Camouflage: Tiny particles help insects evade predators
The wings of leafhoppers – tiny insects that feed on the sap in plants – are coated with hollow spheres called brochosomes. These structures are extremely small, typically ranging from 200 to 600 nanometers in diameter (Figure 1), and have fascinated entomologists since they were discovered in the 1950s (Tulloch et al., 1952; Tulloch and Shapiro, 1954).
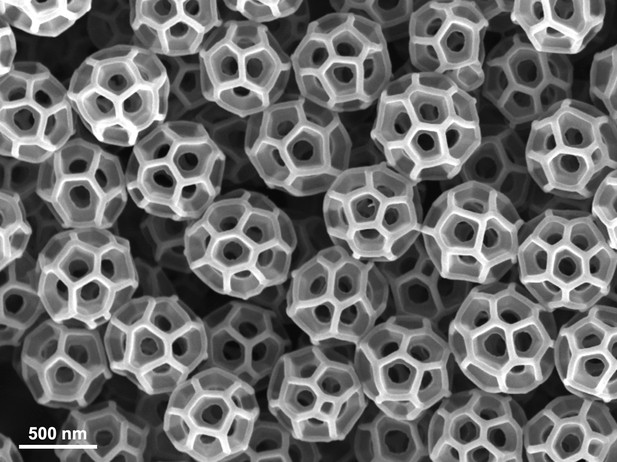
The structure of brochosomes.
A scanning-electron microscope image showing brochosomes produced by the leafhopper species Gyponana serpenta. The name brochosome comes from the Greek words for net (brochos) and body (soma), and the near-spherical shape of these nanoparticles resembles the structure of Buckminsterfullerene (a much smaller molecule formed of 60 carbon atoms). Wu et al. demonstrated how brochosomes reduce the reflection of ultraviolet light, thus making it more difficult for various predators to see leafhoppers.
Image credit: Lin Wang and Tak-Sing Wong (CC BY 4.0).
Brochosomes are secreted through droplets and are spread across the body by the hind legs of the insect during a repetitive grooming process (Rakitov, 1996). They also have properties that are thought to help leafhoppers perform various roles, such as repelling water and keeping themselves clean (Rakitov and Gorb, 2013a; Rakitov and Gorb, 2013b).
It has long been believed that brochosomes may also provide a camouflage coating that allows leafhoppers to hide from predators, such as birds and jumping spiders (Swain, 1936). These predators are able to see a broad range of wavelengths, including ultraviolet light (Cronin and Bok, 2016). This creates intense evolutionary pressure for insects like leafhoppers to develop mechanisms that reduce their visibility.
Previous research revealed that brochosomes reflect light poorly at both visible and ultraviolet wavelengths (Yang et al., 2017; Wang et al., 2024b). Now, in eLife, Wei Wu, Jian-Ping Chen and colleagues at Ningbo University and the Zhejiang Academy of Agricultural Sciences report the first direct evidence that this camouflage function protects leafhoppers from jumping spiders (Wu et al., 2024).
To investigate how effective brochosomes are at reducing reflection at ultraviolet wavelengths, Wu et al. studied the forewings of leafhoppers 5 and 25 days after they had emerged from their eggs and developed into adults. Their findings showed that 5-day-old leafhoppers, which have high levels of brochosome coverage (90%), exhibited less reflectance than 25-day-old leafhoppers, which have lower levels of coverage (10–40%).
Wu et al. then measured the hunting efficiency of the jumping spiders against 5-day-old and 25-day-old leafhoppers. This revealed that leafhoppers with less brochosomes were captured more quickly than those with more extensive coverage. In addition, the percentage of leafhoppers killed by the spider (known as the predation rate) was higher for populations with reduced brochosome coverage (66.7% vs 33% for males; 81.7% vs 18.3% for females). These findings provide direct evidence that brochosomes enhance leafhopper survival by reducing the reflection of ultraviolet light, making it more difficult for predators to see the leafhoppers.
Another important contribution by Wu et al. was to identify four novel structural proteins that help to synthesize brochosomes. Blocking the production of these proteins, using a tool called RNA interference, altered the shape of the brochosomes. Moreover, leafhopper wings coated with these misshapen nanoparticles reflected more ultraviolet light, leading to a higher rate of predation. Further analysis investigating the ancestry of the four genes that code for these proteins suggests that brochosomes may have evolved through gene duplication events and by accumulating genetic changes over time.
