Autoimmunity: The epigenetic trajectory of type 1 regulatory T cells
Autoimmunity – when the immune system attacks healthy tissues in an individual – is often due to the failure of regulatory mechanisms to suppress immune cells that react to healthy tissue, which are known as ‘autoreactive’. Historically, steroids have been used to treat autoimmunity by dampening the activity of the entire immune system. However, this systemic suppression makes individuals more susceptible to infection. More recently, immunotherapies have been designed to target specific cell types or signaling pathways. While these therapeutics can block autoreactive cells, they often still block non-autoreactive cells too. A better understanding of how immune cells are regulated could help to develop therapies that specifically target autoreactive cells.
Several cell types are involved in suppressing immune cell activity, with perhaps the most characterized being regulatory T cells (or Tregs for short). These cells can suppress other immune cells indirectly by producing cytokines and directly through interacting with co-stimulatory molecules on the surface of cells that initiate immune responses. Less is known about a distinct subset of Tregs called type 1 regulatory T cells (known as TR1 cells for short) and their role in suppressing immune responses (Roncarolo et al., 2018). Now, in eLife, Pere Santamaria and colleagues from the Institut D’Investigacions Biomèdiques August Pi I Sunyer and The University of Calgary – including Josep Garnica as first author – report insights into the mechanisms regulating the gene activity required for TR1 cells to develop (Garnica et al., 2024).
Previous work from the same laboratory showed that T follicular helper (TFH) cells – which are involved in helping B cells generate antibodies – can be re-programmed into TR1 cells when exposed to nanoparticles coated with disease-specific peptides. These nanoparticles function as a delivery system, presenting the peptides to TFH cells as an approach for treating autoimmunity (Clemente-Casares et al., 2016). The resulting TR1 cells reversed autoimmunity without impairing normal functioning of the immune system (Singha et al., 2017; Umeshappa et al., 2019; Solé et al., 2023a). A key transcription factor, known as BLIMP-1 was identified as the master regulator of this conversion process (Solé et al., 2023b).
Building on this previous work, Garnica et al. employed several tools to examine how the control of gene activity, or ‘epigenetics’, affects the re-programming of TFH cells into TR1 cells. The accessibility of chromatin – a complex of DNA and proteins that compacts DNA into chromosomes – can influence the likelihood of gene expression. Analyzing chromatin accessibility in each of the cell types showed that several chromatin regions close during conversion, while several others open. Furthermore, thousands of genes were expressed differently between TFH and TR1 cells. Indeed, the upregulated genes corresponded to regions of chromatin that remained accessible, while the downregulated genes corresponded to chromatin regions that closed during the conversion process.
Garnica et al. next looked at post-translational changes to DNA and the histone proteins that bind to it to see if they also affect gene expression during TFH to TR1 cell conversion (Figure 1). For the genes upregulated in TR1 cells but not expressed in TFH cells (despite having accessible chromatin), their analysis revealed that these genes already had their respective histone marks at the TFH stage. Another way to control gene expression is to modify DNA by adding a methyl group via a process called methylation, which tends to make genes less likely to be expressed. Similarly to the histone markers, the methylation status of TR1 cells was already present at the TFH cell stage, suggesting that changes in gene expression during conversion are not due to DNA methylation changes.
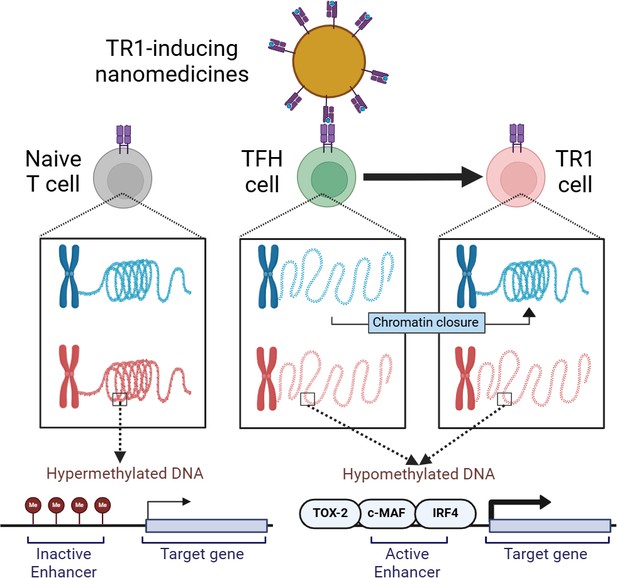
How epigenetics drives the conversion of T follicular helper cells into immunosuppressive Type 1 regulatory T cells.
