The Smc5/6 complex counteracts R-loop formation at highly transcribed genes in cooperation with RNase H2
Figures

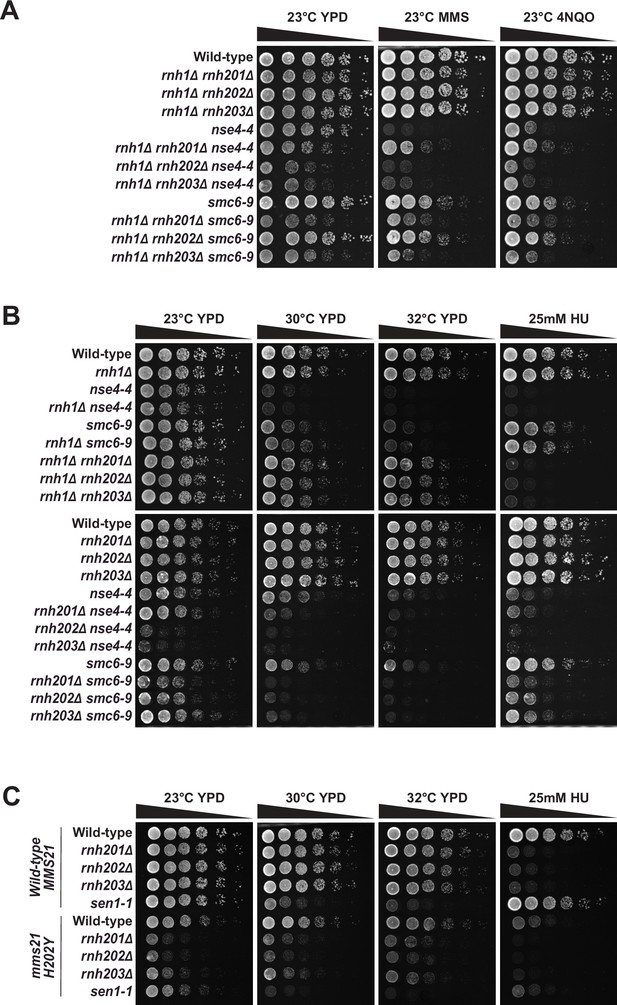
Synthetic enhancement of RNase H enzyme defects by mutations affecting Smc5/6 complex activity.
(A) Schematic model showing the mechanism of RNase H mediated RNA-DNA hybrid degradation. The crystal structures of the RNase H1 and RNase H2 complex used in this representation are PDB: 2QK9 (Nowotny et al., 2007) and PDB: 3PUF (Figiel et al., 2011), respectively. (B) Schematic representation of the Smc5/6 complex showing its subunits and the corresponding mutant alleles used in this study. The crystal structure of the Smc5/6 complex used in this representation is PDB: 7QCD (Hallett et al., 2022). (C) Growth of nse4-4 rnh1Δ rnh201Δ and smc6-9 rnh1Δ rnh201Δ haploid spores after sporulation and germination of heterozygous diploid strains at 23 °C. The viability of the haploid spores was scored after 3 days of germination. (D) The proliferation capacity of combination mutants affecting Smc5/6 complex and RNase H activity was monitored after dilution on solid medium and growth under various conditions (indicated on top of the growth medium). Concentration of HU used was 12.5 mM. YPD 23 °C, 30 °C, 32 °C, and HU plates were grown in temperature-controlled incubators for ~48 hr,~28 hr,~26 hr, and ~72 hr respectively, before scanning the plates.
Growth phenotype of yeast strains carrying mutations affecting RNA-DNA hybrid metabolism enzymes and SMC5/6 complex components.
(A) The proliferation capacity of double mutant strains defective for the Smc5/6 complex and RNase H activity was assessed in the presence of DNA-damaging agents (0.005% methyl methanesulfonate [MMS] and 0.03 μM 4NQO at 23 °C). (B) Proliferation capacity of yeast mutants defective for the Smc5/6 complex and only one subtype of RNase H enzyme at 23 °C, 30 °C, and 32 °C and in the presence of HU at 23 °C. (C) Growth phenotype of yeast strains carrying mutations that inactivate Mms21 E3-ligase activity and RNase H/Sen1 enzymes at 23 °C, 30 °C, and 32 °C and in the presence of HU at 23 °C.

RNA-DNA hybrid accumulation in cells defective for Smc5/6 complex and RNase H activity.