This study represents an important step in understanding the broader biological functions of brochosomes. By demonstrating how these structures protect leafhoppers from their natural predators, Wu et al. provide compelling evidence of the role of brochosomes in antireflective camouflage. An intriguing open question remains: do variations in the size and geometry of brochosomes help leafhoppers evade specific predators in different environments? The techniques developed in this work could help answer this and other questions.
Beyond their importance in biology, brochosomes offer a blueprint for new optical materials that could be useful for applications in solar energy, advanced coatings and camouflage technology. However, replicating the complex structure of brochosomes using synthetic methods at scale remains difficult (Wang et al., 2024a). The work of Wu et al. could help to inspire novel approaches for addressing these challenges.
References
-
Photoreception and vision in the ultravioletThe Journal of Experimental Biology 219:2790–2801.https://doi.org/10.1242/jeb.128769
-
Post-moulting behaviour associated with Malpighian tubule secretions in leafhoppers and treehoppers (Auchenorrhyncha: Membracoidea)Eur. J. Entomology 93:167–184.
-
Brochosomal coats turn leafhopper (Insecta, Hemiptera, Cicadellidae) integument to superhydrophobic stateProceedings of the Royal Society B. Biological Sciences 280:20122391.https://doi.org/10.1098/rspb.2012.2391
-
Brochosomes protect leafhoppers (Insecta, Hemiptera, Cicadellidae) from sticky exudatesJournal of the Royal Society Interface 10:20130445.https://doi.org/10.1098/rsif.2013.0445
-
Notes on the oviposition and life history of the leafhopper Oncometopta undata FabrEntomological News 47:264–266.
-
The occurrence of ultramicroscopic bodies with leafhoppers and mosquitoesBull. Brooklyn Entomol. Soc 47:41–42.
-
Ultra-antireflective synthetic brochosomesNature Communications 8:1285.https://doi.org/10.1038/s41467-017-01404-8
Article and author information
Author details
Publication history
Copyright
© 2024, Wang and Wong
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 611
- views
-
- 65
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Ecology
- Evolutionary Biology
Eurasia has undergone substantial tectonic, geological, and climatic changes throughout the Cenozoic, primarily associated with tectonic plate collisions and a global cooling trend. The evolution of present-day biodiversity unfolded in this dynamic environment, characterised by intricate interactions of abiotic factors. However, comprehensive, large-scale reconstructions illustrating the extent of these influences are lacking. We reconstructed the evolutionary history of the freshwater fish family Nemacheilidae across Eurasia and spanning most of the Cenozoic on the base of 471 specimens representing 279 species and 37 genera plus outgroup samples. Molecular phylogeny using six genes uncovered six major clades within the family, along with numerous unresolved taxonomic issues. Dating of cladogenetic events and ancestral range estimation traced the origin of Nemacheilidae to Indochina around 48 mya. Subsequently, one branch of Nemacheilidae colonised eastern, central, and northern Asia, as well as Europe, while another branch expanded into the Burmese region, the Indian subcontinent, the Near East, and northeast Africa. These expansions were facilitated by tectonic connections, favourable climatic conditions, and orogenic processes. Conversely, aridification emerged as the primary cause of extinction events. Our study marks the first comprehensive reconstruction of the evolution of Eurasian freshwater biodiversity on a continental scale and across deep geological time.
-
- Ecology
- Neuroscience
Prey must balance predator avoidance with feeding, a central dilemma in prey refuge theory. Additionally, prey must assess predatory imminence—how close threats are in space and time. Predatory imminence theory classifies defensive behaviors into three defense modes: pre-encounter, post-encounter, and circa-strike, corresponding to increasing levels of threat—–suspecting, detecting, and contacting a predator. Although predatory risk often varies in spatial distribution and imminence, how these factors intersect to influence defensive behaviors is poorly understood. Integrating these factors into a naturalistic environment enables comprehensive analysis of multiple defense modes in consistent conditions. Here, we combine prey refuge and predatory imminence theories to develop a model system of nematode defensive behaviors, with Caenorhabditis elegans as prey and Pristionchus pacificus as predator. In a foraging environment comprised of a food-rich, high-risk patch and a food-poor, low-risk refuge, C. elegans innately exhibits circa-strike behaviors. With experience, it learns post- and pre-encounter behaviors that proactively anticipate threats. These defense modes intensify with predator lethality, with only life-threatening predators capable of eliciting all three modes. SEB-3 receptors and NLP-49 peptides, key stress regulators, vary in their impact and interdependence across defense modes. Overall, our model system reveals fine-grained insights into how stress-related signaling regulates defensive behaviors.






