Cytosolic S100A8/A9 promotes Ca2+ supply at LFA-1 adhesion clusters during neutrophil recruitment
Figures
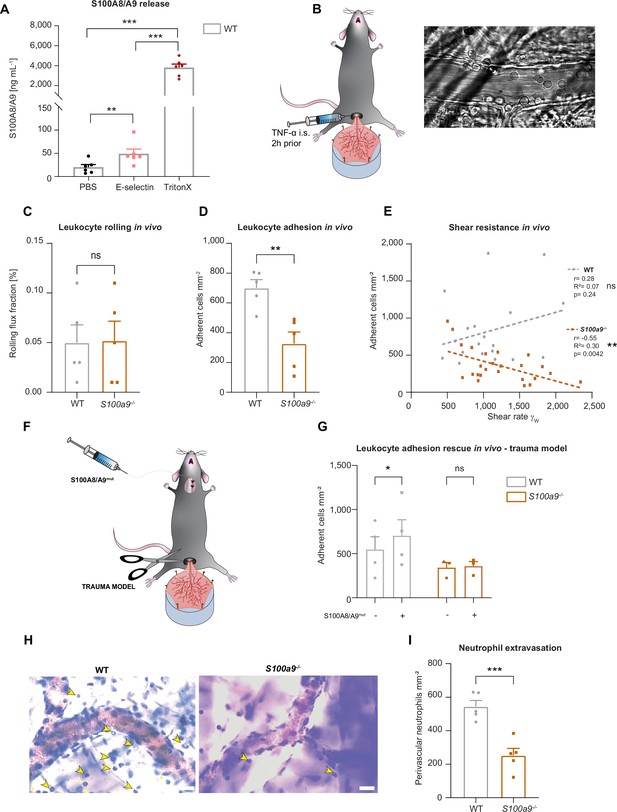
Cytosolic S100A8/A9 regulates leukocyte recruitment in vivo regardless of extracellular S100A8/A9.
(A) Enzyme-linked immunosorbent assay (ELISA) measurements of S100A8/A9 levels in supernatants of wildtype (WT) bone marrow neutrophils stimulated for 10 min with PBS, E-selectin, or lysed with Triton X-100 (mean + SEM, n=6 mice per group, RM one-way analysis of variance [ANOVA], Holm-Sidak’s multiple comparison). (B) Schematic model of the mouse cremaster muscle preparation for intravital microscopy and representative picture of a vessel showing rolling and adherent cells. WT and S100a9-/- mice were stimulated intrascrotally (i.s.) with TNF-α 2 hr prior to cremaster muscle post-capillary venules imaging by intravital microscopy. Quantification of (C) number or rolling (rolling flux fraction) and (D) number of adherent neutrophils per vessel surface of WT and S100a9-/- mice (mean + SEM, n=5 mice per group, 25 [WT] and 30 [S100a9-/-] vessels, unpaired Student’s t-test). (E) Correlation between physiological vessel shear rates and number of adherent neutrophils in WT and S100a9-/- mice (n=25 [WT] and 30 [S100a9-/-] vessels of 5 mice per group, Pearson correlation). (F) Schematic model of sterile inflammation induced by exteriorizing WT and S100a9-/- cremaster muscles. (G) Analysis of number of adherent leukocytes by intravital microscopy before and after S100A8/A9mut intra-arterial injection (mean + SEM, n=3 mice per group, 3 [WT] and 3 [S100a9-/-] vessels, two-way ANOVA, Sidak’s multiple comparison). (H) Representative Giemsa staining micrographs of TNF-α stimulated WT and S100a9-/- cremaster muscles (representative micrographs, scale bar = 30 µm, arrows: transmigrated neutrophils) and (I) quantification of number of perivascular neutrophils (mean + SEM, n=5 mice per group, 56 [WT] and 55 [S100a9-/-] vessels, unpaired Student’s t-test). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
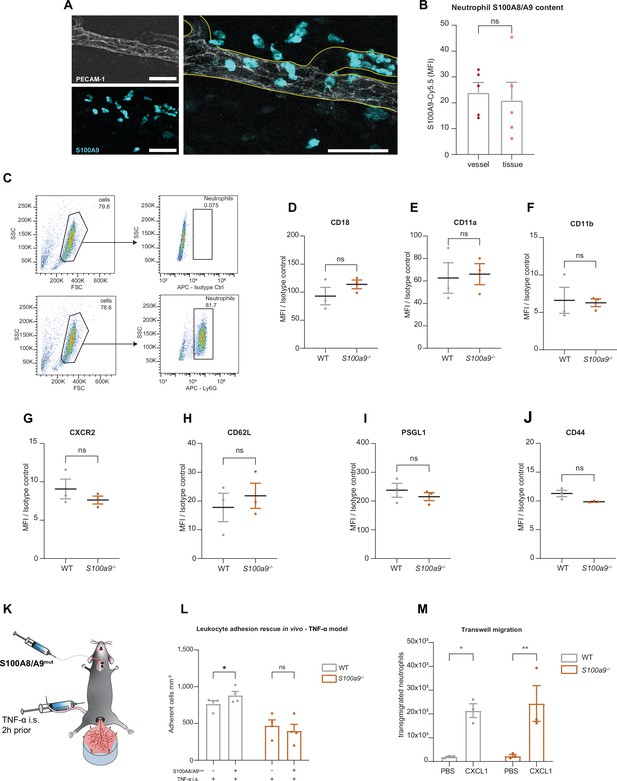
Cytosolic S100A8/A9 is indispensable for neutrophil recruitment in vivo.