(A) The abundance of RNA-DNA hybrids in chromosomes was monitored using the S9.6 antibody by indirect immunofluorescence microscopy on chromosome spreads prepared from wild-type (WT), single- and double-mutant yeast strains grown at 23 °C. Representative spreads are shown, with DNA stained in blue (DAPI) and orange foci representing RNA-DNA hybrid structures detected by the S9.6 antibody. Quantification of nuclei containing S9.6 foci (>10 foci per nucleus) is shown below the images. 100-200 nuclei were visualized and manually counted for each replicate to obtain the fraction of nuclei with detectable RNA-DNA hybrids. Data represent the mean and SE of three independent experiments. *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). Scale bar, 5 μm. (B) RNA-DNA hybrid were monitored and quantified as described above in the presence and absence of ectopic overexpression of RNase H1 at 23 °C in strains carrying rnh1Δ rnh201Δ, nse4-4 rnh1Δ rnh201Δ, and nse4-4 rnh1Δ rnh203Δ mutations. Scale bar, 5 μm.
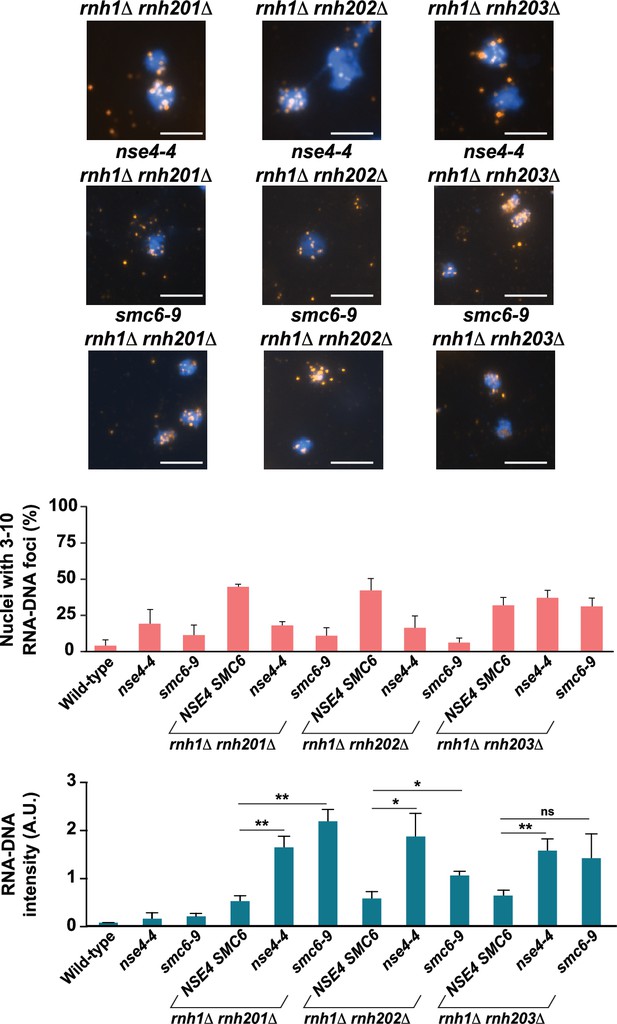
Direct and indirect quantification of R-loops in cells defective for Smc5/6 and RNase H activity.
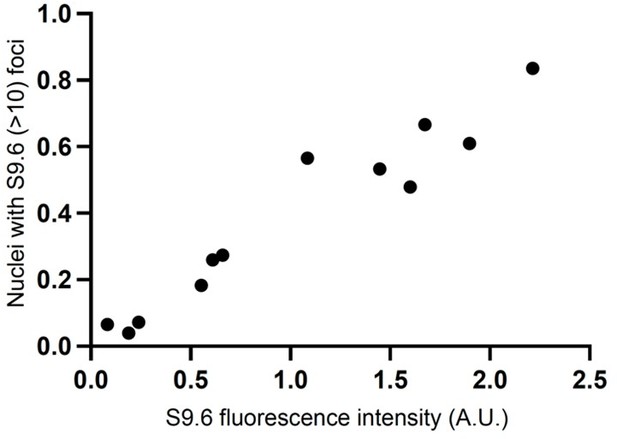
Quantification of nuclei containing 3–10 RNA-DNA/S9.6 foci in single- and double-mutant strains grown at 23 °C as shown in Figure 2 (Top). Representative images of chromosome spreads from RNase H mutant strains are replicated in Figure 2A. Quantification of fluorescence intensity of RNA-DNA hybrid structures (arbitrary units; A.U.) normalized to DAPI signal in single- and double-mutant yeast strains shown in Figure 2 (bottom).

