Amphibian mast cells serve as barriers to chytrid fungus infections
Figures
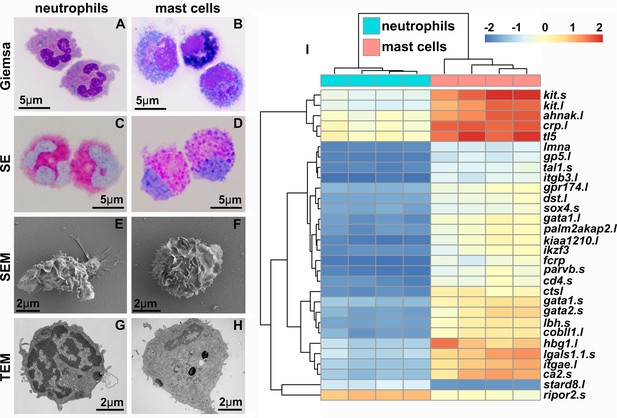
X. laevis bone marrow-derived mast cells possess archetypal mast cell cytology and transcriptional profiles.
Neutrophils (A, C, E, G) and mast cells (B, D, F, H) were stained with Giemsa (A, B) and Leder to visualize specific esterase activity (SE) (C, D) or imaged with scanning and transmission electron microscopy (SEM: E, F and TEM: G, H). (I) Heat map of the top 30 differentially expressed genes (DEGs) identified with RNA sequencing analyses of X. laevis mast cell (N = 4) and neutrophil (N = 4) cultures. Log2fold change in expression represented as color scale.
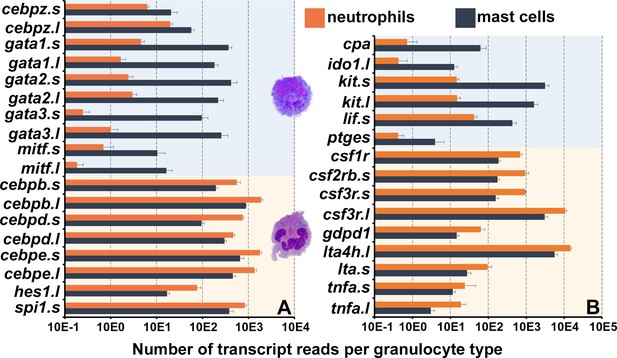
Frog mast cells and neutrophils possess gene profiles similar to their mammalian counterparts.
The differentially expressed genes from the RNA sequencing analyses of X. laevis mast cells and neutrophil cultures were profiled for those encoding (A) transcription factors associated with mast cell- or neutrophil-specific lineages and (B) granulocyte antimicrobial components and growth factor receptor genes. All depicted genes were significantly differentially expressed between the two populations, N = 4 per group.
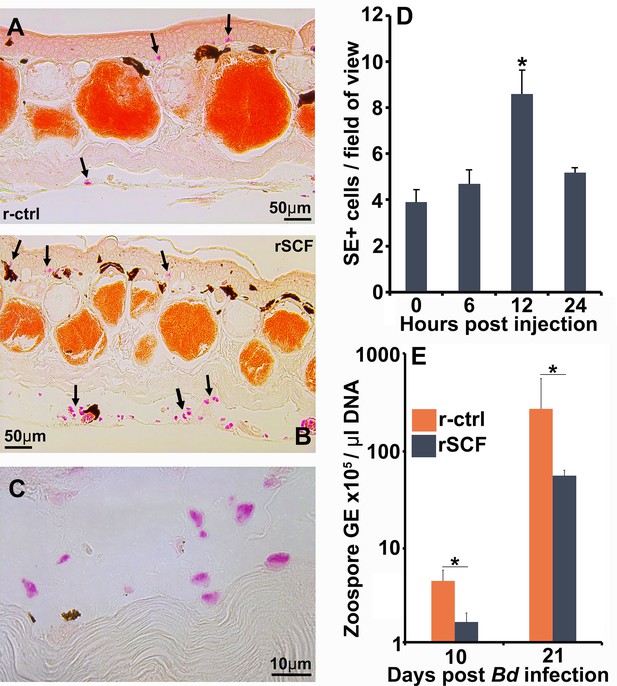
Enriching frog cutaneous mast cells lowers Bd loads.
