Dimeric R25CPTH(1–34) activates the parathyroid hormone-1 receptor in vitro and stimulates bone formation in osteoporotic female mice
Figures
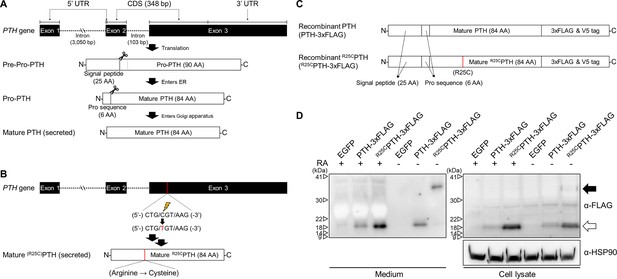
Formation of R25C mutant PTH(1–84) dimer.
(A) Schematic representation of PTH gene structure and expression. (B) Schematic representation of R56Cpre-pro-PTH(1–115) (in mature form, R25CPTH(1–84)) gene structure and expression. (C) Schematic representation of recombinant PTH proteins. (D) In vitro dimerization of R25CPTH. Recombinant protein constructs were transfected into HEK293T cells, and expression of PTH-3xFLAG and R25CPTH3xFLAG in culture medium or cell lysate was demonstrated by western blot. The result confirms the presence of dimeric R25CPTH (*bp: base pairs; *AA: amino acids; *RA: reducing agent).
-
Figure 1—source data 1
The original files of the raw membranes correspond to Figure 1D.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-data1-v1.zip
-
Figure 1—source data 2
The uncropped membranes correspond to Figure 1D indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-data2-v1.zip
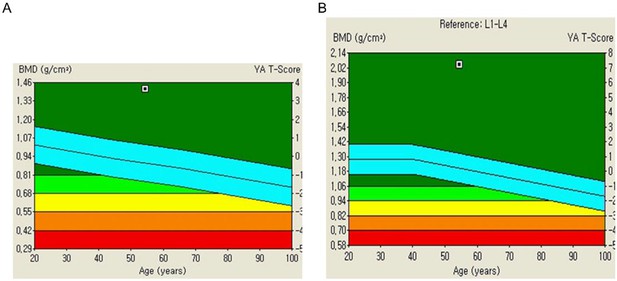
Bone mineral density (BMD) analysis in a patient affected by homogeneous R25CPTH mutation.
Trabecular bone score (TBS) is a measure in bone density assessments, evaluating bone tissue microarchitecture. Derived from dual-energy X-ray absorptiometry (DXA) scans, TBS aids in fracture risk determination. The T-score, present on bone density reports, reflects bone mass deviation from the norm within the same age group. (A) The TBS report demonstrating a significantly elevated BMD in the lumbar spine of the patient. (B) The TBS report demonstrating a significantly elevated BMD in the femur of the patient. The left Y-axis indicates BMD (g/cm2), while the right Y-axis presents T-score. T-score interpretation is as follows. Normal or lower risk of fractures: –1.0 and above; low bone density or osteopenia: between –1.0 and –2.5, indicating weaker bones than normal but not classified as osteoporotic; osteoporosis: –2.5 and below, signifying a substantial fracture risk due to very low bone density. Light blue indicates average T-score of normal population ±1.
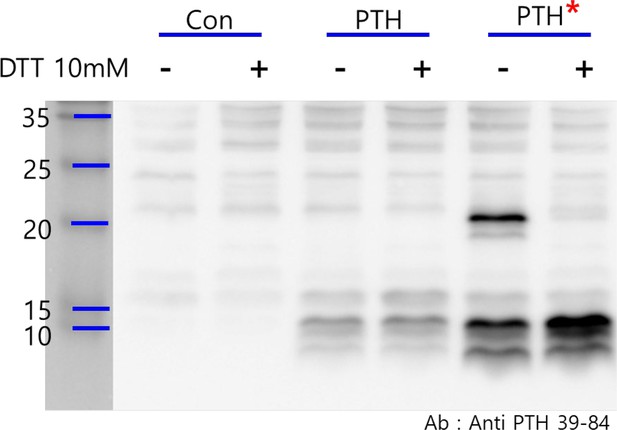
Identification of dimeric R25CPTH (1–84) peptide.
HEK293T cells were separately transfected with three plasmids, pcDNA3.0 empty vector, pcDNA3.0-(pre-pro-PTH)-IRES, and pcDNA3.0(R56Cpre-pro-PTH)-IRES. Total cell lysates were extracted from each transfected group and divided into two types of samples, reduced and non-reduced. The results revealed a specific dimeric formation of R25CPTH(1–84). The primary antibody used in this experiment binds to the 39–84 region of mature PTH(1–84) (QuidelOrtho, 21-3010, PTH Antibody (Center 39–84)). 10 mM DTT was used for reducing agent.