(A) Representative confocal images of S100A9 intensity in intravascular and extravascular neutrophils after TNF-α stimulation of wildtype (WT) cremaster muscle tissues (scale bars = 50 μm) and (B) quantification (mean + SEM, n=5 mice per group, 602 [intravascular] and 326 [extravasated] neutrophils, unpaired Student’s t-test). (C) WT neutrophils’ purity assessment and gating strategy (equivalent for S100a9-/- neutrophils) and flow cytometry analysis of (D) CD18, (E) CD11a, (F) CD11b (G) CXCR2, (H) CD62L, (I) PSGL1, and (J) CD44 surface levels of WT and S100a9-/- neutrophils (mean + SEM, n=3 mice per group, unpaired Student’s t-test). (K) Schematic model of the adhesion rescue experiments in TNF-α stimulated WT and S100a9-/- cremaster muscles by intra-arterial application of mutS100A8/A9 (aa exchange N70A+E79A). (L) Quantification of number of adherent WT and S100a9-/- leukocytes mm–2 in the same vessel before and after mutS100A8/A9 i.v. injection (mean + SEM, n=4 mice per group, 4 [WT] and 4 [S100a9-/-] vessels, two-way analysis of variance (ANOVA), Sidak’s multiple comparison). (M) Number of transmigrated WT and S100a9-/- neutrophils in a classical transwell assay upon stimulation with PBS or CXCL1 (mean + SEM, n=3 mice per group, two-way ANOVA, Sidak’s multiple comparison). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
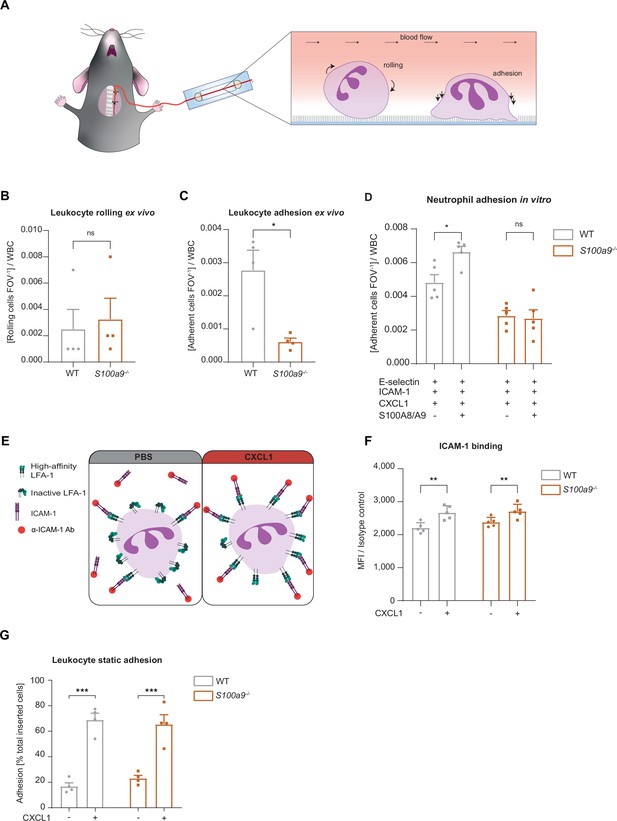
Loss of cytosolic S100A8/A9 impairs neutrophil adhesion under flow conditions without affecting β2 integrin activation.
