Human airway macrophages are metabolically reprogrammed by IFN-γ resulting in glycolysis-dependent functional plasticity
Figures
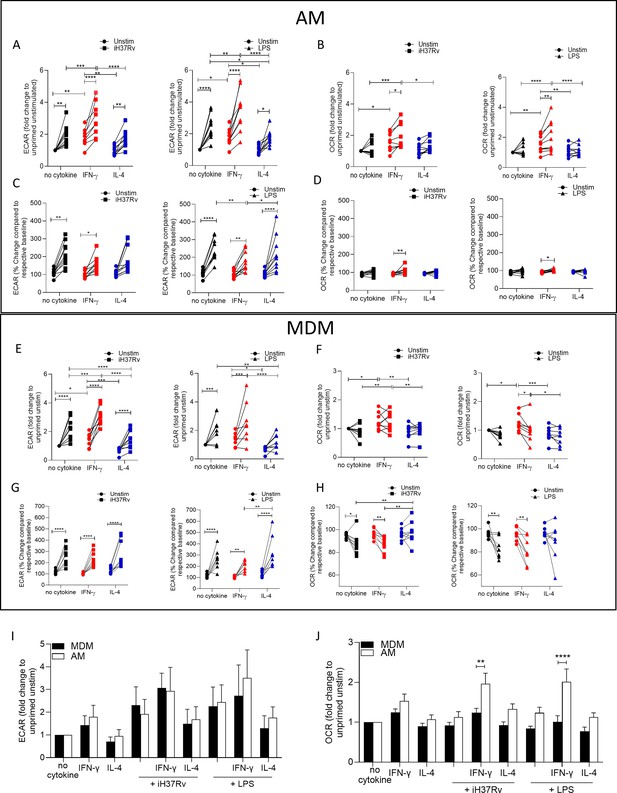
IFN-γ increases energetic metabolism in the AM but enhances ‘Warburg’-like metabolism in MDM in response to inflammatory stimuli.
Human AM (A–D) were isolated from bronchoalveolar lavage fluid. PBMC were isolated from buffy coats and MDM (E–H) were differentiated and adherence purified for 7 days in 10% human serum. Cells were left unprimed (black) or primed with IFN-γ (red) or IL-4 (blue; both 10 ng/ml) for 24 hr. Baseline measurements of the Extracellular Acidification Rate (ECAR) and the Oxygen Consumption Rate (OCR) were established before AM or MDM were stimulated with medium (circle), irradiated Mtb H37Rv (iH37Rv; MOI 1–10; square) or LPS (100 ng/ml; triangle), in the Seahorse XFe24 Analyzer, then monitored at 20 min intervals. At 150 min, post stimulation fold change in ECAR (A, E, I) and OCR (B, F, J) was analysed, and percentage change (from baseline of the respective treatment group) was also calculated for ECAR (C, G) and OCR (D, H) at 150 min. A direct comparison of AM (white bar) and MDM (black bar) was also assessed at 150 min (I, J). Each linked data point represents the average of technical duplicates for one individual biological donor (MDM; n=8–9, AM; n=9–10). Statistically significant differences were determined using two-way ANOVA with a Tukey (A–H) or Bonferroni post-test (I–J); *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.001.
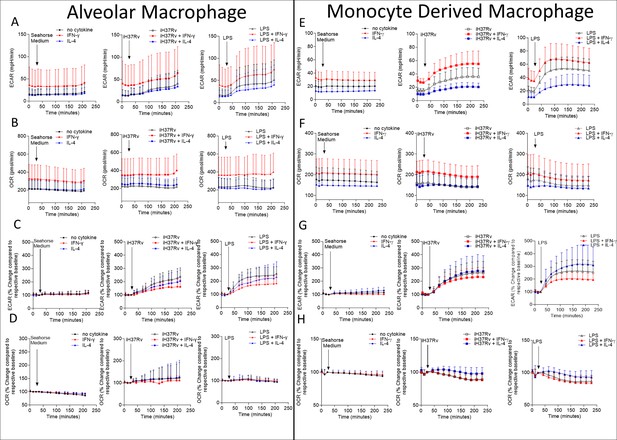
Human AM and MDM seahorse traces for ECAR and OCR.
