a variable of interest in the analysis of the data, nor does it interfere with reporting of the main effect of treatment (which typically takes sex into account). This has been clarified in the bulleted list.
f. LHS of Figure 1: interaction significant – why not comment on the importance of a sex differences of this size?
We wish to distinguish between the communication of sex differences reported by confirmatory studies and exploratory studies. Authors of confirmatory studies are beholden to comment on the clinical or biological importance (or lack thereof) of the sex difference under investigation, moving that information one step closer to translation. Exploratory studies may chance upon a sex difference that was never hypothesized and has never before been reported. The primary responsibility in that case is to consider whether the finding warrants replication rather than to speculate about its clinical or biological significance. To require investigators to comment on translational implications of exploratory findings would be to encourage post hoc rationalization of sex differences, which is particularly problematic for researchers whose main interests do not lie in that area.
g. Is it clear, that you should be operating on the LHS when your goal is a generalisable estimate?
The left-hand side of the figure summarizes best practices when the study is not designed and likely underpowered to detect a sex by treatment interaction—in this case, whether the effect is generalizable is not being rigorously tested. In exploratory research, males and females have been included in numbers generally too small to test the effect of treatment separately within each sex. Typically, what is being tested (on both sides of the figure) is a main effect of a treatment or exposure, and although such an effect may not be detectable within each sex due to small sample size (left side), it can be detected when the sexes are considered together in a factorial analysis.
3. The following statements are judgemental and at odds with the research for the preclinical field.
"some loss of integrity … due to investigator … disinterest".
"After all, … sex differences.. nor even a passing interest".
These statements have been removed and the paragraph rewritten to clarify our rationale for the piece.
4. The manuscript talks about power and the challenges in detecting sex differences but doesn't reference nor consider an important paper exploring this topic (Phillips et al., 2023). This paper shows that when the intervention effect is very different (opposite direction or only in one sex) the power does pass from the main effect to the interaction term providing evidence that it studying the two sexes separately. How does this finding intersect with their arguments?
Phillips B, Haschler TN, Karp NA (2023) Statistical simulations show that scientists need not increase overall sample size by default when including both sexes in in vivo studies. PLoS Biol 21(6): e3002129. https://doi.org/10.1371/journal.pbio.3002129
We have added a brief discussion of a scenario in which a significant interaction can help researchers detect effects that may differ between the sexes. Phillips et al. (2023) is now cited.
5. Page 4: "Here we seek to … address some of the methodological barriers " There have been discussions on the barriers in the preclinical space (eg Karp and Reavey 2019 British J Pharmacology 176:4107-4118) that are not considered.
It wasn't clear whether it was aimed at preclinical or clinical research to me. For example – most referencing come from clinical. Being confounded by gender is only relevant to clinical. I think it needs to be clear on scope – analytical and design barriers? It doesn't cover ethical (3Rs, reproductive safety concerns), welfare or recruitment challenges.
This sentence has been rewritten: “Here, we seek to highlight and help address this methodological barrier by proposing “best practices” in study design, data analysis, and presentation of findings, emphasizing principles common to preclinical, clinical, and population science.”
6. The authors make many statements that should be supported by published data – even and especially when it concerns a topic of current political interest and pressure (the authors explicitly discuss these dangers themselves). An example is the statement about the "historical exclusion of women in biomedical studies".
A reference has been added about the historical exclusion of women and other references have been added throughout as appropriate.
Or the requirement to include both sexes in pre-clinical studies (which implies that it was not done before, that males were mostly used – which is not supported by the literature).
A reference to the SABV policy announcement has been added, as well as literature supporting a male bias in preclinical work prior to 2016.
Another example is that "similar policies have been implemented in Canada and Europe and many journals", which is only partially supported by the chosen literature.
A reference to White et al. 2021 has been added, which covers the policies in Canada and Europe. A reference to the UK policy has also been added. The “many journals” refers to the SAGER guidelines for which Heidari, et al. is referenced.