(A) Schematic representation of blood harvesting from wildtype (WT) and S100a9-/- mice via a carotid artery catheter and perfusion into self-made flow cambers coated with E-selectin, ICAM-1, and CXCL1. Analysis of (B) number of rolling and (C) number of adherent leukocytes FOV–1 (mean + SEM, n=4 mice per group, 10 [WT] and 12 [S100a9-/-] flow chambers, paired Student’s t-test). (D) Number of adherent leukocytes FOV–1 in self-made flow chambers coated with E-selectin, ICAM-1, CXCL1, and additionally with extracellular S100A8/A9 (mean + SEM, n=5 mice per group, ≥12 [WT] and 14 [S100a9-/-] flow chambers, two-way analysis of variance (ANOVA), Sidak’s multiple comparison). (E) Schematic representation of the soluble ICAM-1 binding assay using bone marrow neutrophils stimulated with PBS control or CXCL1 (10 nM) assessed by (F) flow cytometry (MFI = median fluorescence intensity, mean + SEM, n=5 mice per group, two-way ANOVA, Sidak’s multiple comparison). (G) Spectroscopy fluorescence intensity analysis of percentage of adherent WT and S100a9-/- neutrophils, seeded for 5 min on ICAM-1 coated plates and stimulated with PBS or CXCL1 (10 nM) for 10 min (mean + SEM, n=4 mice per group, two-way ANOVA, Sidak’s multiple comparison). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
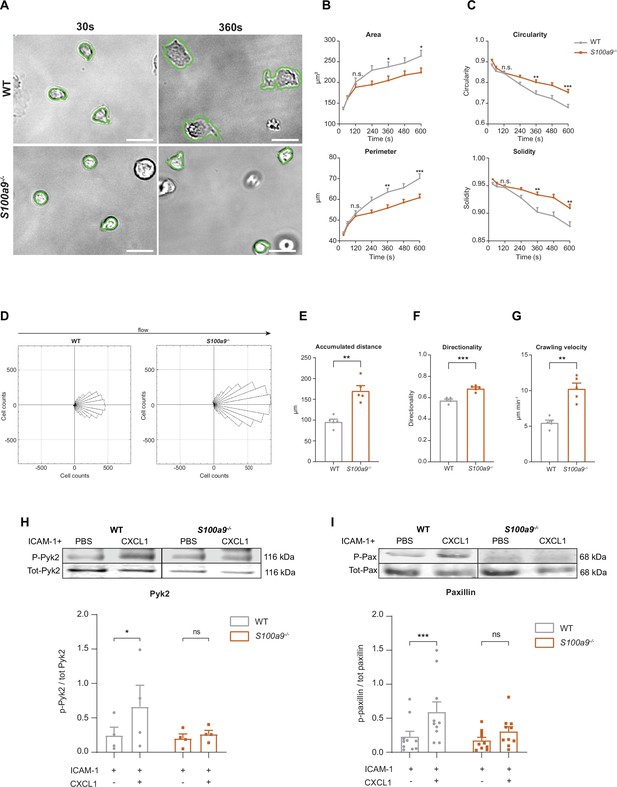
Cytosolic S100A8/A9 is crucial for neutrophil spreading, crawling, and post-arrest modifications under flow.
(A) Representative bright-field pictures of wildtype (WT) and S100a9-/- neutrophils spreading over E-selectin, ICAM-1, and CXCL1 coated glass capillaries (scale bars = 10 μm). Analysis of cell shape parameters (B) area, perimeter, (C) circularity (4π * [area/perimeter]) and solidity (area/convex area) over time (mean + SEM, n=103 [WT] and 96 [S100a9-/-] neutrophils of 4 mice per group, unpaired Student’s t-test). (D) Rose plot diagrams representative of migratory crawling trajectories of WT and S100a9-/- neutrophils in flow chambers coated with E-selectin, ICAM-1, and CXCL1 under flow (2 dyne cm–2). Analysis of (E) crawling distance, (F) directionality of migration, and (G) crawling velocity of WT and S100a9-/- neutrophils (mean + SEM, n=5 mice per group, 113 [WT] and 109 [S100a9-/-] cells, paired Student’s t-test). Western blot analysis of ICAM-1-induced (H) Pyk2 and (I) paxillin phosphorylation of WT and S100a9-/- neutrophils upon CXCL1 stimulation (10 nM) (mean + SEM, representative western blot of n≥4 mice per group, two-way analysis of variance (ANOVA), Sidak’s multiple comparison). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
-
Figure 3—source data 1
Pyk2 and paxillin western blot data for Figure 3H and I.
