Hypersensitive intercellular responses of endometrial stromal cells drive invasion in endometriosis
Figures
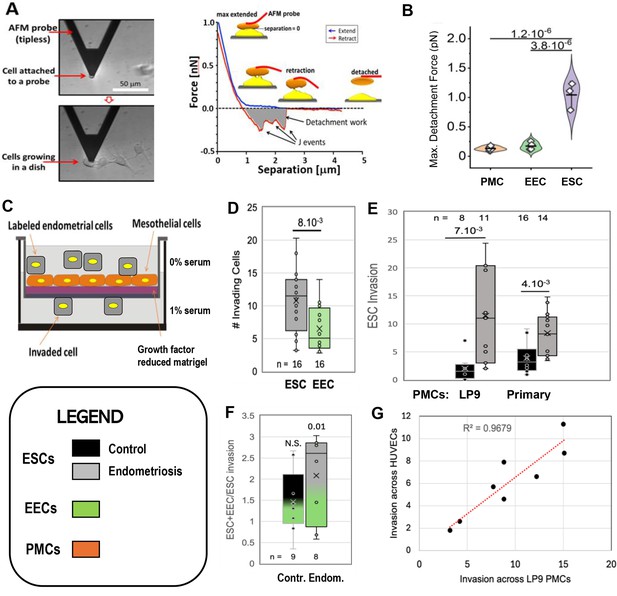
Characterization of endometrial cells from control and endometriosis patients.
Adhesiveness. (A) The force needed to separate a cell attached to an atomic force microscope (AFM) cantilever tip (left) from peritoneal mesothelial cells (PMCs) growing on a dish (left) was calculated from a force/distance curve (right). (B) LP9 PMCs show similar adhesion to one another as to endometrial epithelial cells (EECs), but much stronger adhesion to endometrial stromal cells (ESCs) (3–6 technical replicates). Invasiveness. (C) A 3-D ex-vivo model measured endometrial cell invasion across a PMC monolayer in a Boyden chamber. (D) Consistent with their lower adhesion, EECs were twofold less invasive than ESCs across all patients. (E) ESCs from Endometriosis patients (n=7) were more invasive than those from controls (n=6), both through an LP9 PMCs (> fivefold difference) or primary PMCs (fourfold difference) derived from both control (n=5) or endometriosis (n=3) patients. (F) Mixes (1:1) of ESCs and EECs from the same control (n=6) or endometriosis (n=7) patients were 1.5 and 2.1-fold more invasive, respectively than ESCs alone (significance values represent the difference of co-cultures from ESCs alone). (G) Invasion of ESCs from eight patients across LP9 PMC or HUVEC monolayers were highly correlated. Number of repeats for each condition in D - F is shown. Significance based on two-tailed t-test. Full data in Figure 1—source data 1. Legend applies to Figures 1—4.
-
Figure 1—source data 1
Data tables, and statistical analyses, for Figure 1B, E and F.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig1-data1-v3.xlsx
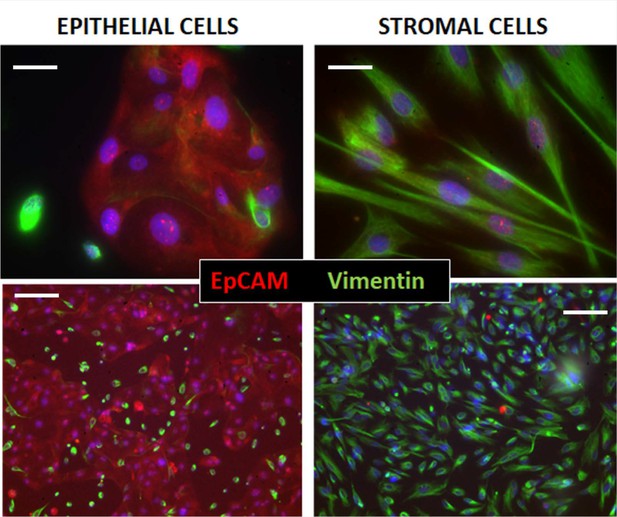
Immunocytochemical assessment of epithelial and stromal cell isolations from patients.