-
Figure 1—figure supplement 2—source data 1
The original files of the raw membranes correspond to Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-figsupp2-data1-v1.zip
-
Figure 1—figure supplement 2—source data 2
The uncropped membranes correspond to Figure 1—figure supplement 2 indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-figsupp2-data2-v1.zip
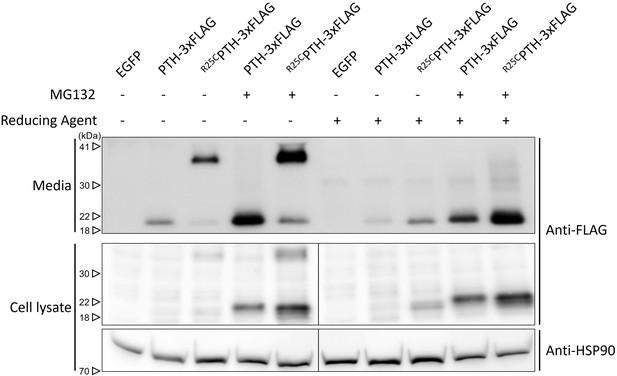
Influence of proteasome inhibitor MG132 on PTH and R25CPTH stability.
To validate whether inhibition of proteasome-mediated degradation can restore the decreased expression level of wild-type PTH compared to R25CPTH under normal conditions, a proteasome inhibition assay using MG132 was conducted. HEK293T cells were transfected with plasmids including control vector, pcDNA3.1-PTH-3xFLAG, and pcDNA3.1-R25CPTH-3xFLAG. After 24 hr of transfection, MG132 was administered for 24 hr, and media as well as total cell lysate were collected for subsequent western blot analysis. Each sample was divided into two types, reduced and non-reduced. The results indicated that both PTH and R25CPTH exhibited increased protein expression levels following MG132 treatment-induced proteasome inhibition. However, the disparity in expression levels between the PTH and R25CPTH remained unchanged.
-
Figure 1—figure supplement 3—source data 1
The original files of the raw membranes correspond to Figure 1—figure supplement 3.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-figsupp3-data1-v1.zip
-
Figure 1—figure supplement 3—source data 2
The uncropped membranes correspond to Figure 1—figure supplement 3 indicating the relevant bands and treatments.
- https://cdn.elifesciences.org/articles/97579/elife-97579-fig1-figsupp3-data2-v1.zip
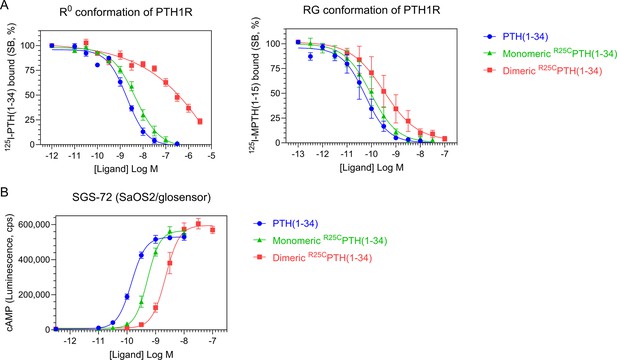
Effect of PTH, monomeric R25CPTH, and dimeric R25CPTH to the PTH1R in vitro.
(A) The binding of PTH(1–34), monomeric R25CPTH(1–34), and dimeric R25CPTH(1–34) to the PTH1R in R0 conformation of RG conformation was assessed by competition methods using 125I-PTH(1–34) and 125I-MPTH(1–15) as radioligand (n=4). (B) Ligand potency for cyclic AMP (cAMP) signaling was assessed in SGS-72 cells, which were derived from SaOS-2 cells modified to express Glosensor cAMP reporter (n=4). The cells were preloaded with luciferin and treated with varying concentrations of PTH(1–34), monomeric R25CPTH(1-34), and dimeric R25CPTH(1–34). Error bars represent mean ± standard error.
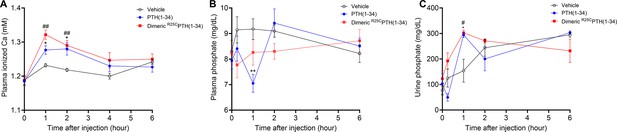
Calcemic and phosphatemic responses by PTH injection in CD1 female mice.