Original membranes corresponding to Figure 3H and I. paxillin, p-paxillin, Pyk2, and p-Pyk2 membranes are depicted and representative blots were then cropped and edited. Rectangle boxes indicate the representative bands used in the figure. Lowest membranes were cut before the staining to save staining solution and fit more membranes at the same time. GAPDH was always employed as an internal control. Chamaleon Duo Pre-stained Protein Ladder was used as molecular weight marker.
- https://cdn.elifesciences.org/articles/96810/elife-96810-fig3-data1-v1.zip
-
Figure 3—source data 2
Pyk2 and paxillin western blot data for Figure 3H and I.
Original membranes corresponding to Figure 3H and I. Paxillin, p-paxillin, Pyk2, and p-Pyk2 original membranes.
- https://cdn.elifesciences.org/articles/96810/elife-96810-fig3-data2-v1.zip
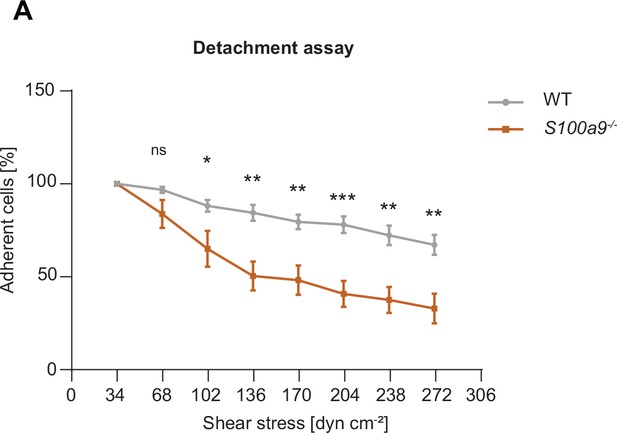
S100A8/A9 deficient cells are more susceptible to increasing shear stress compared to WT cells.
(A) Analysis of number of adherent WT and S100a9-/- neutrophils under flow as percentage related to the initial number of adherent neutrophils in E-selectin, ICAM-1, and CXCL1 coated flow chambers at indicated shear stress levels. Shear stress was increased every 30sec. [mean + SEM, n=3 mice per group, 3 (WT) and 3 (S100a9-/-) flow chambers, unpaired Student’s t-test]. ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
Neutrophil functional crawling depends on S100A8/A9.
WT and S100a9-/- neutrophils were seeded into E-selectin, ICAM-1 and CXCL1 coated flow chambers, allowed to settle down for 3min and then physiological shear stress (2dyne cm−2) was applied and crawling parameters were analyzed (representative time-lapse movies, frame interval 5sec, scale bar=10μm, time=min).
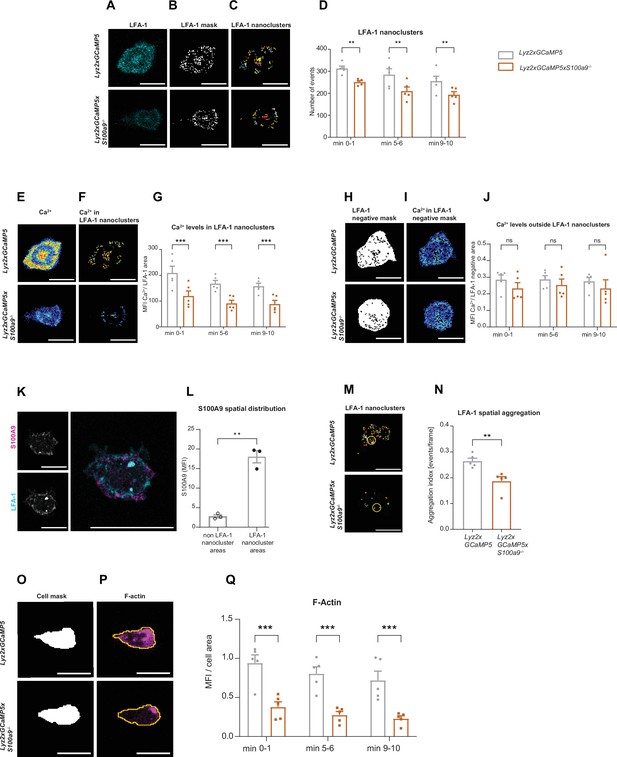
Cytosolic S100A8/A9 drives neutrophil cytoskeletal rearrangement by regulating LFA-1 nanocluster formation and Ca2+ availability within the clusters.