Cell separations from an endometriosis and control patient taken at 40 x (top) and 10 x (bottom), indicate the purity of the isolations, using double staining with EpCAM or cytokeratin 7 antibodies for Epithelial cells (red), and Vimentin for stromal cells (green). Nuclei are stained blue with DAPI in both. Bars are 20 µm (top) and 100 µm (bottom).
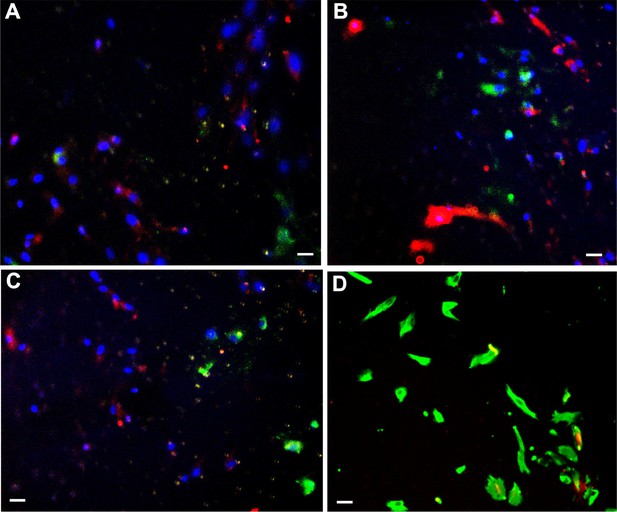
Relative invasiveness of endometrial stromal cells (ESCs) and endometrial epithelial cells (EECs).
(accompanies Figure 1F) (A–C) ESCs [labeled with DiI (red)] and EECs [labeled with DiO (green)] were mixed in equal numbers prior to invasion across a peritoneal mesothelial cell (PMC) monolayer. Invading cells were visualized with DAPI (blue) to stain the nuclei. As DiI and DiO are lipophilic dyes, they stain in a non-uniform punctate pattern. The two-cell types tended to invade in clusters. ESCs formed the majority of the invasive cells (A and C), although in some regions EECs represented 50% of the invasive species. (D) In some experiments, rather than pre-labeling, the cells were fixed and stained with Vimentin for ESCs (green) and cytokeratin 7 for EECs (red). The more uniform labeling clearly demonstrates the predominance of ESCs in the invasive cells. Scale bars are 10 µm.
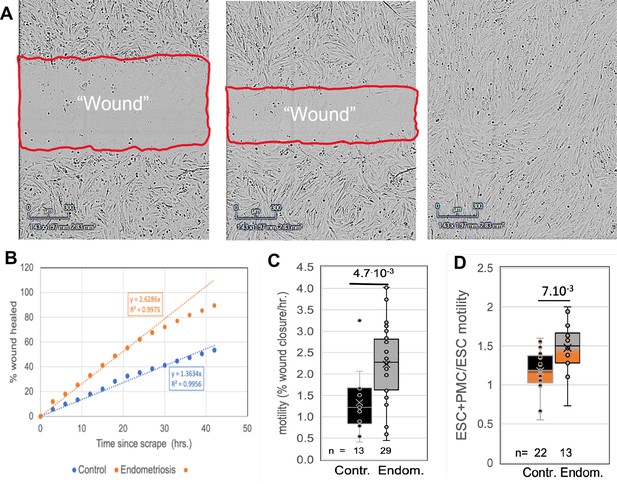
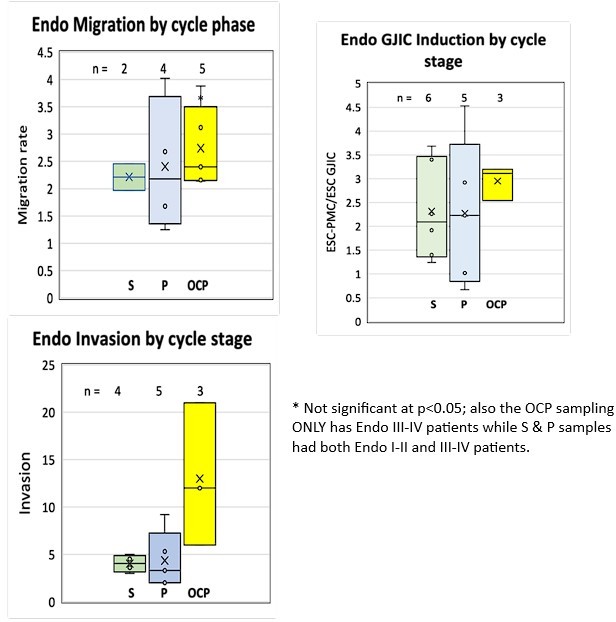
Comparisons of motility of patient endometrial stromal cells (ESCs).