(A) Plasma calcemic response after injection (n=6). Both PTH(1–34) and dimeric R25CPTH(1–34) significantly elevate ionized calcium levels in plasma at 1–2 hr post-injection. After 2 hr post-injection, plasma ionized calcium level gradually restored to baseline levels similar to those of the vehicle group. (B) Plasma phosphatemic response after injection (n=12). Following PTH(1–34) injection, plasma phosphate levels significantly decrease at 1 hr post-injection, subsequently returning to baseline akin to those of the vehicle group. Conversely, dimeric R25CPTH(1–34) injection shows no significant alteration in phosphatemic response but demonstrates a tendency toward a slight decrease in phosphate levels, gradually restoring to baseline levels akin to those of the vehicle group. (C) Urine phosphatemic response after injection (n=6). The urine phosphate levels markedly increased at 1 hr post-injection for both PTH(1–34) and dimeric R25CPTH(1–34), followed by a return to baseline levels akin to those of the vehicle group. This analysis was conducted using 9-week-old female CD1 mice. The mice were administered PTH(1–34) and dimeric R25CPTH(1–34) at a concentration of 50 nmol/kg for each compound. Error bars represent mean ± standard error. p-Values were determined using the two-way ANOVA to compare the mean of each test cell with the mean of the control cell at the same time point. * denotes p-value<0.05 for PTH(1–34) compared to vehicle, ** denotes p-value<0.01 for PTH(1–34) compared to vehicle, # denotes p-value<0.05 for dimeric R25CPTH(1–34) compared to vehicle, ## denotes p-value<0.01 for dimeric R25CPTH(1–34) compared to vehicle.
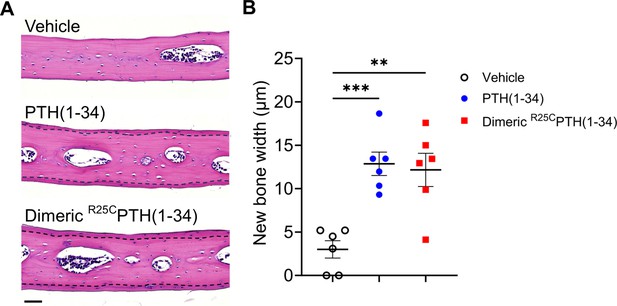
Effect of dimeric R25CPTH(1–34) in calvarial injection model.
(A) Dissections of the calvarial bones. Calvarial injections were performed on 8-week-old male C57BL/6 mice (N=6 per group) that received daily administrations of vehicle, PTH(1–34), or dimeric R25CPTH(1–34) for 6 days. Following a 10-day treatment period, histological sections of calvariae, stained with hematoxylin (blue-purple, indicating cell nuclei) and eosin (pink, representing bone matrix), were obtained. The area of new bone formation, with more intense staining compared to the existing bone tissue, is denoted by the dotted line. Scale bar = 50 µm. (B) Quantification of new bone width in calvarial injection model. The result showed a significant increase in new bone width following injections of both PTH(1–34) and dimeric R25CPTH(1–34) compared to the vehicle group. p-Values were obtained using the one-way ANOVA to compare the mean of each column with the mean of a control column. ** indicates p-value<0.01 against vehicle, *** indicates p-value<0.001 against vehicle.
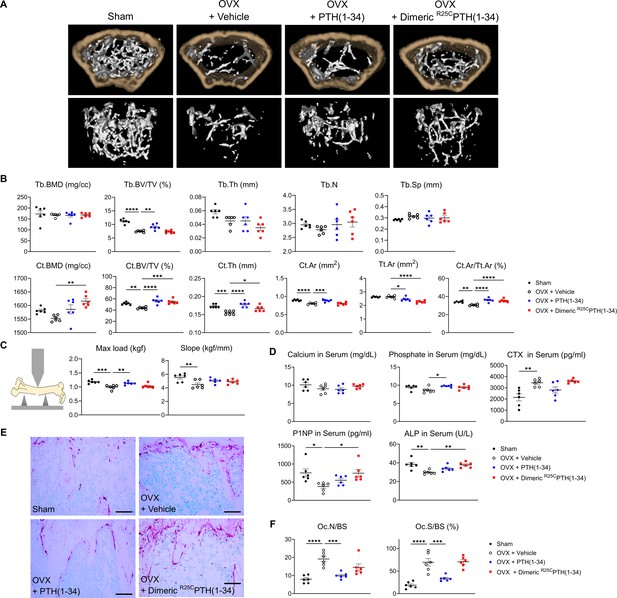
Impact of R25CPTH(1–34) on bone turnover.