Human AM (A–D) or MDM (E–H) were left unprimed or primed with IFN-γ or IL-4 (both 10 ng/ml) for 24 hr. Baseline measurements of the Extracellular Acidification Rate (ECAR) and the Oxygen Consumption Rate (OCR) were established before AM or MDM were stimulated with medium, with irradiated H37Rv (iH37Rv; MOI 1–10) or LPS (100 ng/ml), in the Seahorse XFe24 Analyzer, then monitored at 20 min intervals. The time course graphs illustrate the collated ECAR (A, E) and OCR (B, F) in unstimulated cells or in response to iH37Rv or LPS in the presence or absence of IFN-γ or IL-4. Time course graphs were also generated for % change ECAR (C,G) and % change OCR (D, H) to account for differences at baselines (MDM; n=8–9, AM; n=9–10, error bars represent standard deviation).
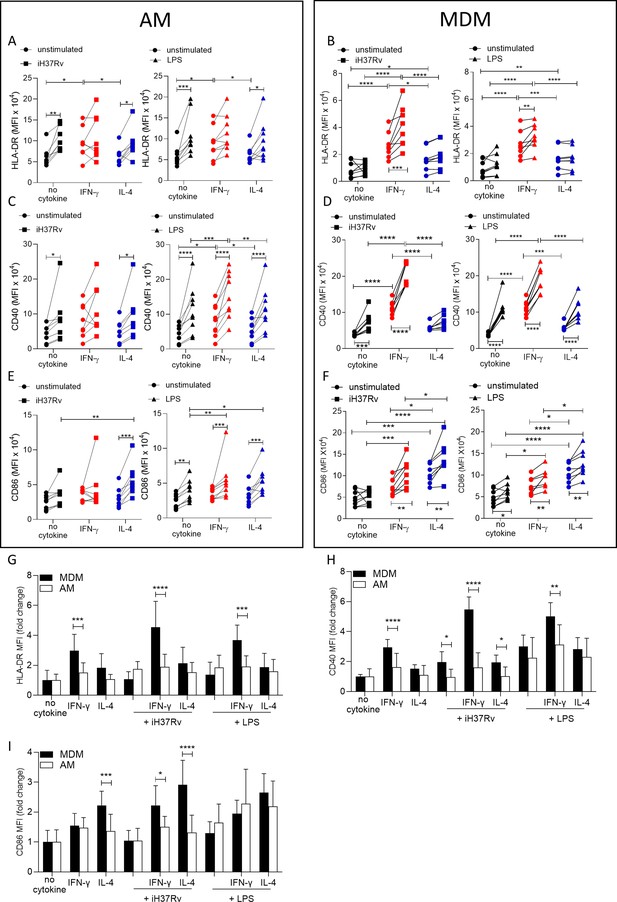
IFN-γ boosts activation marker expression on MDM to a greater extent than AM.
Human AM (A, C, E) isolated from bronchoalveolar lavage fluid. PBMC were isolated from buffy coats and MDM (B, D, F) were differentiated and adherence purified for 7 days in 10% human serum. Cells were left unprimed (black) or primed with IFN-γ (red) or IL-4 (blue) (both 10 ng/ml) for 24 hr. AM or MDM were left unstimulated (circle) or stimulated with iH37Rv (MOI 1–10; square) or LPS (100 ng/ml; triangle). After 24 hr, cells were detached from the plates by cooling and gentle scraping and stained for HLAR-DR (A, B), CD40 (C, D), CD86 (E, F) and analysed by flow cytometry. Fold change of HLA-DR (G), CD40 (H) and CD86 (I) was calculated for AM (white bar) and MDM (black bar) based on the average of their respective no cytokine controls. Each linked data point represents the average of technical duplicates for one individual biological donor (n=8–9). Statistically significant differences were determined using two-way ANOVA with a Tukey (A–F) or Bonferroni post-test (G–I); *p≤0.05, **p≤0.01, p***≤0.001, ****p≤0.001.