Representative images of specific esterase (SE) stained (A) control and (B) mast cell-enriched skin 12 hr post injection (hpi). (C) We confirmed the enriched population was composed of mono-morphonuclear cells. (D) Mast cell enrichment was optimized across several time points by quantifying SE-positive cells per field of view under ×40 magnification. Results represent means ± SEM from three animals per time point (two experimental repeats). (E) Mast cell-enriched and control dorsal skins were collected from X. laevis 10 and 21 dpi. Bd loads are represented as the number of zoospore genomic equivalents (GE) × 105 per μL of total input DNA. Time points were analyzed independently. Results represent means ± SEM from seven animals per experimental group (N = 7). Asterisks indicate significance: p<0.05 by (D) one-way ANOVA with Tukey post hoc analysis or (E) Student’s t-test.
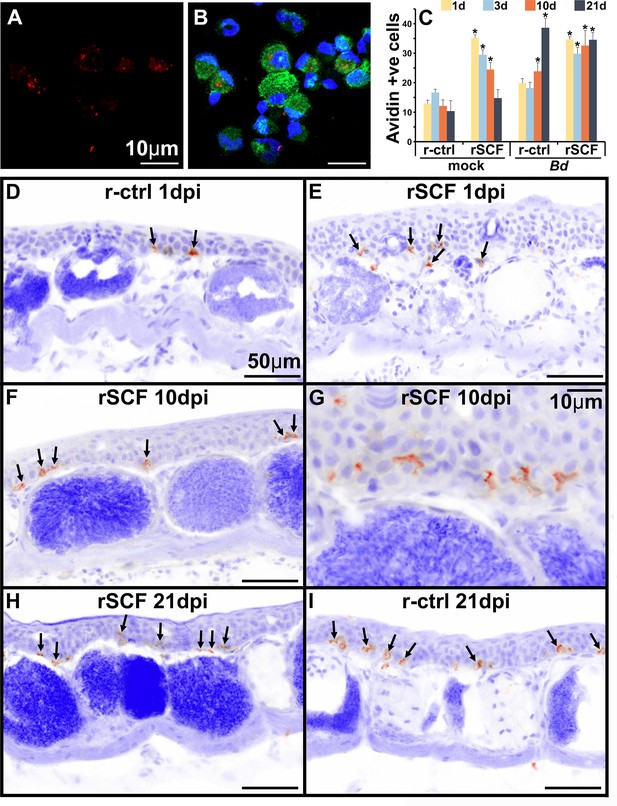
Heparin content and skin localization of frog mast cells.
Frogs were administered r-ctrl or rSCF subcutaneously, mock or Bd-challenged, and their skins examined after 1, 3, 10, and 21 days post infection (dpi). (A, B) Representative images (cultures derived from five individual frogs) of bone marrow-derived frog mast cells, stained with fluorescently labeled avidin to visualize heparin-containing granules (avidin: red; nuclei: blue; actin: green). (C–I) Skin tissue from control (r-ctrl) or mast cell-enriched (rSCF), mock- (not shown) and Bd-infected (D–I) X. laevis were stained with fluorescently labeled avidin to visualize mast cells therein (N = 6 animals per treatment group). Images were inverted in ImageJ for greater contrast and visibility. (C) Heparin-containing mast cells were enumerated and depicted as means ± SEM of heparin-positive cells per field of view, N = 6 animals per treatment group. Asterisks indicate statistical significance from r-ctrl: p<0.05. Representative images of heparin-containing mast cells in the skins of (D) r-ctrl animals 1 dpi; (E) rSCF-administered frogs 1 dpi; (F, G) rSCF-administered frogs 10 dpi; (H) rSCF-administered frogs 21 dpi; and (I) r-ctrl-treated animals 21 dpi with Bd.