(A) Motility was measured by rates of wound clousre in in an incucyte system (images at 0, 24 and 48 hr after scraping). (B) Motility is measured by fits to the linear portion of the wound closure over time. (C) ESCs from endometriosis patients (n=10) show higher the motility than from control patients (n=9). (D) Mixing LP9 peritoneal mesothelial cell s (PMCs) with ESCs further increases motility of Endometriosis ESCs, while little effect is seen in control ESCs. The number of repeats for each condition is shown. Significance based on two-tailed t-tests. Full data in Figure 2—source data 1.
-
Figure 2—source data 1
Original data tables and statistical analyses for Figure 2C and D.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig2-data1-v3.xlsx
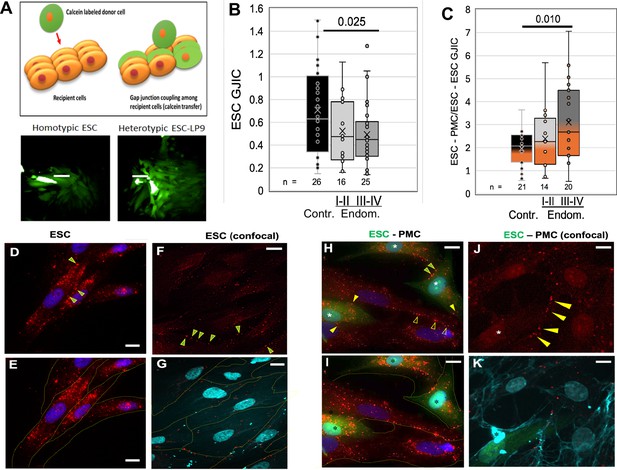
Coupling between endometrial stromal cells (ESCs) and peritoneal mesothelial cells (PMCs) is induced in endometriosis.
(A) Gap junction intercellular coupling (GJIC) was measured by a modified ‘parachute assay’ where calcein-loaded donors are dropped onto a monolayer of acceptors of either the same (homocellular) or different (heterocellular) cell type, and calcein transfer is measured as a linear increase in fluorescent acceptor/donor ratio over time. Scale bars are 50 µM. (B) GJIC between eutopic ESCs decreased progressively with the disease, reaching significance in Endometriosis III-IV patients. (C) Heterocellular ESC-PMC GJIC was induced compared to ESC homocellular coupling and this increased with disease progression to 2–4.5-fold in Endometriosis III-IV patients. (D–K) Immunocytochemical staining of Cx43 (red), with cell outlines from phase (yellow) or membrite labeling (blue) superimposed in the lower panels. ESCs alone showed some labeling between cells (arrowheads), but most Cx43 was in intracellular pools (D–G). By contrast, in mixed cultures of PMCs with ESCs [labeled with cell tracker green (*)] there is less intracellular Cx43 labeing, and punctate staining of GJs between cells is increased in frequency [Arrowheads: ESC-ESC (green in yellow); PMC-PMC (hollow yellow); ESC-PMC (solid yellow)] (H–K). Nuclei are stained with DAPI. Scale bars are 10 μm. Number of repeats for each condition are shown in B and C, with 8–10 patients in each group. Significance based on two-tailed t-tests. Full data in Figure 3—source data 1.