Supplementary flow cytometry gating strategy.
PBMC were isolated from buffy coats and MDM were differentiated and adherence purified for 7 days in 10% human serum. Cells were placed in ice cold PBS and detached with gentle scraping. Cells were Fc blocked, treated with viability dye and stained with fluorochrome-conjugated antibodies specific for CD14, CD68, HLA-DR, CD40, and CD86. Cells were fixed with 2% PFA and analysed by flow cytometry. MDM were gated on the basis of forward (FSc) and side scatter (SSc), doublets were excluded using forwards scatter area versus width, live cells were gated on the basis of ZombieNearIR exclusion. MDM were CD14 and CD68 double positive. The expression of HLA-DR or CD40 were quantified by median fluorescence intensity (MFI). Representative dot plots show staining of unstimulated MDM and histograms show overlaid fluorescence of unstimulated cells compared with Mtb (iH37Rv) stimulated cells in the presence or absence of IFN-γ.
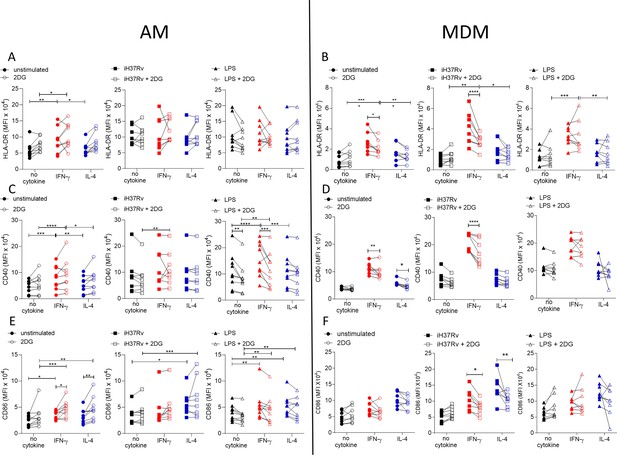
Glycolysis is required for IFN-γ induced expression of activation markers by MDM and not AM.
Human AM (A, C, E) isolated from bronchoalveolar lavage fluid. PBMC were isolated from buffy coats and MDM (B, D, F) were differentiated and adherence purified for 7 days in 10% human serum. Cells were left unprimed (black) or primed with IFN-γ (red) or IL-4 (blue) (both 10 ng/ml) for 24 hr. Cells were left untreated (solid) or treated with 2DG (5 mM; empty) 1 hr prior to stimulation with iH37Rv (MOI 1–10; square) or LPS (100 ng/ml; triangle) or left unstimulated (circle). After 24 hr, cells were detached from the plates by cooling and gentle scraping and stained for HLAR-DR (A, B), CD40 (C, D), CD86 (E, F) and analysed by flow cytometry. Each linked data point represents the average of technical duplicates for one individual biological donor (n=8–9). Statistically significant differences were determined using two-way ANOVA with a Tukey post-test (A–F); *p≤0.05, **p≤0.01, p***≤0.001, ****p≤0.001.