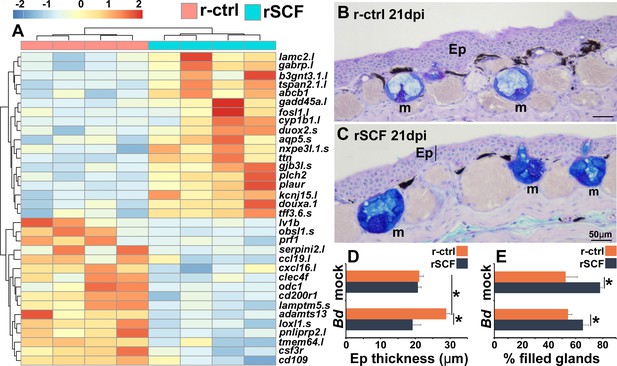
Consequences of cutaneous mast cell enrichment.
(A) RNAseq analysis of skin tissue from control (r-ctrl) or mast cell-enriched (rSCF) Bd-infected X. laevis at 21 days post infection (dpi). Heat map of the top 30 differentially expressed genes (DEGs), numbers matched to colors represent log2 fold change in expression, N = 4 r-ctrl-treated, Bd-infected and 4 rSCF-treated, Bd-infected skin samples. (B, C) Representative images of control and mast cell-enriched, Bd-infected skins, 21 dpi, demonstrating differences in epidermal thickening and mucus gland filling. Mucin content was visualized in cutaneous mucus glands with Alcian Blue/PAS stain. Mucus glands are denoted by ‘m’, and epithelia are denoted by ‘Ep’. ImageJ software was used to determine the means ± SEM of (D) skin epithelial thickness and (E) percent mucus gland filling (N = 6). Asterisks indicate significance: p<0.05 by one-way ANOVA with Tukey post hoc analysis.
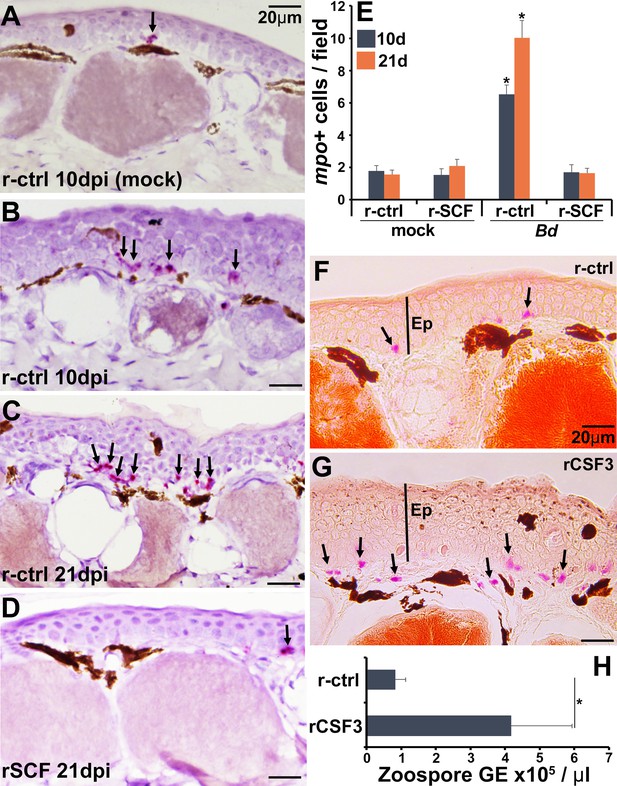
Consequences of cutaneous neutrophil enrichment.