(A) Representative confocal images of LFA-1 staining in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- crawling neutrophils on E-selectin, ICAM-1, and CXCL1 coated flow chambers (scale bar = 10 μm). (B) Segmentation of LFA-1 signals through automatic thresholding (scale bars = 10 μm). (C) Size-excluded LFA-1 nanoclusters of 0.15 μm2 minimum size from previously thresholded images (scale bars = 10 μm). (D) Single-cell analysis of average number of LFA-1 nanoclusters in min 0–1, 5–6, and 9–10 of analysis of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (mean + SEM, n=5 mice per group, 56 [WT] and 54 [S100a9-/-] neutrophils, two-way analysis of variance (ANOVA), Sidak’s multiple comparison). (E) Representative confocal images of Ca2+ signals in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (scale bars = 10 μm) and (F) Ca2+ signals in the previously segmented LFA-1 nanoclusters (scale bars = 10 μm). (G) Quantification of subcellular Ca2+ levels in the LFA-1 nanocluster area in min 0–1, 5–6, and 9–10 in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (mean + SEM, n=5 mice per group, 56 [WT] and 54 [S100a9-/-] cells, two-way ANOVA, Sidak’s multiple comparison). (H) Segmented LFA-1 cluster negative areas (scale bars = 10 μm) and (I) representative confocal images of Ca2+ signals in the LFA-1 cluster negative areas (scale bars = 10 μm) (E–I, scale bar color code: 0=black, 255=white). (J) Analysis of cytosolic Ca2+ levels in the LFA-1 cluster negative areas in min 0–1, 5–6, and 9–10 of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (mean + SEM, n=5 mice per group, 56 [WT] and 54 [S100a9-/-] neutrophils, two-way ANOVA, Sidak’s multiple comparison). (K) Representative confocal images showing S100A9 localization at LFA-1 nanocluster areas in stimulated WT neutrophils (scale bars = 10 μm). (L) Quantitative analysis of S100A9 levels in positive LFA-1 nanocluster areas compared to non-LFA-1 nanocluster areas in stimulated WT neutrophils. (mean + SEM, n=3 mice, 26 [WT] neutrophils, paired Student’s t-test). (M) Representative confocal micrographs of LFA-1 nanocluster spatial aggregation in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils, within 10 μm2 area and minimum 10 LFA-1 nanoclusters considered (≥10 LFA-1 nanoclusters within 10 µm2, yellow circles = spatial aggregation area, scale bars = 10 μm). (N) Analysis of spatially aggregated LFA-1 nanoclusters of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (mean + SEM, n=5 mice per group, 56 [WT] and 54 [S100a9-/-] cells, unpaired Student’s t-test). (O) Segmentation of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophil area through Lyz2 channel automatic thresholding (scale bars = 10 μm) and (P) representative confocal images of respective F-actin signals (scale bars = 10 μm). (Q) Analysis of F-actin intensity normalized to the cell area in min 0–1, 5–6, and 9–10 of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (mean + SEM, n=5 mice per group, 74 [WT] and 66 [S100a9-/-] cells, two-way ANOVA, Sidak’s multiple comparison). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
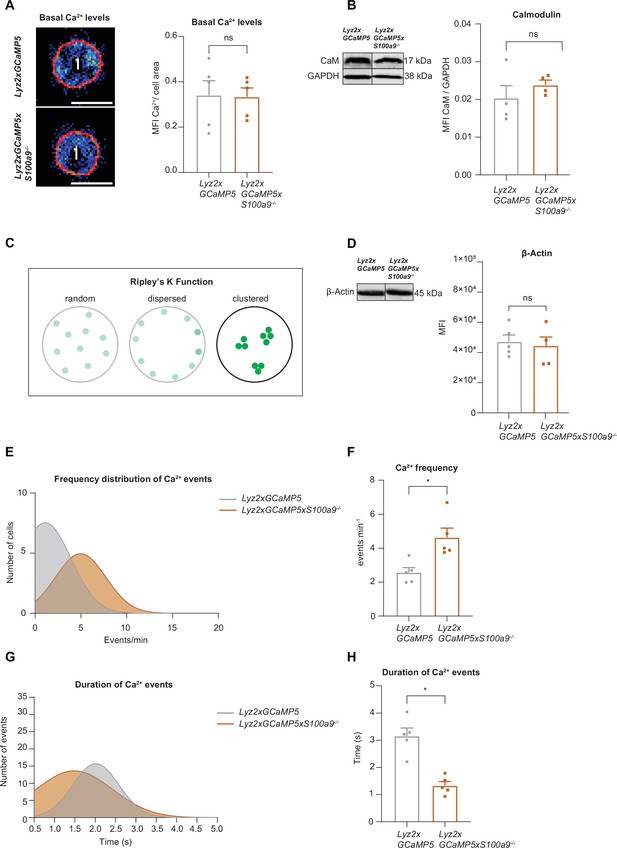
S100A8/A9 deficient cells display higher frequencies but shorter duration of Ca2+ waves compared to WT cells.