Quantification of R-loop abundance in cells defective for Smc5/6 and RNase H activity.
(A) Mutations in the Smc5/6 complex components increase the levels of RNA-DNA hybrids at rDNA genes and telomeres in the absence RNase H activity. DRIP was performed at TEL06R, rDNA 18S, and rDNA 21S loci with the S9.6 antibody using genomic DNA prepared from asynchronous cultures of WT, single- and double-mutant yeast strains grown at 23 °C. Data represents the mean and SE of at least three independent experiments. *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). (B) Detection of R-loops using AID-induced Rad52 foci formation. (Top Left) Schematic illustration of AID-induced R-loop mutagenesis and subsequent Rad52 activation. (Top Right) Representative images of cells carrying Rad52-GFP foci in the absence (–AID) or presence of AID (+AID). (Bottom) Quantification of cells showing Rad52-GFP foci after AID overexpression at 23 °C in WT, single- and double-mutant yeast strains. About 100 cells were visualized and manually counted for each replicate to obtain the fraction of cells with detectable Rad52-GFP foci. Data represent the mean and SE of three independent experiments. *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). Scale bar, 10 μm.
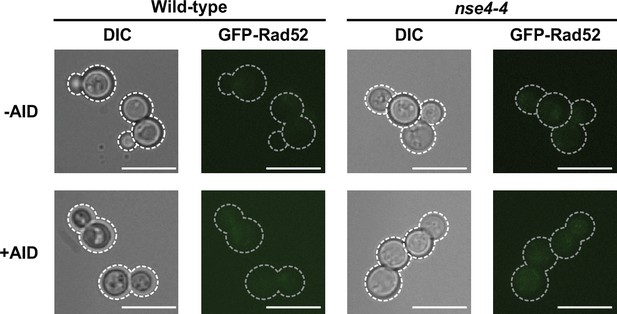
Detection of R-loops using activation-induced cytosine deaminase (AID)-induced Rad52 foci formation (extended data from Figure 3).
Representative images of cells carrying Rad52-GFP foci in the absence (–AID) or presence of AID (+AID) in wild-type and nse4-4 mutant yeast cells grown at 23 °C. Scale bar, 10 μm.

R-loops formed at highly transcribed genes and telomeres are endogenous targets for the Smc5/6 complex.
(A) Schematic representation of various cellular mechanisms responsible for RNA-DNA hybrid formation in chromosomes and relevant proteins/mutants implicated in each process. (B-C) Proliferation capacity of yeast strains carrying the specified mutations was monitored by dilution assay as described in Figure 1. The growth temperatures and the presence of specific DNA-damaging agents (MMS concentration was 0.005%; HU concentration was 25 mM in panel (B) and 100 mM in panel (C)) in the growth medium are indicated on top of the images. YPD 23 °C, 30 °C, 32 °C, MMS, and HU plates were grown in temperature-controlled incubators for ~48 hr,~28 hr,~26 hr,~48 hr, and ~72 hr, respectively, before scanning the plates.
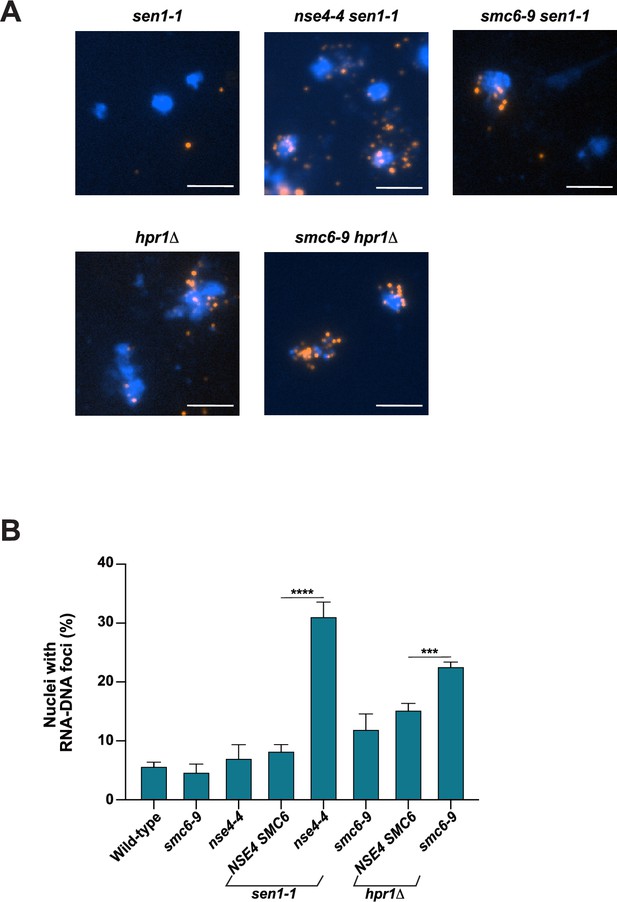
RNA-DNA hybrid accumulation in cells defective for Smc5/6 complex and Sen1 helicase or THO-complex activity.