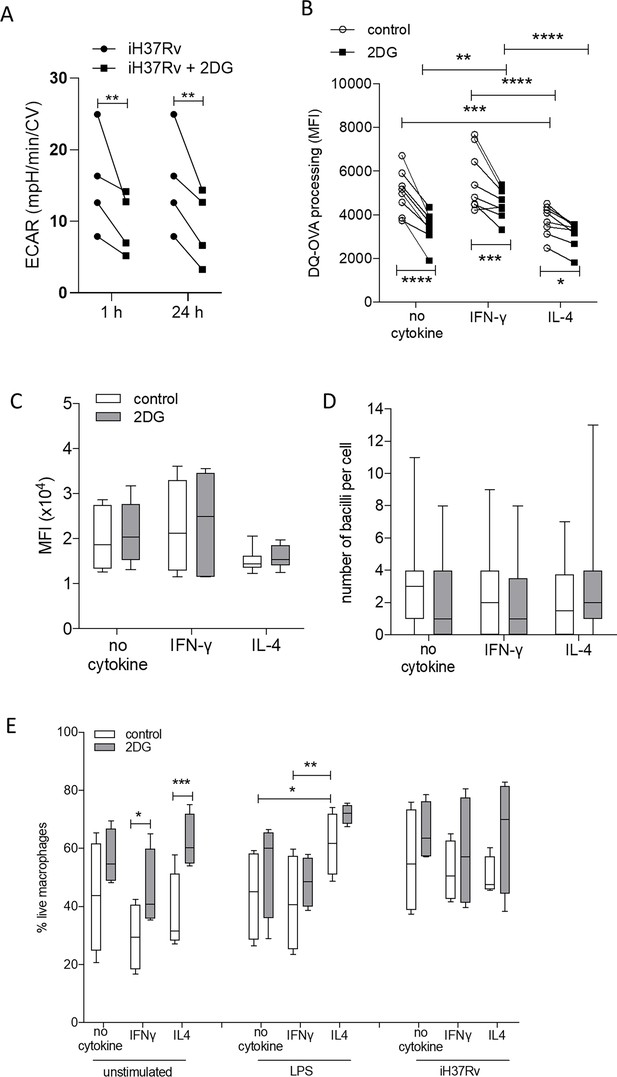
2DG reduces ECAR and antigen-processing but does not reduce phagocytosis or cell viability.
PBMC were isolated from buffy coats and MDM were differentiated and adherence purified for 7 days in 10% human serum. MDM were left untreated or treated with 2DG (5 mM) for 1 or 24 hr. MDM were stimulated with iH37Rv and ECAR was assessed at indicated time points (A). MDM were primed with IFN-γ or IL-4 (both 10 ng/ml) for 24 hr. MDM were treated with 2DG (5 mM) for 1 hr prior to treatment with DQ-Ovalbumin (B; 500 ng/ml; 30 min), fluorescent latex beads (C; 15 min) or iH37Rv (E; MOI 1–10; 180 min). MDM were then detached with ice cold PBS, scraped and analysed by flow cytometry. Alternatively, 1 hr after 2DG MDM were stimulated with iH37Rv (MOI 1–10) or LPS (100 ng/ml) for 24 hr. MDM were then scraped and stained with Zombie NIR for cell viability (E), measured by flow cytometry. Each data point represents one individual donor. Statistically significant differences were determined using two-way ANOVA with a Tukey post-test; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
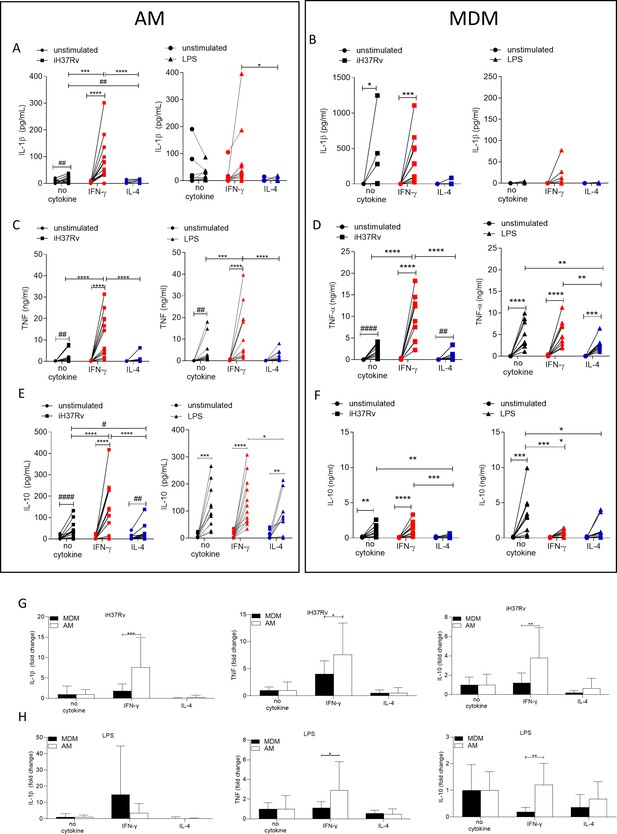
IFN-γ enhances cytokine production more in AM compared with MDM.