(A–E) Frogs were administered with r-ctrl or rSCF, mock- or Bd-infected, and examined in situ for neutrophil content via by RNAScope analyses of myeloperoxidase (mpo) transcripts. Representative images of (A) r-ctrl-injected frog skins 10 days post mock infection; (B) r-ctrl-injected frog skins 10 days post Bd infection; (C) r-ctrl-injected frog skins 21 days post Bd infection; and (D) r-SCF-administered frog skins 21 days post Bd infection. (E) Means ± SEM of mpo-positive neutrophils per field of view of r-ctrl- and rSCF-administered, mock- or Bd-challenged frog skins, 10 or 21 days post infection (dpi), (N = 6). Asterisks indicate significance from control: p<0.05 by one-way ANOVA with Tukey post hoc analysis. Representative specific-esterase staining of skin tissues from frogs were administered with (F) r-ctrl or (G) rCSF3, N = 4. Arrows denote specific esterase-positive cells. Ep: epithelium. (H) Bd loads in control and neutrophil-enriched skin tissues 7 dpi, N = 6. Asterisks indicate significance: p<0.05 by one-way ANOVA with Tukey post hoc analysis.
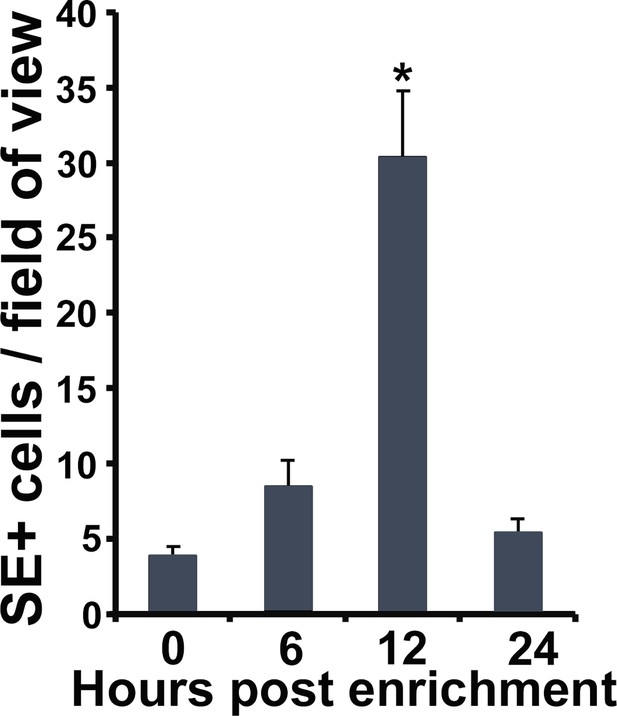
Enrichment of neutrophils in frog skins.
Frogs were administered with rCSF3 subcutaneously and their skins examined for specific esterase activity at 0, 6, 12, and 24 hr post injection. Results are means ± SEM of specific esterase (SE)-positive cells per field of view from four animals per time point (N = 4). Asterisks indicate significance: p<0.05 by one-way ANOVA with Tukey post hoc analysis.