(A–B) The abundance of RNA-DNA hybrids in nuclei was monitored using the S9.6 antibody by indirect immunofluorescence microscopy on chromosome spreads prepared from wild-type (WT), single- and double-mutant yeast strains grown at 23 °C. Representative chromosome spread images (as in Figure 2A) and quantification of nuclei containing S9.6 foci are shown in panels A and B, respectively. At least 100 nuclei were visualized and manually counted for each replicate to obtain the fraction of nuclei with detectable RNA-DNA hybrids. Data represent the mean and SE of three independent experiments. *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). Scale bar, 5 μm.

R-loops are high-affinity substrates for the Smc5/6 complex.
(A) Coomassie blue stained gel showing the purified human SMC5/6 complex used in R/D-loop binding experiments. All the subunits of the SMC5/6 complex migrate in SDS-PAGE at the positions of the native full-length proteins. (B) Schematic representation of the reaction steps for the production of [32P]-labeled R/D-loop substrates and their expected behavior in electromobility shift assays (EMSAs) on a 6% native polyacrylamide gel. Blue and pink strands represent DNA and RNA, respectively, while the asterisk indicates the 32P label introduced at the end of the RNA/DNA strand. (C–D) Competitive EMSA assay to evaluate the R-loop and D-loop binding specificity of human SMC5/6 complex. [32P]-radiolabeled probe (40 nM) and 100 x molar excess Poly [d(I-C)] were confined together with gradually increasing concentrations of SMC5/6 complex. The concentrations of SMC5/6 complex used in the assays are represented by the triangles on top of the gels and correspond to the following values (in nM): 0, 6.25, 12.5, 25, 50, and 100. Positions of unbound substrates and SMC5/6-bound R/D-loop substrates are marked by cartoon illustrations on the side of the gels.
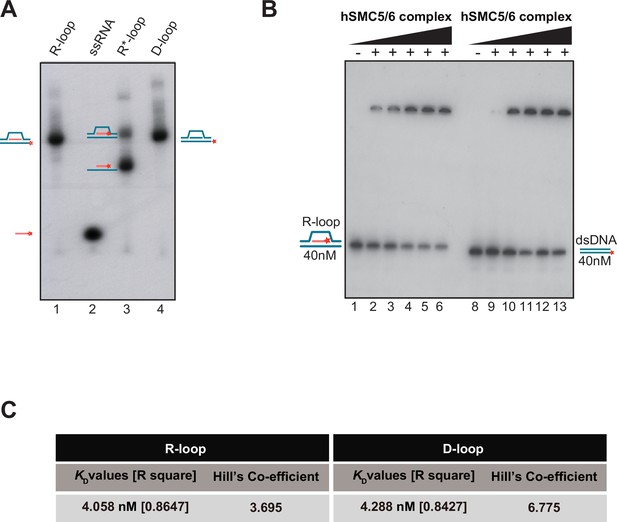
R-loop binding affinity for the SMC5/6 complex.
(A) Purification and electrophoretic behavior of γ-32P-labeled RNA-DNA substrates. Lane 1: R-loop substrate where DNA strand 2 (DD 4264) is radiolabeled at its 5’-end with [γ-32P] ATP; Lane 2: ssRNA oligo (DD 4265) radiolabeled with [γ-32P] ATP; Lane 3: R*-loop substrate (top band) containing a 32P-radiolabeled RNA oligo (DD 4265). Note that a linear DNA-RNA hybrid can be produced as a byproduct of the reannealing reaction (bottom band); Lane 4: D-loop where DNA strand 2 (DD 4264) is radiolabeled at its 5’-end with 32P. All the probes were quantified (nM) using a scintillation counter. The positions of individual probes after migration are marked on both sides of the gel. (B) Electrophoretic mobility shift assay (EMSA) were performed with purified human SMC5/6 complex (6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM) using R-loop (lanes 1–6) and linear double-strand (dsDNA) (lanes 8–13). (C) SMC5/6 complex affinity constants observed for R-loop and D-loop binding reactions are shown in Figure 5.
