Regulation of food intake by mechanosensory ion channels in enteric neurons
Abstract
Regulation of food intake is fundamental to energy homeostasis in animals. The contribution of non-nutritive and metabolic signals in regulating feeding is unclear. Here we show that enteric neurons play a major role in regulating feeding through specialized mechanosensory ion channels in Drosophila. Modulating activities of a specific subset of enteric neurons, the posterior enteric neurons (PENs), results in sixfold changes in food intake. Deficiency of the mechanosensory ion channel PPK1 gene or RNAi knockdown of its expression in the PENS result in a similar increase in food intake, which can be rescued by expression of wild-type PPK1 in the same neurons. Finally, pharmacological inhibition of the mechanosensory ion channel phenocopies the result of genetic interrogation. Together, our study provides the first molecular genetic evidence that mechanosensory ion channels in the enteric neurons are involved in regulating feeding, offering an enticing alternative to current therapeutic strategy for weight control.
https://doi.org/10.7554/eLife.04402.001eLife digest
Around one third of children and two thirds of adults in the US are thought to be overweight or obese. By increasing the risk of disorders such as heart disease, stroke, diabetes and some types of cancer, obesity has become one of the leading causes of preventable death worldwide and accounts for an increasing proportion of all spending on healthcare.
Given the high costs of obesity for individuals and society, there is widespread interest in the development of drugs to aid weight loss. Three compounds are currently approved for this purpose, and they work either by reducing the body's ability to absorb fat or by acting on the brain to suppress appetite. However, all three have significant side effects.
Now, on the basis of experiments in fruit flies, Olds and Xu suggest an alternative strategy, namely targeting the ‘stretch-sensitive’ ion channels in the neurons in the digestive system that signal to the brain that the body has ingested enough food. By artificially activating these ion channels, it might be possible to induce feelings of fullness after smaller quantities of food have been consumed.
These ion channels—known as PPK1 ion channels—are present on posterior enteric neurons, which wrap around the muscles of the gut. Silencing these neurons caused fruit flies to eat too much, whereas activating them caused the flies to eat less. Deleting the gene that encodes the PPK1 ion channel had the same effect as silencing neurons, suggesting that drugs that act directly on PPK1 could help to regulate food intake. Consistent with this, insects ate more when their food was supplemented with a chemical that blocked the PPK1 ion channels.
By showing that PPK1 ion channels can be targeted pharmacologically, Olds and Xu have opened up a new avenue of anti-obesity research. A drug that can activate the equivalent ion channel in mammals would have the potential to aid weight loss, while avoiding the side effects associated with compounds that act directly on the brain.
https://doi.org/10.7554/eLife.04402.002Introduction
Historically, the sensation of fullness has been documented as far back as Homer's Odyssey. Pioneering work by Cannon and Washburn revealed a correlation between stomach expansion and satiety in humans (Cannon and Washburn, 1911), which was later confirmed in rodents (Hargrave and Kinzig, 2012). Recently, several groups have shown that feeding-related neurons are sensitive to satiety state but not nutrients in Drosophila (Marella et al., 2012; Dus et al., 2013; Pool et al., 2014). These studies argue that non-metabolic inputs such as mechanic tension could regulate feeding.
Results and discussion
Recent studies in Drosophila have identified neuronal regulation of food intake and nutrient sensing in the central nervous system (Marella et al., 2012; Dus et al., 2013). However, the potential contribution of the enteric neurons in the gastrointestinal tract has not been explored. To investigate this, we utilized four previously characterized Gal4 lines that are expressed in enteric neurons in the different parts of the Drosophila digestive system (Figure 1A,B). While the GMR48A05-Gal4 neurons project to the proventriculus and the anterior midgut, the GMR51F12-Gal4 neurons project to the anterior midgut and the crop (Figure 1A,B) (Jenett et al., 2012). In contrast, both HGN1-Gal4 and Ilp7-Gal4 neurons project to two posterior regions in the gut: (1) the hindgut pylorus and the connecting posterior midgut and anterior hindgut, and (2) the rectum pylorus and the rectum (referred to as the posterior enteric neurons or the PENs, Figure 1A,B) (Cognigni et al., 2011).