Carefully check all statements throughout the manuscript as to whether they need literature support.
References have been added where needed.
7. The terms sex and gender should be appropriately introduced at the very beginning (see e.g., Clayton and Tannebaum 2016). They are still mixed up frequently in literature, with using gender regularly for research animals.
The definitions of sex and gender are in flux and, although we agree the terms are sometimes used incorrectly, we believe it is beyond the scope of the present paper to delve into these definitions (any that we could choose would be oversimplified and quickly outdated). At the end of the introduction, we acknowledge that like sex, gender is also critically important in human research and we point out that all of our recommendations about how sex should be handled as a variable also apply to gender, where relevant.
8. This reviewer disagrees with the statement (page 19) that when authors consider sex differences (statistically significant) not interesting, they should present aggregated data. This approach deprives the readers of making their own judgments.
We now use “entire sample” instead of “aggregated” and have clarified that our recommendation pertains only to the visual presentation of results (e.g., figures), not data analysis or presentation of statistics.
Regarding the recommendation of different graphical approaches depending on whether the sex difference is “interesting”; we have reworded this entire section to remove the word “interesting” and to emphasize that in most cases, a significant sex-by-treatment interaction warrants graphing the results from females and males separately. In some cases the author may judge a significant interaction from an exploratory approach to be irrelevant to the research question or of trivial magnitude (e.g., in the case of very large sample sizes), and may choose to put all of the results onto the same graph. This does not deprive the interested reader of making their own judgments because all of the statistics relevant to sex, including main effects, interactions, and their magnitudes, should always be presented elsewhere in the main text, and if the journal requires separate graphs, they should be included in the supplemental material.
9. Some specific points:
a. "As a result of the policies to increase inclusion of both sexes, women are now represented in most clinical trials; preclinical research increasingly includes female animals" – References for animal studies are missing.
These references have been added.
b. The extremely high percentage of sex differences in gene-deficient animals (see Karp 2017 Nat Comm) points towards possible sex differences in a large number of human-centric studies as well.
A reference to Karp et al., 2017 has been added.
c. Statistically sound (or field standards) for group sizes in exploratory research are available – would not now be the time to call for increases in group sizes (exploratory) to make SABV analysis more sound?
We remain agnostic about whether researchers should or should not increase their sample sizes so that they can properly test for sex differences. One of our main goals with the manuscript is to point out that not all studies are powered to do so, which is not itself an issue unless researchers use approaches and/or draw conclusions that are not appropriate for exploratory research. That is, we do not condemn exploratory approaches – on the contrary, we hope to provide guidance about how to make exploratory research more rigorous. We also point out that (as perhaps another reviewer noted) the SABV policy does not require comparing the sexes. This point has been clarified in the manuscript.
d. In the consideration phase: Does one not start with the decision of confirmatory versus exploratory?
Yes, that is stated as the first step in the Consideration phase in the text. We have emphasized this by adding a bullet point in the Consideration phase at the top of the decision tree in Figure 1. We have added emphasis in the text that this decision is made very early in the research process. This point is also made at the end of the section on Consideration.
e. While for preclinical research the operationalization of sex is well-explained, it would be helpful to similarly give guidance on clinical/observational research.
We have added a mention of how sex is typically measured in clinical studies; as this is a controversial and fast-evolving area, we prefer to highlight the principle rather than detail recommendations. We now direct the reader to more in-depth discussions of this topic.
f. A clearer description of block and/versus factorial design may benefit the reader, as well as pointing to respective literature for animal, human, and epidemiological studies to help implement it.
We have added a description of factorial designs for pre-clinical studies and sex-stratified randomization for clinical trials. We include references for these methods.
10. The statement at the top of p6 reads "sex is the only subgroup analysis mandated by the federal government." I have not read every piece of guidance and policy from the ORWH or NIH on the SABV policy, but I have heard a number of advocates of the policy state unequivocally that the SABV policy does not *require* researchers to compare males and females, so this may need to be given more nuance.