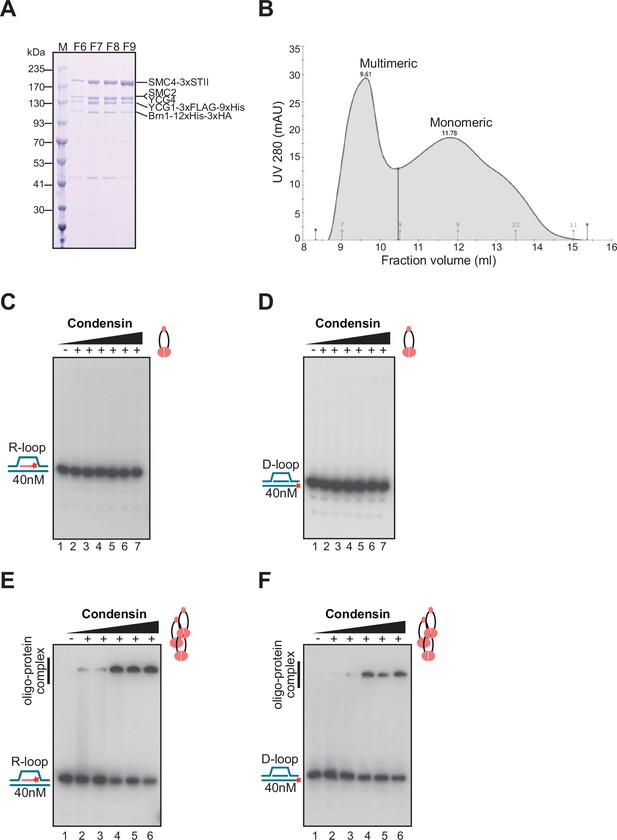
R-loop binding properties of the condensin holoenzyme.
(A) Coomassie blue stained gel showing purified yeast condensin fractions used in R/D-loop binding assays. All the subunits of condensin migrated in SDS-PAGE at the positions of the native full-length proteins. (B) The elution profile shows two major peaks of condensin holoenzyme corresponding to multimeric protein fractions (F6–F7) and monomeric fractions (F9–F11). Electrophoretic mobility shift assay (EMSA) were performed with monomeric yeast condensin holoenzyme using (C) R-loop (D) D-loop. (E–F) EMSA were performed with multimeric yeast condensin holoenzyme using R-loop and D-loop substrates. The concentrations of protein used in EMSA are: 6.25 nM, 12.5 nM, 25 nM, 50 nM, and 100 nM, respectively. Cartoon illustrations on the side of the gels mark the positions of unbound substrates and condensin bound to R/D-loop substrates.

The SMC5/6 complex stimulates the degradation of R-loops by RNase H2.
(A) Coomassie blue-stained gel showing the purity of recombinant RNase H2 (Chon et al., 2009) and SMC5/6 complex Serrano et al., 2020 used in R-loop degradation assays. All the components of the SMC5/6 and RNase H2 holoenzymes migrate in SDS-PAGE at the positions expected for the native/full-length subunits of their respective complexes. (B) Schematic representation of the steps involved in the production of a radiolabeled R-loop probe and in the RNase H2 degradation assay. Blue and pink strands represent DNA and RNA, respectively, while the asterisk marks the 32P label introduced in the RNA strand of the R-loop structure. (C) R-loop degradation assay conducted in the presence of human RNase H2 (0.15 nM) and increasing concentration of human SMC5/6 complex (1.25 nM, 2.5 nM, 5 nM, 10 nM, 20 nM). The bar graph (next to the gel) shows the quantification of the degradation assay. Individual bars report the mean and SE of four independent experiments. *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test).
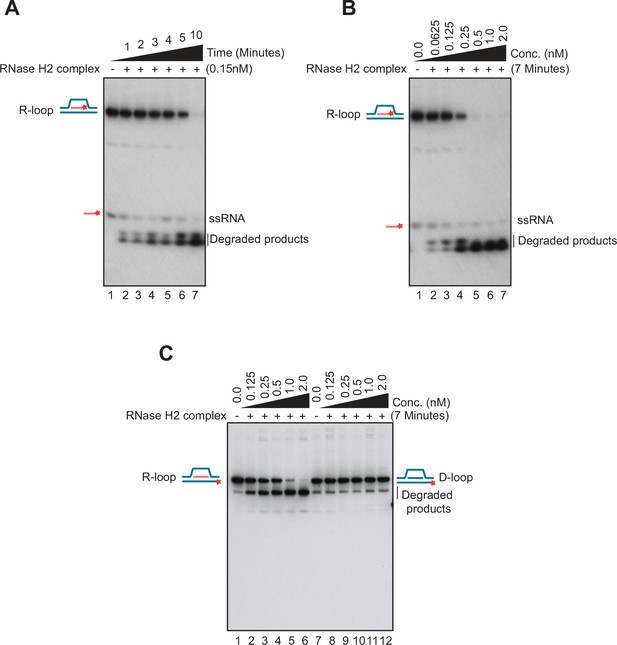
Biochemical properties of nucleic acid substrates and RNase H2 enzyme used in this study.