Human AM (A, C, E) isolated from bronchoalveolar lavage fluid. PBMC were isolated from buffy coats and MDM (B, D, F) were differentiated and adherence purified for 7 days in 10% human serum. Cells were left unprimed (black) or primed with IFN-γ (red) or IL-4 (blue) (both 10 ng/ml) for 24 hr. AM or MDM were left unstimulated (circle) or stimulated iH37Rv (MOI 1–10; square) or LPS (100 ng/ml; triangle). Supernatants were harvested 24 hr after stimulation and concentrations of IL-1β (A, B), TNF (C, D), and IL-10 (E, F) were quantified by ELISA. Fold change in IL-1β, TNF and IL-10 was calculated for AM and MDM based on the average of respective no cytokine controls for iH37Rv (G) and LPS (H). Each linked data point represents the average of technical duplicates for one individual biological donor (AM; n=12–13, MDM; n=8–10). Statistically significant differences were determined using two-way ANOVA with a Tukey (A–F) or Bonferroni post-test (G–H); *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001 or #p≤0.05, ##p≤0.01, ####p≤0.0001 (where IFN-γ-treated data sets were excluded for post-test analysis to analyse statistical differences between no cytokine and IL-4-treated data sets).
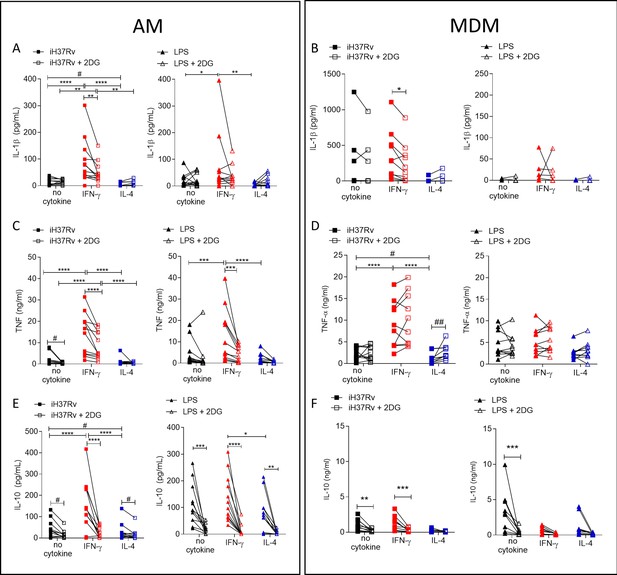
Cytokine secretion by AM is more reliant on glycolysis than MDM.
Human AM (A, C, E) isolated from bronchoalveolar lavage fluid. PBMC were isolated from buffy coats and MDM (B, D, F) were differentiated and adherence purified for 7 days in 10% human serum. Cells were left unprimed (black) or primed with IFN-γ (red) or IL-4 (blue; both 10 ng/ml) for 24 hr. Cells were left untreated (solid) treated with 2DG (5 mM; empty) for 1 hr prior to stimulation with iH37Rv (MOI 1–10; square) or LPS (100 ng/ml; triangle) or left unstimulated (circle). Supernatants were harvested 24 hr after stimulation and concentrations of IL-1β (A, B), TNF (C, D) and IL-10 (E, F) were quantified by ELISA. Each linked data point represents the average of technical duplicates for one individual biological donor (AM; n=12–13, MDM; n=8–10). Statistically significant differences were determined using two-way ANOVA with a Tukey post-test (A–D); *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001 or #p≤0.05, ##p≤0.01 (where IFN-γ primed data sets were excluded for post-test analysis to analyse statistical differences between no cytokine and IL-4 treated data sets).