(A) Analysis of basal Ca2+ levels normalized to the cell area of bone marrow derived Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils (scale bars = 10 μm) seeded on poly-L-lysine coated slides [mean + SEM, n=234 (WT) and 192 (S100a9-/-) neutrophils of 5 mice per group, paired Student’s t-test]. (B) Representative western blot images and quantification of total calmodulin levels normalized to GAPDH signal of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils [mean + SEM, representative western blot of n=4 mice per group, unpaired Student’s t-test]. (C) Schematic representation of the Ripley’s K used to determine spatial LFA-1 nanocluster correlation. (D) Representative western blot images and quantification of total actin levels (β-Actin) of Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils [mean + SEM, representative western blot of n≥4 mice per group, unpaired Student’s t-test]. (E) Histogram of Ca2+ event frequency distribution and (F) quantification of Ca2+ event mean frequency in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils [mean + SEM, n=5 mice per group, paired Student’s t-test]. (G) Histogram of Ca2+ event duration and (H) quantification of average Ca2+ event duration in Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils [mean + SEM, n=5 mice per group, paired Student’s t-test]. ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
-
Figure 4—figure supplement 1—source data 1
Calmodulin and β-actin western blot data for Figure 4—figure supplement 1.
Original membranes corresponding to Figure 4—figure supplement 1B and D. Calmodulin (CaM), GAPDH, and β-actin membranes are depicted and representative blots were then cropped and edited. Rectangle boxes indicate the representative bands used in the figure. Chamaleon Duo Pre-stained Protein Ladder was used as molecular weight marker.
- https://cdn.elifesciences.org/articles/96810/elife-96810-fig4-figsupp1-data1-v1.zip
-
Figure 4—figure supplement 1—source data 2
Calmodulin and β-actin western blot data for Figure 4—figure supplement 1.
Original membranes corresponding to Figure 4—figure supplement 1B and D. Calmodulin (CaM) and β-actin original membranes.
- https://cdn.elifesciences.org/articles/96810/elife-96810-fig4-figsupp1-data2-v1.zip
S100A8/A9 is essential for LFA-1 nanocluster formation and turnover.
Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils were seeded into E-selectin, ICAM-1 and CXCL1 coated flow chambers, allowed to settle down for 3min and then physiological shear stress (2dyne cm−2) was applied and number of LFA-1 nanoclusters was quantified at min 0-1, min 5-6 and min 9-10 of analysis (representative segmented and thresholded confocal microscopy movies of LFA-1 signals, scale bar=10μm).
S100A8/A9 increases Ca2+ levels at the LFA-1 nanocluster sites.
Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils were seeded into E-selectin, ICAM-1 and CXCL1 coated flow chambers, allowed to settle down for 3min and then physiological shear stress (2dyne cm−2) was applied and Ca2+ MFI was analyzed in the LFA-1 nanocluster areas at min 0-1, min 5-6 and min 9-10 of analysis [representative movies of Ca2+ oscillations in the LFA-1 segmented areas (“LFA-1 mask”), scale bar=10μm].
S100A8/A9 induces F-actin polymerization.
Lyz2xGCaMP5 and Lyz2xGCaMP5xS100a9-/- neutrophils were seeded into E-selectin, ICAM-1 and CXCL1 coated flow chambers, allowed to settle down for 3min and then physiological shear stress (2dyne cm−2) was applied and F-actin MFI was analyzed in the overall cell area at min 0-1, min 5-6 and min 9-10 of analysis [representative movies of F-actin intensity in the Lyz2 segmented areas (“cell mask”), scale bar=10μm].