(A) An R-loop substrate containing a 32P-labeled RNA moiety (40 nM) was incubated with 0.15 nM of RNase H2 complex in a time course experiment (t=1, 2, 3, 4, 5, and 10 min). The positions of the substrate and reaction products are marked as described above. Data are representative of three independent experiments. (B) The R-loop substrate described above (40 nM) was incubated with increasing concentrations of RNase H2 complex (0, 0.0625, 0.125, 0.25, 0.5, 1, and 2 nM) for 7 min. Data are representative of three independent experiments. (C) R-loop degradation assay with R-loop substrate containing a 32P-labeled in the DNA strand was performed in the presence of an increasing concentration of RNase H2 complex (as indicated on top of lanes 1–6). Similar reactions were carried out with a D-loop substrate (lanes 7–12) as a negative control for RNase H2 activity. The positions reached by the substrate and reaction products after electrophoresis are marked on both sides of the gels. Data are representative of three independent experiments.
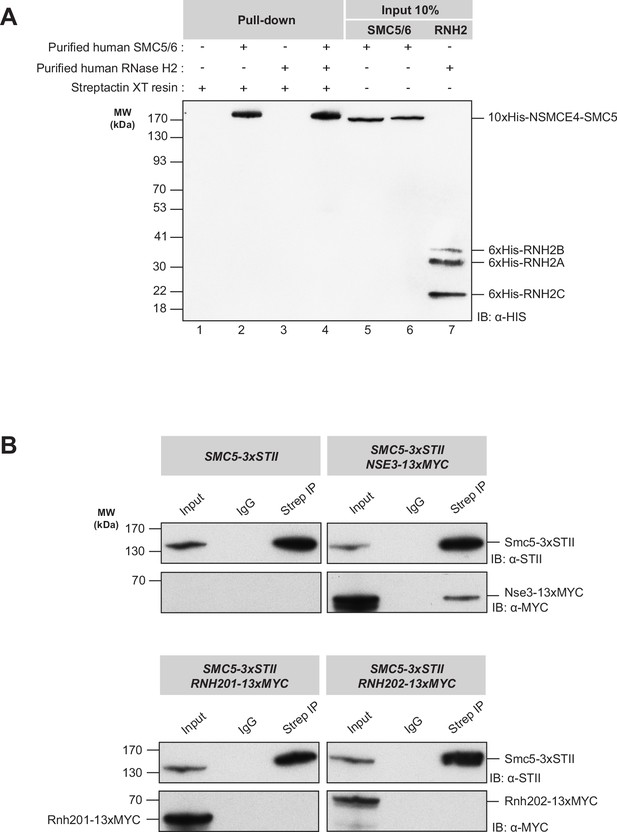
Protein-protein binding experiments with human SMC5/6 complex and RNase H2 enzyme.
(A) Pull-down experiment to assess the possible binding of the SMC5/6 complex to RNase H2. Lane 1: negative control with Strep-Tactin XT sepharose beads incubated in binding buffer alone. Lanes 2 and 3: positive controls with individual protein complexes plus beads incubated in the binding buffer. Lane 4: Pull-down experiment showing only the SMC5/6 complex and no detectable interaction with RNase H2. Lanes 5–7: 10% input for the Smc5/6 complex (lanes 5 and 6) and the RNase H2 (lane 7), respectively. (B) Co-immunoprecipitation assay to test the association of yeast Smc5 with RNase H2 in cell extracts prepared at 23 °C. For each panel, the first lane shows the whole cell extract loaded as an input (~5%). The second lane is a negative control (pull-down with IgG antibody). The third lane shows the immunoprecipitation with the anti-Strep antibody. For negative control, we used a strain where only the Smc5 subunit was tagged (top left). The positive control experiment shows that the Nse3 subunit (tagged with 13xMyc) of the Smc5/6 complex is associated with Smc5 under the co-immunoprecipitation conditions (top left). Both co-IPs with extracts containing tagged Rnh201 and Rnh202 show no detectable association connecting Smc5 to the RNase H2 enzyme (bottom panels).