TR1-relevant chromatin regions (blue) are inaccessible in naïve T cells (gray; left) and the DNA at relevant sites is highly methylated in order to keep them inactive. On the other hand, the same sites in TFH cells (green; middle) become active through demethylation. This ensures TFH cells are poised to be converted into TR1 cells (pink) when TR1-inducing nanomedicines (yellow; top) activate them through self-surface molecules (blue and pink structures). This leads to chromatin closure in certain regions and expression of active enhancer-containing genes through transcription factors such as TOX-2, c-MAF, and IRF4. TR1: T-regulatory type-1 cell, TFH cell: T-follicular helper cell.
This figure was created with BioRender.com.
These findings prompted Garnica et al. to hypothesize that the conversion process is fueled by changes in the expression and binding of transcription factors that either stabilize TFH cells or promote TR1 cells. Intriguingly, losing TFH-specific transcription factor gene expression during conversion was associated with chromatin closure. For the relevant chromatin regions that remain open during the conversion process, TR1 cells inherited stretches of DNA, called enhancers, from their TFH precursors, which increase gene expression. Whereas in naïve T cells these genes were highly methylated and inaccessible, in TFH and TR1 cells they were less methylated and instead were enriched in binding sites for TFH-transcription factors such as TOX-2, IRF4, and c-MAF. Taken together, the findings suggest that the TR1 transcriptional program is genetically imprinted at the TFH cell stage.
While Garnica et al. have shown the ability of TR1 cells to reverse autoimmunity, TR1 cells have also been shown to hinder some cancer immunotherapies (Sultan et al., 2024). TR1 cells that developed in response to high doses of antigen impaired tumor rejection, even when immune checkpoint therapy was used to boost the immune response against the cancer cells. Therefore, TR1 cells are a double-edged sword in preventing undesirable autoimmunity and simultaneously suppressing desirable immune responses.
Therapeutically, one could imagine harnessing the beneficial anti-autoimmune effects of TR1 cells in an antigen-specific manner while avoiding their detrimental pro-cancer activity. However, doing so requires a thorough understanding of TR1 development and function in both homeostasis and disease. The findings of Garnica et al. provide the first robust examination of the epigenetic changes that occur during TFH-to-TR1 conversion. Current research by the same team is ongoing to examine how different transcription factors affect the transcriptional and chromatin changes during TFH-TR1 conversion.
References
-
A T follicular helper cell origin for T regulatory type 1 cellsCellular & Molecular Immunology 20:489–511.https://doi.org/10.1038/s41423-023-00989-z
Article and author information
Author details
Publication history
Copyright
© 2024, Turicek and Wan
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 474
- views
-
- 51
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Immunology and Inflammation
FOXP3-expressing regulatory T (Treg) cells play a pivotal role in maintaining immune homeostasis and tolerance, with their activation being crucial for preventing various inflammatory responses. However, the mechanisms governing the epigenetic program in Treg cells during their dynamic activation remain unclear. In this study, we demonstrate that CXXC-finger protein 1 (CXXC1) interacts with the transcription factor FOXP3 and facilitates the regulation of target genes by modulating H3K4me3 deposition. Cxxc1 deletion in Treg cells leads to severe inflammatory disease and spontaneous T cell activation, with impaired immunosuppressive function. As a transcriptional regulator, CXXC1 promotes the expression of key Treg functional markers under steady-state conditions, which are essential for the maintenance of Treg cell homeostasis and their suppressive functions. Epigenetically, CXXC1 binds to the genomic regulatory regions of Treg program genes in mouse Treg cells, overlapping with FOXP3-binding sites. Given its critical role in Treg cell homeostasis, CXXC1 presents itself as a promising therapeutic target for autoimmune diseases.
-
- Immunology and Inflammation
- Microbiology and Infectious Disease
The members of the Mycobacterium tuberculosis complex (MTBC) causing human tuberculosis comprise 10 phylogenetic lineages that differ in their geographical distribution. The human consequences of this phylogenetic diversity remain poorly understood. Here, we assessed the phenotypic properties at the host-pathogen interface of 14 clinical strains representing five major MTBC lineages. Using a human in vitro granuloma model combined with bacterial load assessment, microscopy, flow cytometry, and multiplexed-bead arrays, we observed considerable intra-lineage diversity. Yet, modern lineages were overall associated with increased growth rate and more pronounced granulomatous responses. MTBC lineages exhibited distinct propensities to accumulate triglyceride lipid droplets—a phenotype associated with dormancy—that was particularly pronounced in lineage 2 and reduced in lineage 3 strains. The most favorable granuloma responses were associated with strong CD4 and CD8 T cell activation as well as inflammatory responses mediated by CXCL9, granzyme B, and TNF. Both of which showed consistent negative correlation with bacterial proliferation across genetically distant MTBC strains of different lineages. Taken together, our data indicate that different virulence strategies and protective immune traits associate with MTBC genetic diversity at lineage and strain level.