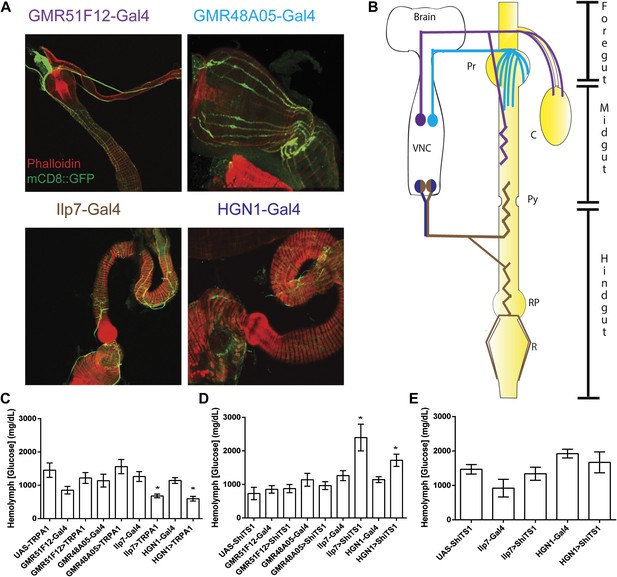
Modulating activities of Drosophila PENs causes metabolic defects.
(A) Enteric neural projections of Gal4 lines tested (red, phalloidin; green, UAS-mCD8::GFP) and their diagram (B). GMR51F12-Gal4 neurons project to the foregut, anterior midgut and crop. GMR48A05-Gal4 neurons project to the proventriculus and anterior midgut. Both HGN1-Gal4 and Ilp7-Gal4 drive expression in the neurons projecting to the posterior midgut, hindgut pylorus, anterior hindgut, rectal pylorus and the rectum. Pr, Proventriculus; C, Crop; Py, Pylorus; RP, Rectal Pylorus; R, Rectum; VNC, Ventral Nerve Cord. The effects of activating (C) or inactivating (D) enteric neurons on hemolymph glucose (GMR51F12-Gal4, GMR48A05-Gal4, Ilp7-Gal4, or HGN1-Gal4; UAS-TRPA1 or UAS-shiTS1) (n = 6–10 replicates of 10 flies). (E) The effect of silencing the PENs in starvation conditions (Ilp7-Gal4 or HGN1-Gal4; UAS-shiTS1) (n = 6–9 replicates of 10 flies). * = p < 0.05, compared to corresponding UAS and Gal4 control. Significances indicated are based on ANOVA and Tukey post-hoc test. Data represent the average ± s.e.m. of the results obtained.
We first used these Gal4 lines to activate specific enteric neurons by expressing the temperature-sensitive ion channel, TRPA1, and measured the effects on hemolymph glucose levels (UAS-TRPA1 [Hamada et al., 2008]). Activation of the Ilp7-Gal4 neurons significantly decreases glucose levels in comparison to the Ilp7-Gal4 and UAS-TRPA1 controls (Figure 1C). Since Ilp7-Gal4 is also expressed in the CNS and in neurons projecting to the reproductive organs (Yang et al., 2008), we did similar experiment using HGN1-Gal4, which expresses in the PENSs, but not in the other Ilp7-Gal4 neurons (Cognigni et al., 2011), and obtained similar results on glucose levels (Figure 1C). We next examined enteric neurons projecting to other regions of the digestive system using GMR48A05-Gal4 and GMR51F12-Gal4 and did not observe any obvious effect (Figure 1C). We then assayed the effects of silencing these neurons by expressing the temperature-sensitive Dynamin, ShiTS1 (UAS-ShiTS1 [Kitamoto, 2013]). Silencing of the PENs using both Ilp7-Gal4 and HGN1-Gal4 increased glucose levels in comparison to the controls, while no effect was observed by silencing the GMR48A05-Gal4 and GMR51F12-Gal4 neurons (Figure 1D). Together, the data indicate that activities of the PENs are integral in the regulation of hemolymph glucose levels.
The change of glucose levels could result from differences in food intake. Previous work by Miguel-Aliaga and colleagues revealed that silencing Ilp7-Gal4 neurons increases defecation (Cognigni et al., 2011), which could be the result of increased feeding. We therefore examined the hypothesis that the activities of the PENs regulate food intake. Consistent with the hypothesis, we silenced the PENs in the absence of food and found that this abolished the gains in glucose levels (Figure 1E). We next investigated this directly using the capillary feeding assay (Ja et al., 2007). Silencing the PENs dramatically increased food intake (Figure 2A), which is consistent with the gains in glucose levels seen earlier. Conversely, activating these neurons caused dramatic decreases in feeding (Figure 2B). Together, manipulation of the activities of the PENs results in an overall six-fold change in feeding in comparison to the controls. This change is significantly larger than alterations by modulation of neuropeptide F signaling (Hong et al., 2012). These data indicate that the activities of the PENs play a prominent role in feeding.