Proposed mode of action for the Smc5/6 complex during R-loop removal from chromosomes.
A nascent RNA transcript synthesized during gene transcription invades separated DNA strands and forms a stable interaction with its complementary DNA stand. The Smc5/6 complex then recognizes the R-loop and associates stably with the RNA-DNA hybrid structure. RNase H2 catalytic activity is stimulated in presence of the Smc5/6 complex. Effective removal of the RNA moiety from the R-loop allows reannealing of complementary ssDNA (left panel). Based on the known DNA compaction activity of the Smc5/6 complex (Serrano et al., 2020), we hypothesize that this enzyme will also contribute to R-loop prevention and/or repair by facilitating reannealing of separated ssDNA formed during gene transcription and/or after removal of RNA from R-loops. Timely reannealing of complementary ssDNA is expected to prevent re-invasion of separated DNA strands by a new RNA transcript. In the absence of RNase H and Smc5/6 complex, the stabilized R-loop will often cause replication stress and DNA double-strand breaks (right panel). Figure prepared using Adobe Illustrator.
Tables
Yeast strains used in this study.
| Figure | Strain name | Relevant genotype details |
|---|---|---|
| Figure 1 | D7528 | MATa |
| D6531 | MATa rnh1::HIS3MX6 rnh201::kanMX6 | |
| D6533 | MATa rnh1::HIS3MX6 rnh202::kanMX6 | |
| D6535 | MATa rnh1::HIS3MX6 rnh203::kanMX6 | |
| D7799 | MATa nse4-4::URA3 | |
| D7055 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh201::kanMX6 | |
| D7057 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh202::kanMX6 | |
| D7084 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh203::kanMX6 | |
| D7795 | MATa smc6-9::NAT | |
| D7159 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh201::kanMX6 | |
| D7357 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh202::kanMX6 | |
| D7157 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh203::kanMX6 | |
| Figure 2A | D7528 | MATa |
| D6531 | MATa rnh1::HIS3MX6 rnh201::kanMX6 | |
| D6533 | MATa rnh1::HIS3MX6 rnh202::kanMX6 | |
| D6535 | MATa rnh1::HIS3MX6 rnh203::kanMX6 | |
| D7799 | MATa nse4-4::URA3 | |
| D7055 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh201::kanMX6 | |
| D7057 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh202::kanMX6 | |
| D7084 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh203::kanMX6 | |
| D7795 | MATa smc6-9::NAT | |
| D7159 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh201::kanMX6 | |
| D7357 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh202::kanMX6 | |
| D7157 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh203::kanMX6 | |
| Figure 2B | D8122 | MATa trp1-1::Pgal1::TRP1 |
| D8120 | MATa trp1-1::Pgal1::RNH1::TRP1 | |
| D8177 | MATa rnh1::HIS3MX6 rnh201::kanMX6 trp1-1::Pgal1::TRP1 | |
| D8407 | MATa rnh1::HIS3MX6 rnh201::kanMX6 trp1-1::Pgal1::RNH1::TRP1 | |
| D8128 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh201::kanMX6 trp1-1::Pgal1::TRP1 | |
| D8119 | MATa smc6-9::NAT rnh1::HIS3MX6 rnh201::kanMX6 trp1-1::Pgal1:: RNH1::TRP1 | |