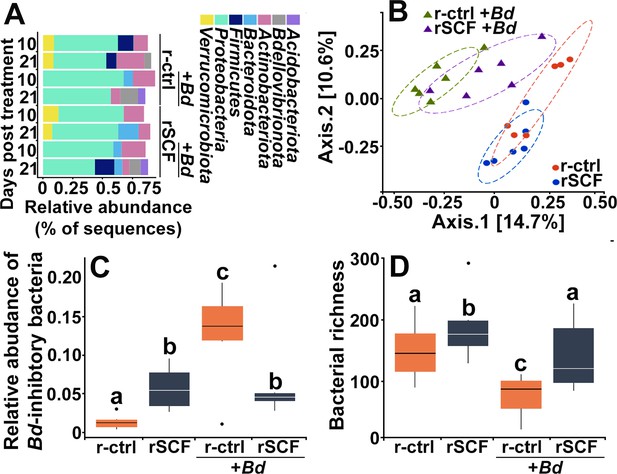
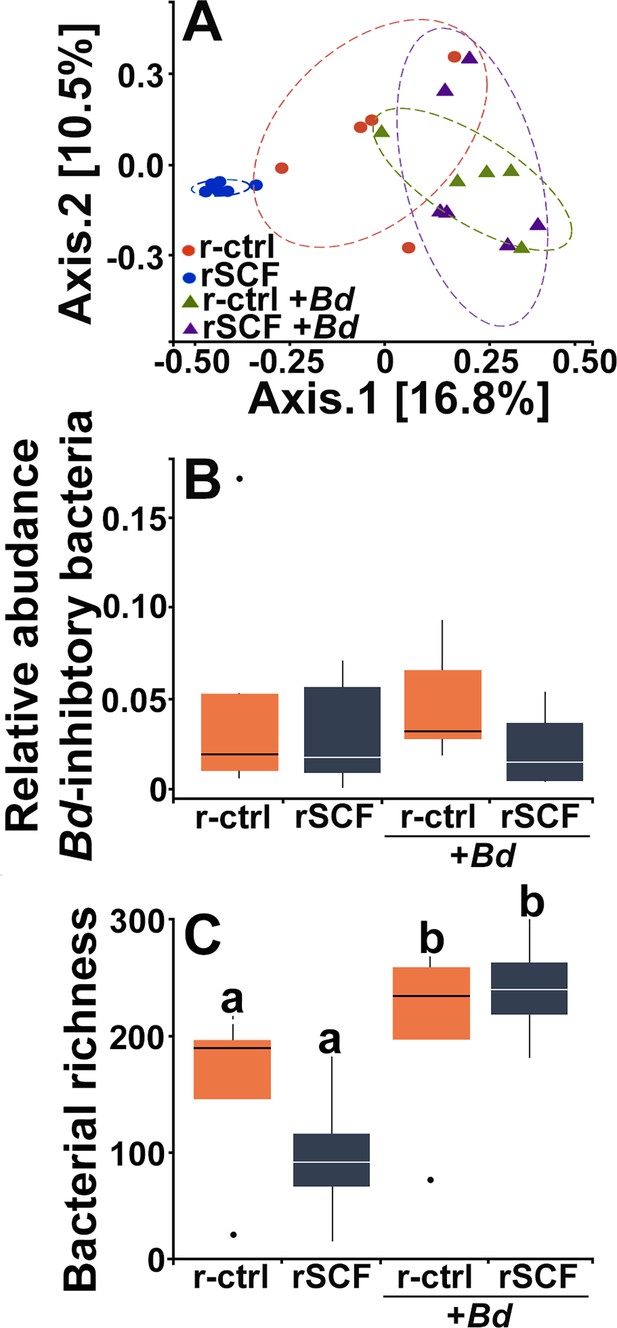
Cutaneous mast cells protect skin microbial communities.
Control (r-ctrl-injected) or mast cell-enriched (rSCF-injected) X. laevis were mock-infected or challenged with Bd for 21 days. (A) Microbial phyla distribution across groups. Low-abundance phyla (<5% relative abundance are not shown). At 10 days post infection (dpi) (B), community composition (Jaccard distances shown with 80% confidence ellipses) differed among all treatments. (C) Relative abundance of Bd-inhibitory bacteria and (D) bacterial richness were examined in control and mast cell-enriched frogs, 10 days post Bd or mock challenge. Letters above bars indicate statistically different groups.
Mast cell enrichment protects frogs from Bd-mediated changes to skin microbiomes.
At 21 days post mock or Bd-challenge, we examined (A) community composition (Jaccard distances shown with 80% confidence ellipses), (B) relative abundance of Bd-inhibitory bacteria, and (C) bacterial richness. Letters above bars indicate statistically distinct groups.