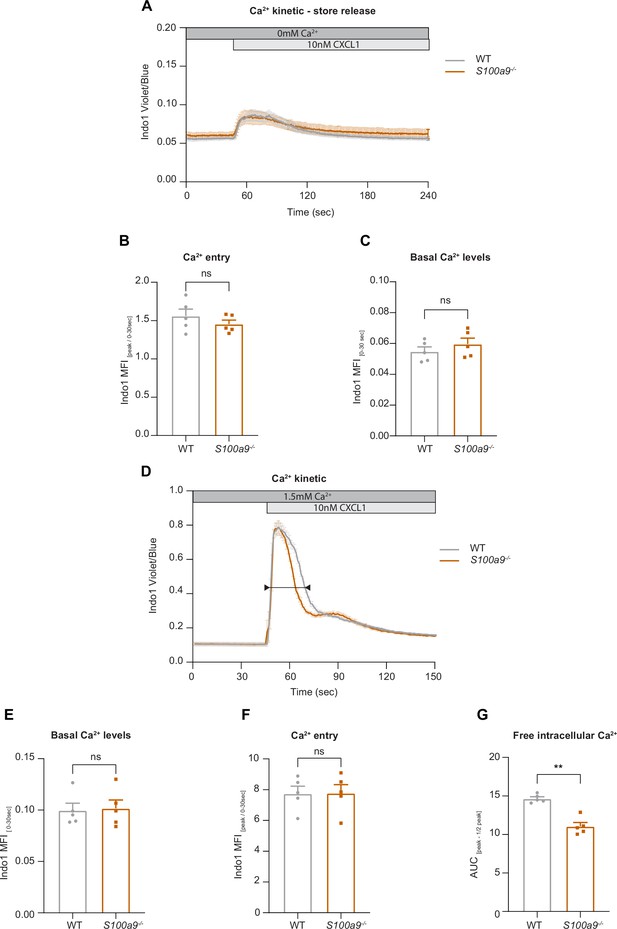
Cytosolic S100A8/A9 is dispensable for chemokine-induced endoplasmic reticulum (ER) store Ca2+ release and for the initial phase of store-operated Ca2+ entry (SOCE).
(A) Average flow cytometry kinetic graphs of Ca2+ store release in the absence of extracellular Ca2+ (Ca2+ free medium) in wildtype (WT) and S100a9-/- neutrophils upon CXCL1 stimulation (traces are shown as mean + SEM, n=5 mice per group). (B) Rapid ER store Ca2+ release (MFI peak/ MFI 0-30s) of WT and S100a9-/- neutrophils (mean + SEM, n=5 mice per group, paired Student’s t-test). (C) Quantification of Ca2+ levels under baseline conditions (MFI 0-30s) (mean + SEM, n=5 mice per group, paired Student’s t-test). (D) Average flow cytometry kinetic graphs of Ca2+ influx in the presence of extracellular Ca2+ (HBSS medium, 1.5 mM Ca2+) of WT and S100a9-/- neutrophils upon CXCL1 stimulation (traces are shown as mean + SEM, n=5 mice per group, double-headed arrow represents the time points of quantification). (E) Ca2+ levels before CXCL1 stimulation (MFI 0-30s) (mean + SEM, n=5 mice per group, paired Student’s t-test). (F) Quantification of ER store Ca2+ release and calcium release-activated channel (CRAC) store-operated Ca2+ entry (MFI peak/ MFI 0-30s) (mean + SEM, n=5 mice per group, paired Student’s t-test). (G) Ca2+ influx after CXCL1 stimulation, from peak to peak half-life (AUCpeak – ½ peak) of WT and S100a9-/- neutrophils (mean + SEM, n=5 mice per group, paired Student’s t-test). ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.

Extracellular S100A8/A9 does not rescue the adhesion defect in Mrp14/- neutrophils.
Analysis of number of adherent leukocytes FOV-1 normalized to the WBC of WT and Mrp14-/- mice. Whole blood was harvested through a carotid artery catheter and perfused with a high precision pump at constant shear rate using flow cambers coated with either E-selectin, ICAM-1 and CXCL1 or E-selectin, ICMA-1, CXCL1 and S100A8/A9. [mean + SEM, n=5 mice per group, 12 (WT) and 14 (Mrp14-/-) flow chambers, 2way ANOVA, Sidak’s multiple comparison]. ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
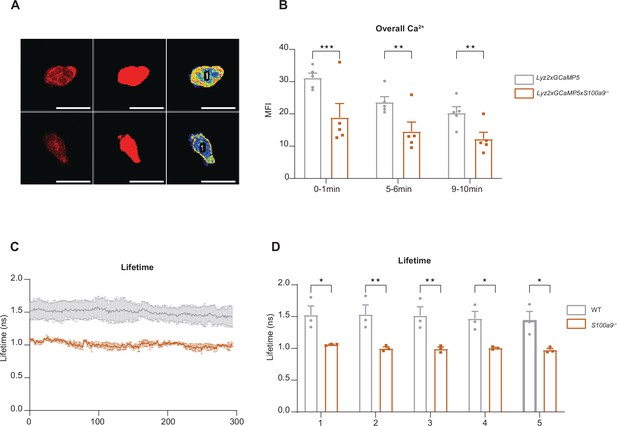
Overall Ca2+ levels in WT and Mrp14-/- neutrophils (A) Representative confocal images of neutrophils from WT Lyz2xGCaMP5 and Mrp14-/- Lyz2xGCaMP5 mice, labeled with Lyz2 td Tomato marker.