In our manuscript we were using the term “subgroup analysis” to mean separate, within-sex analyses of disaggregated data. This practice is not the same thing as comparing males and females. The sentence has been removed to avoid confusion.
I think it could also be very valuable for the authors to expand more here on the dangers of such an emphasis on 'sex differences' and sex-specific interventions, as many researchers engaging in this type of approach fail to see the problems that almost inevitably arise from uncritically implementing findings based on a M-F binary.
We have expanded this section of the manuscript to say a bit more about the risks of such uncritical implementations of findings.
11. It could be useful for the authors to expand and offer a few more examples for their bullet point on page 8 about considering whether sex must be binarized -- this will likely be a new possibility for many readers and they could probably benefit from a little more explanation and examples.
Two additional examples are now provided. We are aware of several other papers in press and under review (by other authors) that cover this topic in detail, and do not wish to expand this part of the paper unless/until we can cite those publications. The ideas are (as the reviewer points out) new and bold and we don’t wish to take credit, only plant the seed.
12. I'd like to see the authors take a stronger stand in the final bullet point about becoming familiar with the literature. People doing exploratory studies should be expected to examine whether relevant findings have already been published; and people doing confirmatory studies absolutely *should* know what the literature already says about findings and possible mechanisms. They've phrased it as "a good idea" but I'd like to see them take a stronger stand.
The recommendation to become familiar with that literature has been moved to the top of the Consideration section and is now more clearly relevant to both confirmatory and exploratory approaches.
13. The point on page 9 about sex category as a proxy, and that it is better to measure sex-related variables where possible, is an absolutely crucial point, perhaps one of the most important points in the paper. I would encourage the authors to emphasize this further, and perhaps revisit it in the conclusions or abstract, to ensure it receives appropriate attention from readers.
This point is now emphasized a bit more in that location; note that it comes up again in the bulleted list under “Collect” and is further emphasized there (several related points underlined). The last paragraph of the manuscript also makes a similar point, and we have expanded that paragraph as well.
14. The final bullet point on page 11, it is not clear to me what "factorial (blocked) sex-stratified randomization" means -- I would suggest offering a concrete example to illustrate.
We expanded the description of sex-stratified randomization typically used in clinical research.
15. In the last bullet point on page 16, the authors briefly suggest that researchers should consider effect sizes and directions of within-sex effects. This is a point that I think warrants greater emphasis and discussion, particularly because researchers focussed on sex differences tend to over-interpret the importance of a difference in means, and neglect to evaluate or discuss the extent of heterogeneity within the groups and overlap between them, leading to hyper-polarized calls for sex-specific intervention that are not justified by the data. It would be valuable to have a paragraph or two discussing the importance of effect size (and measures of it) and warning against the tendency to call for sex-specific intervention whenever there is a difference in means.
The context for the bullet point referenced here is to present an alternative to comparing p values – instead, we recommend that researchers look at the directions of the effects and their relative sizes to make a judgment call about the extent to which the sexes “differ” instead of comparing within-sex p-values. The point about effect sizes generally, namely acknowledging overlap between the sexes, is made much more strongly in the section on interpretation, where we recommend contextualizing the magnitudes of all differences, commenting on whether they are biologically or clinically important, discussing overlap, and avoiding over-interpretations. We warn against essentialism and reification of sex and the tendency to inflate the importance of difference. In response to this comment, we have expanded that section to make this point even more strongly.
https://doi.org/10.7554/eLife.90623.sa2Article and author information
Author details
Funding
National Institutes of Health (U54AG062334)
- Donna L Maney
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
The authors thank Mara Hampson for assistance preparing the manuscript for submission, and Andrew Brown and Colby Vorland for fruitful discussions.