-
Figure 3—source data 1
Original data tables and statistical analyses for Figure 3B and C.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig3-data1-v3.xlsx

Invasiveness of endometrial stromal cells (ESCs) is dependent on Cx43 gap junction intercellular coupling (GJIC).
(A) GAP27 peptide: Averaging ESCs from control (black bars, n=3) and endometriosis patients (gray bars, n=6), the invasion was inhibited by a peptide inhibitor of GJ channels, GAP27 (percent Gap Junction Intercellular Coupling (GJIC) compared to untreated shown below each bar). (B-D) shRNA: (B) Infection of a doxycycline-inducible Cx43 shRNA into endometriosis ESCs or LP9 peritoneal mesothelial cells (PMCs) reduced levels of Cx43 protein (arrow) compared to Laminin controls (doublet at ~66 kD), while expression of DN or wt Cx43 increased Cx43 expression levels. (% of untreated shown below gel) (see Figure 4—source data 1 and Figure 4—source data 2). (C) Cx43 shRNA inhibited GJIC by >90% compared to scrambled shRNA in infected LP9 PMCs (black bars, n=3) and Endometriosis ESCs (Gray bars, n=4). (D) Invasiveness was inhibited by ~85% in Cx43 shRNA infected compared to uninfected neighbors, whether expressed in ESCs (n=7), or PMCs (n=2). DN Cx43 inhibited invasiveness by 98% when expressed in ESCs, and 65% when expressed in PMCs, where ~70% of the monolayer was infected. N represents independent tests with different shRNAs, with 10 technical replicates of each. Significance based on two-tailed t-tests. Full data in Figure 4—source data 3.
-
Figure 4—source data 1
Original full unannotated image of SDS gel in Figure 4B.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig4-data1-v3.zip
-
Figure 4—source data 2
Annotated image of gel in Figure 4B with labels and bands highlighted.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig4-data2-v3.pdf
-
Figure 4—source data 3
Original data table and statistical analysis for Figure 4C and D.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig4-data3-v3.xlsx
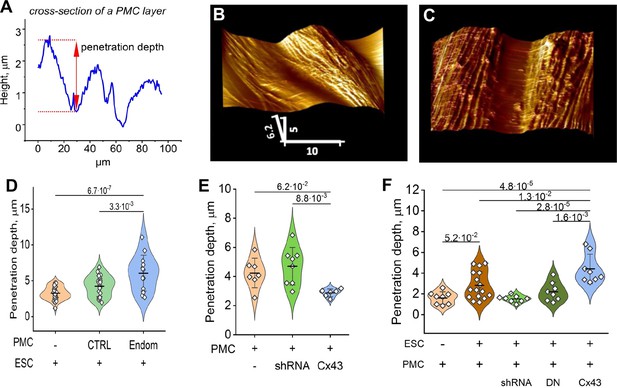
Endometrial stromal cells (ESCs) induce GJ-dependent disruption of the barrier function of a peritoneal mesothelial cell (PMC) monolayer.
(A) Probing the topological surface of a PMC monolayer using an AFM probe under constant force allows the identification of sites of intercellular contact (where penetration of the probe is maximal). (B–C) 3-D reconstructions of the surface of an LP9 PMC monolayer alone (B) or in the presence of ESCs which induce the opening of wide gaps (C) (Scale bars in μm). (D) Penetration depth between PMCs increased more with ESCs from endometriosis than control patients. (E) PMC monolayer integrity (i.e. lower penetrance) is reduced by Cx43 shRNA KD and enhanced by Cx43 overexpression. (F) In contrast, when ESCs are dropped onto a PMC monolayer, the increased penetrance that is induced is eliminated by the expression of Cx43shRNA or DNCx43 in the PMCs and is enhanced by Cx43 overexpression. Each dot in D-F represents a single image analysis. Significance based on two-tailed t-test. Full data in Figure 5—source data 1,Figure 5—source data 2 and Figure 5—source data 3.