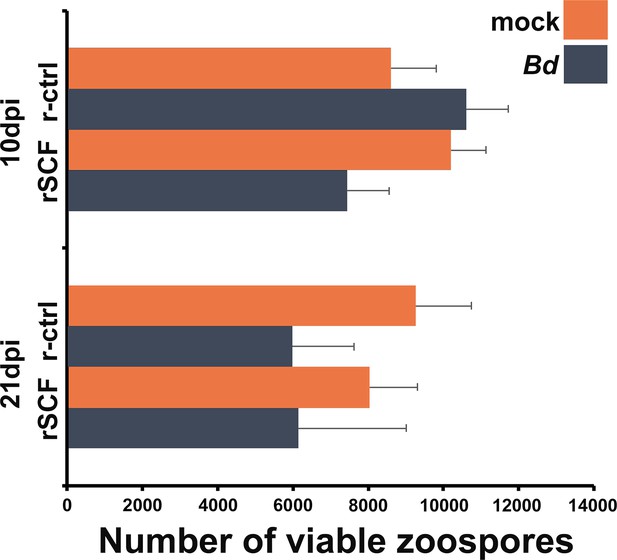
Mucosomes from mast cell-enriched frogs do not confer Bd killing.
Differences in mucosome-killing capacities were determined by incubating zoospores with total mucosome contents for 16 hr. Mucosomes were acquired from 10- or 21-day mock- or Bd-infected X. laevis that were injected with rSCF (mast cell-enriched) or r-ctrl (control). Results are mean ± SEM and were analyzed via a two-way ANOVA; 10- and 21-day experimental groups were analyzed independently; alpha set at 0.05. N = 5 experimental animals per treatment group for 10 days post infection (dpi) analyses and N = 8 experimental animals per treatment group for 21 dpi analyses.
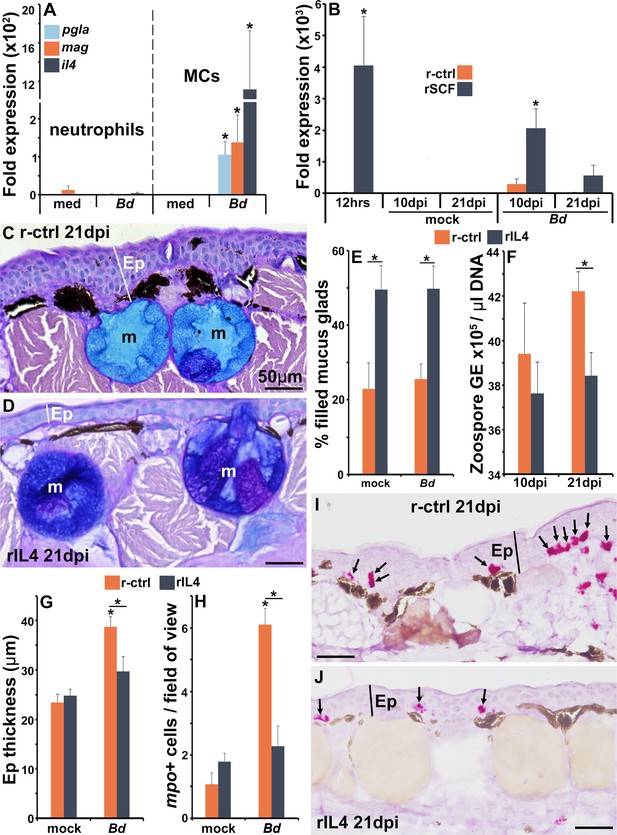
The roles of IL4 in mast cell-mediated skin anti-Bd protection.