| D8130 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh203::kanMX6 trp1-1::Pgal1::TRP1 | |
| D8221 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh203::kanMX6 trp1-1::Pgal1:: RNH1::TRP1 | |
| Figure 3A | D7528 | MATa |
| D6535 | MATa rnh1::HIS3MX6 rnh203::kanMX6 | |
| D7084 | MATa nse4-4::URA3 rnh1::HIS3MX6 rnh203::kanMX6 | |
| Figure 3B | D8852 | MATa rnh1::HIS3MX6 rnh201::kanMX6 RAD52=EGFP::KanMX6 [p6-YCplac111] |
| D8849 | MATa rnh1::HIS3MX6 rnh201::kanMX6 nse4-4::URA3 RAD52=EGFP::KanMX6 [p6-YCplac111] | |
| D8611 | MATa rnh1::HIS3MX6 rnh201::kanMX6 RAD52=EGFP::KanMX6 [p1895-pESC-LEU-HsAIDSc] | |
| D8613 | MATa rnh1::HIS3MX6 rnh201::kanMX6 nse4-4::URA3 RAD52=EGFP::KanMX6 [p1895-pESC-LEU-HsAIDSc] | |
| Figure 4, Figure 4—figure supplement 1 | D8457 | MATa hpr1::HIS3MX6 |
| D8534 | MATa smc6-9::NAT hpr1::HIS3MX6 | |
| D8368 | MATa sen1-1::Tadh1::HIS3MX6 | |
| D8376 | MATa sen1-1::Tadh1::HIS3MX6 nse4-4::URA3 | |
| D8438 | MATa sen1-1::Tadh1::HIS3MX6 smc6-9::NAT | |
| D8573 | MATa sen1-3[R1605K]::Tadh1::HIS3MX6 | |
| D8603 | MATa sen1-3[R1605K]::Tadh1::HIS3MX6 nse4-4::URA3 | |
| D8601 | MATa sen1-3[R1605K]::Tadh1::HIS3MX6 smc6-9::NAT | |
| D6537 | MATa pol2[M644G] | |
| D8645 | MATa pol2[M644G] nse4-4::URA3 | |
| D8643 | MATa pol2[M644G] smc6-9::NAT | |
| D8691 | MATa rat1-1 | |
| D8789 | MATa rat1-1 nse4-4::URA3 | |
| D8783 | MATa rat1-1 smc6-9::NAT | |
| D8844 | MATa rat1-1 sen1-1::Tadh1::HIS3MX6 smc6-9::NAT | |
| Figure 1—figure supplement 1B | D7528 | MATa |
| D9636 | MATa rnh1::HIS3MX6 | |
| D9613 | MATa nse4-4::URA3 rnh1::HIS3MX6 | |
| D9607 | MATa smc6-9::NAT rnh1::HIS3MX6 | |
| D9637 | MATa rnh201::kanMX6 | |
| D9638 | MATa rnh202::kanMX6 | |
| D9639 | MATa rnh203::kanMX6 | |
| D9615 | MATa nse4-4::URA3 rnh201::kanMX6 | |
| D9617 | MATa nse4-4::URA3 rnh202::kanMX6 | |
| D9620 | MATa nse4-4::URA3 rnh203::kanMX6 | |
| D9605 | MATa smc6-9::NAT rnh201::kanMX6 | |
| D9609 | MATa smc6-9::NAT rnh202::kanMX6 | |
| D9612 | MATa smc6-9::NAT rnh203::kanMX6 | |
| Figure 1—figure supplement 1C | D7528 | MATa |
| D6531 | MATa rnh1::HIS3MX6 rnh201::kanMX6 | |
| D6533 | MATa rnh1::HIS3MX6 rnh202::kanMX6 | |
| D6535 | MATa rnh1::HIS3MX6 rnh203::kanMX6 | |
| D8368 | MATa sen1-1::Tadh1::HIS3MX6 | |
| D7671 | MATa rad5-535 mms21-H202Y::URAMX6 | |
| D9633 | MATa rnh1::HIS3 rnh201::kanMX6 mms21-H202Y::URAMX6 | |
| D9632 | MATa rnh1::HIS3 rnh202::kanMX6 mms21-H202Y::URAMX6 | |
| D9650 | MATa rnh1::HIS3 rnh203::kanMX6 mms21-H202Y::URAMX6 | |
| D9634 | MATa sen1-1::Tadh1::HIS3MX6 mms21-H202Y::URAMX6 | |
| Figure 3—figure supplement 1 | D9105 | MATa RAD52=EGFP::KanMX6 [p6-YCplac111] |
| D9101 | MATa nse4-4::URA3 RAD52=EGFP::KanMX6 [p6-YCplac111] | |
| D9107 | MATa RAD52=EGFP::KanMX6 [p1895-pESC-LEU-HsAIDSc] | |
| D9105 | MATa nse4-4::URA3 RAD52=EGFP::KanMX6 [p1895-pESC-LEU-HsAIDSc] | |
| Figure 6—figure supplement 2 | D9272 | MATa rad5-535 SMC5=3xSTII::TRP1 |
| D9274 | MATa rad5-535 SMC5=3xSTII::TRP1 RNH201=13xMYC::kanMx6 | |
| D9585 | MATa rad5-535 SMC5=3xSTI::TRP1 RNH202=13xMYC::kanMX6 | |
| D9264 | MATa rad5-535 SMC5=3xSTII::TRP1 NSE3=13MYC::HIS3MX6 |