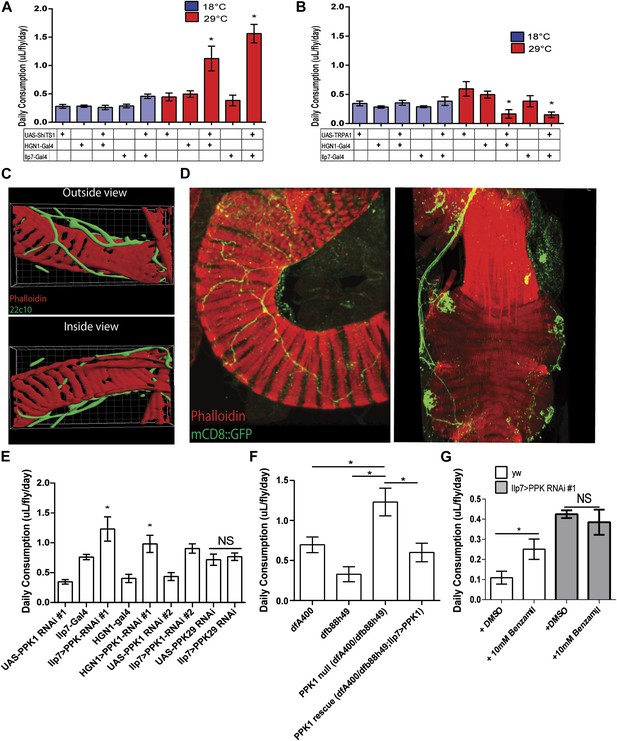
PPK1 functions in Drosophila PENs to regulate feeding.
(A–B) Results of capillary feeding assays by either inactivating (A, Ilp7-Gal4 or HGN1-Gal4; UAS-shiTS1) or activating (B, Ilp7-Gal4 or HGN1-Gal4; UAS-TRPA1) the PENs (n = 4–8 replicates). (C) Outside and inside views of the hindgut (red, phalloidin, muscle) with posterior enteric neuron projections (green, 22C10). (D) PPK1 expresses in the PENs projecting to the hindgut pylorus (left) and rectum (right) (PPK1-Gal4;UAS-mCD8::GFP). (E) The effect of PPK1 knock-down on food intake (Ilp7-Gal4 or HGN1-Gal4, UAS-PPK1-RNAi#1 or UAS-PPK1-RNAi#2) (n = 3–8 replicates). (F) Food intake results for PPK1 deficiency (dfb88h49/dfA400) and rescued animals (dfb88h49/dfA400; Ilp7-Gal4, UAS-PPK1) (n = 4–7 replicates). (G) Food intake results when PPK1 is inhibited using benzamil in wild-type or Ilp7 > PPK1 RNAi #1 flies (n = 8–10 replicates). * = p < 0.05, compared to corresponding UAS and Gal4 control or indicated controls. Significances indicated are based on ANOVA and Tukey post-hoc test. Data represent the average ± s.e.m. of the results obtained.
As described above, experiments in mammals indicated that mechanic tension in the gastrointestinal tract could be a satiety signal (Cannon and Washburn, 1911; Hargrave and Kinzig, 2012). To determine whether the role of the PENs in feeding is related to mechanosensing activity, we first examined the projections of these neurons and found that they tightly wrap around the muscle layer rather than projecting into the lumen of the gut (Figure 2C). This anatomy favors an involvement of mechanosensory activity rather than in detecting nutritional signals in gastrointestinal tract.