-
Figure 5—source data 1
Original data table and statistical analysis table for Figure 5D.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig5-data1-v3.xlsx
-
Figure 5—source data 2
Original data table and statistical analysis table for Figure 5E.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig5-data2-v3.xlsx
-
Figure 5—source data 3
Original data table and statistical analysis table for Figure 5E.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig5-data3-v3.xlsx
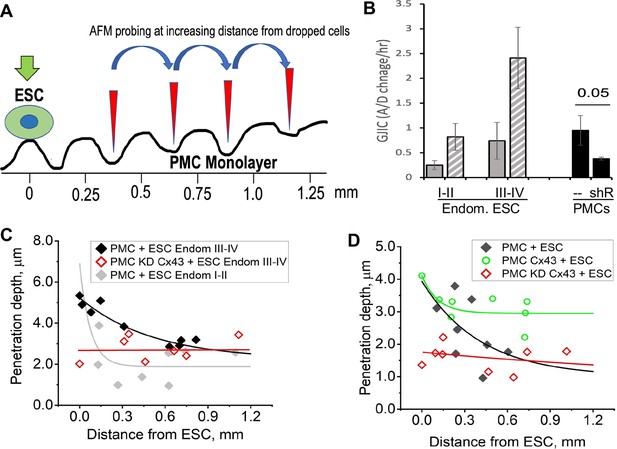
Disruption of the mesothelial barrier by endometrial stromal cells (ESCs) is propagated through mesothelial gap junctions.
(A) Using a constant force of 1 nN, the AFM tip was moved over the peritoneal mesothelial cell (PMC) monolayer progressively further away from a dropped DiI labeled ESC. (B) ESCs from an endometriosis III-IV patient showed greater homocellular (solid gray) and induced heterocellular gap junction intercellular coupling (GJIC) with PMCs (striped gray) than those from an endometriosis I-II patient. GJIC of LP9 PMCs was also measured and shown to be decreased by 60% through an expression of Cx43shRNA (black). (C) Penetration through the LP9 PMC monolayer decayed with distance from the dropped ESC much faster in the poorly coupled Endo I-II ESCs (gray) than the better coupled Endo III-IV ESCs (black). Penetration of the monolayer was eliminated by KD of Cx43 in PMCs (red). (D) Conversely, the decay in penetration of the PMC monolayer induced by Endo III-IV ESC cells (black) was greatly reduced by over-expression of Cx43 in PMCs (green). Full data in Figure 6—source data 1.
-
Figure 6—source data 1
Original data sets for Figure 6C and D.
- https://cdn.elifesciences.org/articles/94778/elife-94778-fig6-data1-v3.xlsx
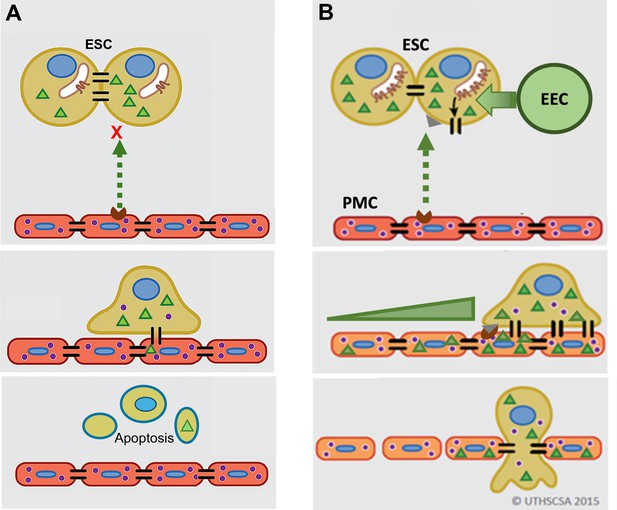
Model of gap junction intercellular coupling (GJIC) induction of trans-mesothelial invasion.