The images illustrate overall cytosolic Ca2+ levels during neutrophil crawling flow chambers coated with E-selectin, ICAM-1, and CXCL1 (scale bars=10μm). (B) Quantitative analysis of total cytosolic Ca2+ intensity in single cells from WT Lyz2xGCaMP5 and Mrp14-/- Lyz2xGCaMP5 neutrophils measured over three time intervals: min 0-1, 5-6 and 9-10 [mean + SEM, n=5 mice per group, 56 (WT) and 54 (Mrp14-/-) neutrophils, 2way ANOVA, Sidak’s multiple comparison]. (C) Representative traces and (D) single cell analysis of total Ca2+ lifetime over the first 5 minutes in WT Lyz2xGCaMP5 and Mrp14-/- Lyz2xGCaMP5 neutrophils crawling on Eselectin, ICAM-1, and CXCL1 coated flow chambers recorded with FLIM microscopy [mean + SEM, n=3 mice per group, 111 (WT) and 95 (Mrp14-/-) neutrophils, 2way ANOVA, Sidak’s multiple comparison]. ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.

Giemsa staining of extravasated leukocyte subsets.
(A) Representative image of Giemsa-stained cremaster muscle tissue post-TNF stimulation. The image clearly differentiates leukocyte subsets (white arrow = neutrophils, yellow arrow = eosinophils, red arrow = monocytes). Scale bar = 50µm.

Static Transmigration assay.
(a) Transmigration of WT and Mrp14-/- neutrophils in static transwell assays (3um pore size, 45min migration time) showing spontaneously migration (PBS) or migration towards CXCL1. [mean + SEM, n=3 mice per group, 2way ANOVA, Sidak’s multiple comparison]. ns, not significant; *p≤0.05, **p≤0.01, ***p≤0.001.
Tables
Microvascular parameters in vivo.
Number of mice, number of vessels, vessel diameter, centerline velocity, wall shear rate and white blood cell (WBC) of TNF-α stimulated wildtype (WT) and S100a9-/- mice, as well as of WT and S100a9-/- mice treated with mutS100A8/A9 without any prior stimulation (trauma model) and also of TNF-α stimulated WT and S100a9-/- mice treated with mutS100A8/A9 (mean + SEM; unpaired Student’s t-test).
| Mice (n) | Venules (n) | Diameter (µm) | Centerline velocity (µm s–1) | Wall shear rate (s–1) | WBC(µl–1) | |
|---|---|---|---|---|---|---|
| WT +TNF-α | 5 | 21 | 32.50+0.50 | 1130+50 | 920+50 | 3580+450 |
| S100a9-/-+TNF-α | 5 | 20 | 34+1.5 | 1320+50 | 1010+50 | 3520+200 |
| ns. (p=0.6065) | ns. (p=0.1534) | ns. (p=0.6091) | ns. (p=0.9723) | |||
| WT +mutS100 A8/A9 | 4 | 4 | 30+5 | 2030+300 | 1800+420 | 5720+800 |
| S100a9-/-+mutS100 A8/A9 | 3 | 3 | 30+2.5 | 1540+350 | 1300+350 | 5460+650 |
| ns. (p=0.8359) | ns. (p=0.3416) | ns. (p=0.3898) | ns. (p=0.7979) | |||
| WT +TNF-α+mutS100 A8/A9 | 4 | 18 | 31+3 | 1330+330 | 1450+400 | 4100+1000 |
| S100a9-/-+TNF-α+mutS100 A8/A9 | 5 | 24 | 30+3 | 1700+130 | 1500+300 | 3900+500 |
| ns. (p=0.6470) | ns. (p=0.8341) | ns. (p=0.3947) | ns. (p=0.6677) |
Microvascular parameters ex vivo.
Number of mice, number of flow chambers, cells per field of view (FOV), and white blood cell (WBC) of ex vivo flow chamber assay (mean + SEM, unpaired Student’s t-test).
| Mice (n) | Flow chambers (n) | Cells FOV–1 | WBC(µl–1) | |
|---|---|---|---|---|
| WT | 4 | 8 | 39+5 | 8630+1,200 |
| S100a9-/- | 4 | 10 | 37+5 | 8600+1,200 |
| ns. (p=0.7332) | ns. (p=0.9772) |