Previous studies in Caenorhabditis elegans, Drosophila, and mice have shown that members of the Degenerin/Epithelial Sodium Channels (DEG/ENaCs) function as a conserved family of mechanosensory ion channels (O'Hagan et al., 2005; Hwang et al., 2007; Zhong et al., 2010). Mutants of the C. elegans DEG/ENaC Mec-4 and its Drosophila homolog, PPK1, are touch-insensitive and the affected neurons fail to generate action potentials in response to mechanic tension (O'Hagan et al., 2005; Hwang et al., 2007; Zhong et al., 2010). This raised the possibility that PPK1 could be involved in regulating feeding in the PENs. We thus examined PPK1 expression using PPK1-Gal4 driving mCD8::GFP and confirmed its presence in the PENs (Figure 2D). To test whether the function of PPK1 in the PENs is involved in the regulation of feeding, we first assayed the effect of RNAi knockdown of PPK1 expression (Ilp7-Gal4 or HGN1-Gal4//UAS-PPK1-RNAi). Knockdown of PPK1 in the PENs, but not knockdown of PPK29, a related family member (Thistle et al., 2012), dramatically increased feeding (Figure 2E), phenocopying the effects of silencing these neurons. Next, we examined the effect of PPK1 deficiency on feeding and found that PPK1 deficient flies have increased food intake (Figure 2F). This feeding defect is rescued by expressing a PPK1 transgene in the PENs (Ilp7-Gal4, UAS- PPK1 (Ainsley et al., 2014), Figure 2F). Finally, pharmacological inhibitor of DEG/ENaCs, benzamil, has been used to antagonize PPK1 in Drosophila and homologs in mice (Liu et al., 2003; Page et al., 2007). We therefore investigated the effect of benzamil on feeding and found it increased food consumption when supplemented in fly food, but not in flies where PPK1 is knocked down (Figure 2G). Together, these data indicate that the mechanosensory ion channel, PPK1, plays a critical role in the PENs for regulating feeding.
The identification of the involvement of the DEG/ENaC mechanosensory ion channels in enteric neurons for regulating feeding lays the groundwork for investigating mechanisms underlying the phenomenon of fullness sensation. Pharmacological interventions on appetite stimulation and suppression are important for many diseases including obesity, cancer, and AIDS. Currently, all three FDA-approved weight-loss drugs have significant side-effects that target either the hypothalamus or fat absorption (Manning et al., 2014). Enteric DEG/ENaCs provide an attractive alternative for drug development due to their druggability, pharmacological accessibility, and fewer side-effect complications than the central nervous system. Overall, our findings indicate an important role of the DEG/ENaC mechanosensory ion channels in the enteric nervous system in food intake and suggest an exciting therapeutic alternative for fighting obesity.
Materials and methods
Fly stocks
Request a detailed protocolFlies were reared at 25°C on standard cornmeal-molasses medium, unless indicated otherwise. The following stocks were used in this study: GMR51F12-Gal4 (Jenett et al., 2012) (Bloomington Stock Center), GMR48A05-Gal4 (Jenett et al., 2012) (Bloomington Stock Center), Ilp7-Gal4 and HGN1-Gal4 were gifts from Dr Irene Miguel-Aliaga (Cognigni et al., 2011), UAS-ShiTS1 was a gift from Toshihiro Kitamoto (Kitamoto, 2013), UAS-TRPA1 (Bloomington Stock Center), 1XUAS-cd8::GFP ([Pfeiffer et al., 2010], Bloomington Stock Center), PPK1-Gal4 ([Ainsley et al., 2014], Bloomington Stock Center), UAS-PPK1 was a gift from Wayne Johnson (Ainsley et al., 2008), UAS-PPK1 RNAi #1 (Bloomington Stock Center), UAS-PPK1 RNAi #2 (Vienna Drosophila RNAi Center, 108683), UAS-PPK29 RNAi (Bloomington Stock Center), b88h49df (Bloomington Stock Center), A400df (Bloomington Stock Center) and yw flies.
Hemolymph glycemia measurements
Request a detailed protocolCrosses were performed at 18°C. 2-day old male flies of the indicated genotypes were incubated at 29°C in groups of ten for 24 hr and starved for 5 hr. Hemolymph was then collected as described (Haselton et al., 2010) and subjected to a glucose assay (Glucose Hexokinase Liquid Stable Reagent, Thermo Scientific, Waltham, Massachusetts, USA).
Capillary feeding assays
Request a detailed protocolFlies were raised at 18°C. Capillary feeding assays were performed as described (Ja et al., 2007) on 2-day old males in groups of four at 29°C for 24 hr. The diet was a 5% yeast extract and 5% sucrose solution. For the benzamil experiment, male yw flies were provided food with 100 mM sucrose supplemented with either 10 mM benzamil or DMSO. The concentration was chosen based upon previous work (Liu et al., 2003). Green food coloring (1:100, McCormick, Sparks, Maryland, USA) was added to the food to track consumption.