The effects of Sham, OVX-control (OVX+vehicle), OVX treated with PTH(1–34) (OVX+PTH(1–34)), and OVX treated with dimeric R25CPTH(1–34) (OVX+dimeric R25CPTH(1–34)) on bone turnover in mice. (A) Femurs obtained from mice in each group were subjected to micro-computed tomography (µ-CT) analyses for the assessment of bone mass. (B) Several parameters of (A) were quantified using µ-CT measurements, including trabecular bone mineral density (Tb.BMD), trabecular bone volume to tissue volume (Tb.BV/TV), trabecular bone thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), cortical bone mineral density (Ct.BMD), cortical bone volume to tissue volume (Ct.BV/TV), cortical thickness (Ct.Th), cortical area (Ct.Ar), total tissue area (Tt.Ar), and cortical area to total tissue area (Ct.Ar/Tt.Ar). (C) A 3D-point bending test was conducted with femurs obtained from mice in each group. The left panel describes a schematic model of a 3D-point bending test. The middle and right panels each indicate the maximum bending load (kgf) and slope (kgf/mm). (D) Serum levels of calcium, phosphorus, CTX, P1NP, and alkaline phosphatase (ALP) were measured for each group using an enzyme-linked immunosorbent assay (ELISA). (E) Tartrate-resistant acid phosphatase (TRAP) staining of histological sections of proximal tibias was carried out to visualize osteoclast activity. Scale bars = 100 µm. (F) Quantification of osteoclast number per bone surface (Oc.N/BS) and osteoclast surface per bone surface (Oc.S/BS) was performed. Each group consisted of six samples (n=6). The error bars indicate mean ± standard error. p-Values were obtained using the one-way ANOVA to compare the mean of each column with the mean of a control column. * indicates p-value<0.05, ** indicates p-value<0.01, *** indicates p-value<0.001, **** indicates p-value<0.0001.
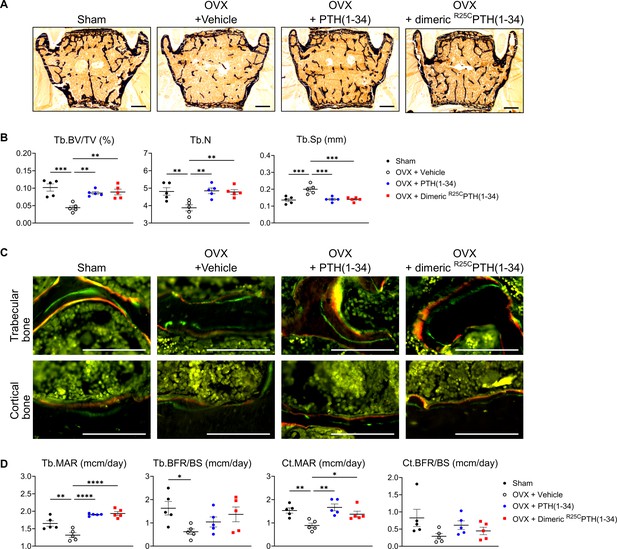
Impact of R25CPTH(1–34) on osteoblast function in vertebrae.
The effects of Sham, OVX-control (OVX+vehicle), OVX treated with PTH(1–34) (OVX+PTH(1–34)), and OVX treated with dimeric R25CPTH(1–34) (OVX+dimeric R25CPTH(1–34)) on osteoblast function in mice. (A) Mineralization of vertebrae obtained from each group was assessed through von Kossa staining. Scale bars = 100 µm. (B) Quantification of trabecular bone parameters including trabecular bone volume to tissue volume (Tb.BV/TV), trabecular number (Tb.N), and trabecular separation (Tb.Sp) was performed using the Bioquant Osteo 2019 v19.9.60 program. (C) Fluorescent microscopic observations of trabecular and cortical bone sections from each group demonstrate the apposition of xylenol (red) and calcein (green) labels. Scale bars = 100 µm. (D) Quantification of trabecular bone parameters such as trabecular bone mineral apposition rate (Tb.MAR), trabecular bone formation rate to bone surface (Tb.BFR/BS), cortical bone MAR (Ct.MAR), and trabecular MAR (Tb.MAR) was carried out using the Bioquant Osteo 2019 v19.9.60 program. Each group consisted of five samples (n=5). The error bars indicate mean ± standard error. p-Values were obtained using the one-way ANOVA to compare the mean of each column with the mean of a control column. * indicates p-value<0.05, ** indicates p-value<0.01, *** indicates p-value<0.001, **** indicates p-value<0.0001.
Additional files
-
Supplementary file 1
Raw data set for statistical analysis.
The numerical data used to generate Figure 2A, B; Figure 3A–C; Figure 4B; Figure 5B–D, F; Figure 6B, D.
- https://cdn.elifesciences.org/articles/97579/elife-97579-supp1-v1.xlsx
-
Supplementary file 2
List of primers used in this study.
- https://cdn.elifesciences.org/articles/97579/elife-97579-supp2-v1.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/97579/elife-97579-mdarchecklist1-v1.docx