(A) Mast cells (MCs) and neutrophils derived from bone marrow of six individual frogs (N = 6) were co-cultured with Bd (five fungal cells per granulocyte) for 6 hr prior to gene expression analyses of the antimicrobial peptide genes PGLa (pgla) and magainin (mag) or interleukin-4 (il4). (B) Il4 gene expression in skins of control and mast cell-enriched, mock- and Bd-infected animals, N = 6. Representative images of frogs administered with (C) r-ctrl or (D) rIL4 and infected with Bd for 21 days, N = 7. Ep: epidermis; m: mucus gland. Means ± SEM of (E) percent mucus gland filling, (F) skin Bd loads, (G) epidermal thickness, and (H) mpo-positive neutrophils, per field of view of r-ctrl- or rIL4-administered, mock- or Bd-challenged frog skins 21 days post infection (dpi) (N = 7). Representative images of mpo-positive neutrophils in (I) control and (J) rIL4-treated frog skins, 21 dpi. Ep: epidermis; arrows: mpo-positive neutrophils. Asterisks indicate significance: p<0.05 by one-way ANOVA with Tukey post hoc analysis.
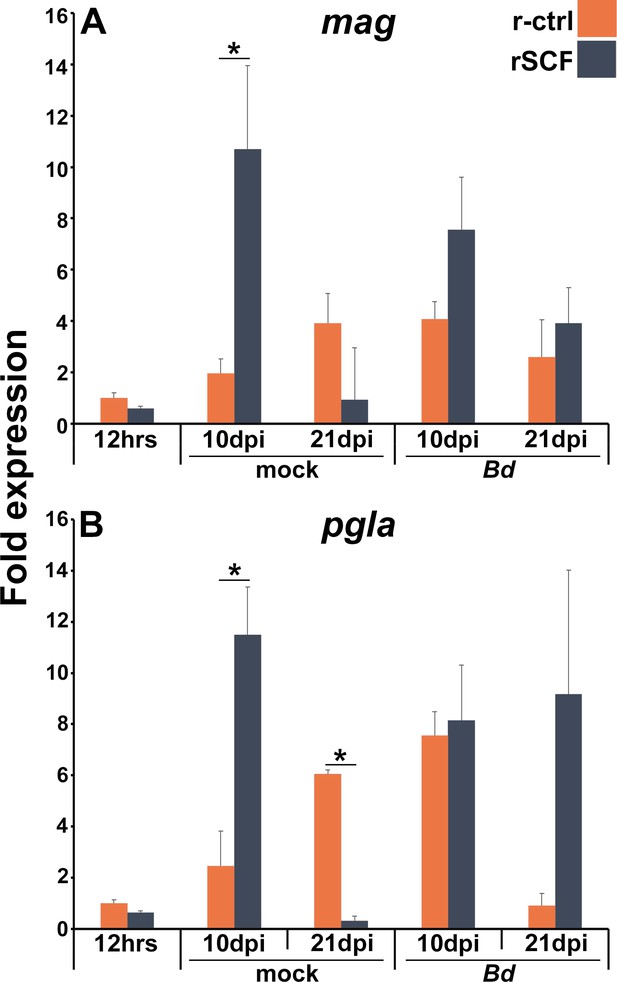
Expression of antimicrobial peptides in mast cell-enriched, Bd-infected frog skins.
(A) Magainin (mag) and (B) PGLa (pgla) expression was examined in control and mast cell-enriched frog skins after 12 hr of enrichment or after 10 or 21 days of mock- or Bd-challenge, N = 6. Asterisks indicate significance: p<0.05 by one-way ANOVA with Tukey post hoc analysis.

IL4 elicits hallmark gene expression but does not protect Bd-infected frogs.
(A) Expression of selected genes in skin following subcutaneous injection of rIL4 (N = 7). (B) Frogs were exposed to Bd and 24 hr later injected subcutaneously with rIL4 or r-ctrl. Skin Bd loads were assessed 9 days following injection (N = 8, per group). Asterisks denote statistical significance, p<0.05 via ANOVA with Tukey post hoc analysis.
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/92168/elife-92168-mdarchecklist1-v1.docx
-
Supplementary file 1
List of primer sequences.
- https://cdn.elifesciences.org/articles/92168/elife-92168-supp1-v1.docx