(A) In healthy patients, when endometrial cells (light brown) encounter a mesothelium (brick red) following arrival in the peritoneum via retrograde menstruation, there is limited GJIC between endometrial stromal cells (ESCs) and peritoneal mesothelial cells (PMCs). ESCs also likely undergo apoptosis. (B) In endometriosis, interactions with mesothelial cells trigger Cx43 trafficking to the cell surface and a significant enhancement of GJIC. The increased GJIC mediates the transfer of signals to PMCs (green triangles), which propagate through the mesothelium, inducing disruption of the adhesive and tight junctions between PMCs, facilitating the invasion of the ESCs. There would also be a passage of signals from PMCs to ESCs (purple dots) that induce further changes in ESCs that could promote invasion (Lin et al., 2022). EECs (green cells) show minimal invasion alone, but can enhance ESC invasion, and in endometriosis invade with ESCs.
Tables
Patient data.
| Patient | Ethnicity | Age | BMI |
|---|---|---|---|
| CONTROL | |||
| 1 | Cauc | 22 | 18.1 |
| 9 | NS | 35 | 27 |
| 10 | Cauc | 36 | 31 |
| 12 | Hisp | 38 | 30 |
| 14 | Hisp | 40 | 29.2 |
| 17 | Cauc | 23 | 32.6 |
| 21 | Hisp | 25 | 28.3 |
| 25 | Afr Am | 33 | 25 |
| 30 | Afr Am/Hisp | 37 | 43.4 |
| 33 * | Hisp | 31 | 39.6 |
| 34 | Cauc | 30 | 38.2 |
| 36 | Cauc | 36 | 33.1 |
| 37 * | Cauc | 28 | 37.3 |
| 38 * | Hisp | 25 | 27.7 |
| 45 * | Cauc | 33 | 38 |
| 47 * | Hisp | 29 | 24.5 |
| H11 | Hisp | 38 | 34 |
| H19 | Cauc | 25 | 28 |
| H20 | Cauc | 45 | 25 |
| H25 | Hisp | 26 | 29 |
| H27 | Cauc | 26 | 19 |
| H47 | Cauc | 24 | 33 |
| ENDOMETRIOSIS I-II | |||
| 4 | Hisp | 35 | 22.5 |
| 16 | Pac Isl | 30 | 28 |
| 23 | Cauc | 25 | 27.4 |
| 24 | Cauc | 35 | 27.6 |
| 26 | Cauc | 24 | 23.1 |
| 27 | Cauc | 31 | 31.3 |
| 31 | Hisp | 30 | 25.8 |
| 32 | Cauc | 39 | 29.9 |
| 35 * | Cuac | 28 | 21.7 |
| 39 * | Hisp/Pac Isl | 25 | 24.2 |
| 43 | Cauc | 25 | 40.2 |
| ENDOMETRIOSIS III-IV | |||
| 2 | Cauc | 26 | |
| 3 | Cauc | 31 | |
| 5 | Afr Am | 28 | 18.9 |
| 6 | Cauc | 41 | 45.1 |
| 7 | Hisp | 40 | 20.3 |
| 13 | Cauc | 37 | 23 |
| 15 | Cauc | 23 | 25 |
| 19 | Cauc | 30 | 22 |
| 40 * | Hisp | 34 | 18.9 |
| 41 | Cauc | 32 | 23 |
| 42 | Cauc | 34 | 21 |
-
OCP, Oral contraceptives; IUD, Intra-uterine device; ES, Early secretory; MS, Mid-secretory; LS, Late Secretory; M, Menstruation.
-
*
PMCs also obtained.