Drosophila immunohistochemistry
Request a detailed protocolDrosophila guts were dissected and fixed as previously described (Cognigni et al., 2011). The following antibodies and fluorescent markers were used: rabbit Anti-GFP antibody (ab290; 1:1000; Abcam, Cambridge, UK). Alexa Fluor 488 Goat Anti-Rabbit IgG (H + L) (A11034; 1:800; Life Technologies, Gaithersburg, MD, USA), mAb22C10 (Developmental Studies Hybridoma Bank, University of Iowa), and Alexa Fluor 633 phalloidin (A22284; 1:250; Life Technologies). For the three-dimensional model of the posterior enteric neuron region, a z-stack series of confocal images were taken from a gut sample immunostained with mAb22C10 and Alexa Fluor 633 phalloidin and then converted into a model using Imaris. All images were acquired using a Zeiss LSM510 and analyzed using Imaris (Bitplane, Zurich, Switzerland).
Statistics analyses
Request a detailed protocolAll data, presented as average ± s.e.m. or average ± s.d., were analyzed with GraphPad Prism 6. Unless indicated otherwise, unpaired Student's t test was used to determine differences between groups in each panel. For the rescue experiment, the results were compared by ANOVA followed by Tukey post hoc test. Data for each experiment met the assumption of the statistical tests. The sample size, as indicated in the figure legends, was chosen based on similar experiments reported previously, and was large enough to eliminate the variance between the groups before testing. No samples or animals were excluded from statistical analysis. All studies had been repeated for more than three times. The experimental groups were allocated randomly, and no blinding was done during allocation.
References
-
Repeated gastric distension alters food intake and neuroendocrine profiles in ratsPhysiology & Behavior 105:975–981.https://doi.org/10.1016/j.physbeh.2011.11.006
-
Nociceptive neurons protect Drosophila larvae from parasitoid waspsCurrent Biology 17:2105–2116.https://doi.org/10.1016/j.cub.2007.11.029
-
Prandiology of Drosophila and the CAFE assayProceedings of the National Academy of Sciences of USA 104:8253–8256.https://doi.org/10.1073/pnas.0702726104
-
Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearanceProceedings of the National Academy of Sciences of USA 100:2128–2133.https://doi.org/10.1073/pnas.252785099
-
Pharmacotherapy for obesity: novel agents and paradigmsTherapeutic Advances in Chronic Disease 5:135–148.https://doi.org/10.1177/2040622314522848
Article and author information
Author details
Funding
Howard Hughes Medical Institute
- Tian Xu
National Institutes of Health (T32 GM007499)
- William H Olds
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Bloomington Stock Center (NIH P40OD018537), VDRC, Wayne Johnson, Toshihiro Kitamoto, and Irene Miguel-Aliaga for fly strains, the Xu lab members for critical reading of the manuscript. WHO is a pre-doctoral fellow and supported by Genetics Training Grant T32 GM007499. TX is a Howard Hughes Medical Institute investigator.
Copyright
© 2014, Olds and Xu
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,865
- views
-
- 441
- downloads
-
- 26
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
When holding visual information temporarily in working memory (WM), the neural representation of the memorandum is distributed across various cortical regions, including visual and frontal cortices. However, the role of stimulus representation in visual and frontal cortices during WM has been controversial. Here, we tested the hypothesis that stimulus representation persists in the frontal cortex to facilitate flexible control demands in WM. During functional MRI, participants flexibly switched between simple WM maintenance of visual stimulus or more complex rule-based categorization of maintained stimulus on a trial-by-trial basis. Our results demonstrated enhanced stimulus representation in the frontal cortex that tracked demands for active WM control and enhanced stimulus representation in the visual cortex that tracked demands for precise WM maintenance. This differential frontal stimulus representation traded off with the newly-generated category representation with varying control demands. Simulation using multi-module recurrent neural networks replicated human neural patterns when stimulus information was preserved for network readout. Altogether, these findings help reconcile the long-standing debate in WM research, and provide empirical and computational evidence that flexible stimulus representation in the frontal cortex during WM serves as a potential neural coding scheme to accommodate the ever-changing environment.
-
- Neuroscience
When navigating environments with changing rules, human brain circuits flexibly adapt how and where we retain information to help us achieve our immediate goals.