Publication history
- Received:
- Accepted:
- Version of Record published:
- Version of Record updated:
Copyright
© 2023, Rich-Edwards and Maney
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,317
- views
-
- 145
- downloads
-
- 12
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
- Microbiology and Infectious Disease
Background:
In many settings, a large fraction of the population has both been vaccinated against and infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hence, quantifying the protection provided by post-infection vaccination has become critical for policy. We aimed to estimate the protective effect against SARS-CoV-2 reinfection of an additional vaccine dose after an initial Omicron variant infection.
Methods:
We report a retrospective, population-based cohort study performed in Shanghai, China, using electronic databases with information on SARS-CoV-2 infections and vaccination history. We compared reinfection incidence by post-infection vaccination status in individuals initially infected during the April–May 2022 Omicron variant surge in Shanghai and who had been vaccinated before that period. Cox models were fit to estimate adjusted hazard ratios (aHRs).
Results:
275,896 individuals were diagnosed with real-time polymerase chain reaction-confirmed SARS-CoV-2 infection in April–May 2022; 199,312/275,896 were included in analyses on the effect of a post-infection vaccine dose. Post-infection vaccination provided protection against reinfection (aHR 0.82; 95% confidence interval 0.79–0.85). For patients who had received one, two, or three vaccine doses before their first infection, hazard ratios for the post-infection vaccination effect were 0.84 (0.76–0.93), 0.87 (0.83–0.90), and 0.96 (0.74–1.23), respectively. Post-infection vaccination within 30 and 90 days before the second Omicron wave provided different degrees of protection (in aHR): 0.51 (0.44–0.58) and 0.67 (0.61–0.74), respectively. Moreover, for all vaccine types, but to different extents, a post-infection dose given to individuals who were fully vaccinated before first infection was protective.
Conclusions:
In previously vaccinated and infected individuals, an additional vaccine dose provided protection against Omicron variant reinfection. These observations will inform future policy decisions on COVID-19 vaccination in China and other countries.
Funding:
This study was funded the Key Discipline Program of Pudong New Area Health System (PWZxk2022-25), the Development and Application of Intelligent Epidemic Surveillance and AI Analysis System (21002411400), the Shanghai Public Health System Construction (GWVI-11.2-XD08), the Shanghai Health Commission Key Disciplines (GWVI-11.1-02), the Shanghai Health Commission Clinical Research Program (20214Y0020), the Shanghai Natural Science Foundation (22ZR1414600), and the Shanghai Young Health Talents Program (2022YQ076).
-
- Epidemiology and Global Health
Background:
The role of circulating metabolites on child development is understudied. We investigated associations between children’s serum metabolome and early childhood development (ECD).
Methods:
Untargeted metabolomics was performed on serum samples of 5004 children aged 6–59 months, a subset of participants from the Brazilian National Survey on Child Nutrition (ENANI-2019). ECD was assessed using the Survey of Well-being of Young Children’s milestones questionnaire. The graded response model was used to estimate developmental age. Developmental quotient (DQ) was calculated as the developmental age divided by chronological age. Partial least square regression selected metabolites with a variable importance projection ≥1. The interaction between significant metabolites and the child’s age was tested.
Results:
Twenty-eight top-ranked metabolites were included in linear regression models adjusted for the child’s nutritional status, diet quality, and infant age. Cresol sulfate (β=–0.07; adjusted-p <0.001), hippuric acid (β=–0.06; adjusted-p <0.001), phenylacetylglutamine (β=–0.06; adjusted-p <0.001), and trimethylamine-N-oxide (β=–0.05; adjusted-p=0.002) showed inverse associations with DQ. We observed opposite directions in the association of DQ for creatinine (for children aged –1 SD: β=–0.05; pP=0.01;+1 SD: β=0.05; p=0.02) and methylhistidine (–1 SD: β = - 0.04; p=0.04;+1 SD: β=0.04; p=0.03).
Conclusions:
Serum biomarkers, including dietary and microbial-derived metabolites involved in the gut-brain axis, may potentially be used to track children at risk for developmental delays.
Funding:
Supported by the Brazilian Ministry of Health and the Brazilian National Research Council.






