Neurodegeneration: A role for RNA knots in Alzheimer’s disease
Memories are fundamental to any human being. They provide a sense of self-identity and shape our perception of the world and our loved ones. This is why neurogenerative diseases involving memory loss, like dementia, are highly debilitating, even when an individual’s physical fitness is not affected.
Alzheimer’s disease is the most common type of dementia, accounting for 60–70% of all cases, and it is projected that around 139 million will be suffering from the disease by 2050 (WHO, 2023). This means that a significant proportion of the world’s population will lose their precious memories in the foreseeable future, resulting in unsustainable pressure on the healthcare system. Given the lack of viable therapies currently available to treat Alzheimer’s, there is an urgent need to identify new molecular targets that can be leveraged to develop innovative therapeutic agents.
Although the exact mechanisms that lead to Alzheimer’s disease are not fully understood, it is well known that an uncontrolled build-up of insoluble protein aggregates in the brain is a hallmark of the disease. Amyloid-beta proteins have been shown to accumulate as clumps outside brain cells, whilst p-tau proteins clump to form neurofibrillary tangles that have been detected in the cytoplasm of neurons and other brain cells (Braak and Braak, 1995; Furman et al., 2017).
In this context, RNA molecules that stabilize protein aggregates are becoming increasingly relevant as potential therapeutic targets (Raguseo et al., 2023; Wolozin and Apicco, 2015). Recently, RNA structures called G-quadruplexes – known for their roles in gene expression and protein production – have emerged as key drivers of aggregate formation in brain cells affected by amyotrophic lateral sclerosis, raising the possibility that they may also be implicated in other neurodegenerative diseases, such as Alzheimer’s disease (Raguseo et al., 2023). Now, in eLife, Scott Horowitz and colleagues from the University of Denver, the Medical University of South Carolina, and the University of Colorado – including Lena Kallweit as first author – report that brain samples taken from Alzheimer’s disease patients show a significant accumulation of RNA G-quadruplex structures (Kallweit et al., 2025).
Kallweit et al. studied brain tissues from deceased people with Alzheimer’s disease aged between 30 and 92 years. Using a combination of immunostaining and fluorescence microscopy, the team showed that older patients had a higher number of RNA G-quadruplexes than younger patients. This connection became even stronger as the disease progressed. The prevalence of RNA G-quadruplexes further increased with the size of the area where p-tau aggregates accumulated.
Besides aging, genetic factors can also contribute to the development of Alzheimer’s disease. It is known that a specific variant of the gene APOE (known as APOE4) is linked to the accumulation of amyloid-beta aggregates (Cicognola et al., 2025). Taking this into account, Kallweit et al. showed that brain samples from patients with the APOE4 variant had a higher number of RNA G-quadruplexes than those without the variant. This suggests that genetic variations known to promote the accumulation of protein aggregates linked to Alzheimer’s disease could also potentially drive the accumulation of RNA G-quadruplex structures.
Taken together, the findings suggest that RNA G-quadruplexes may have an active role in neurodegeneration and support a model in which RNA G-quadruplexes could become tangled with p-tau proteins, leading to insoluble aggregates inside brain cells (Figure 1). This idea is further supported by previous research showing that RNA G-quadruplexes increase the aggregation of p-tau in experiments with cultured cells (Zwierzchowski-Zarate et al., 2022). This suggests that accumulating RNA G-quadruplexes in brain cells, especially when combined with genetic variations such as APOE4 and aging, may promote the formation of p-tau aggregates that drive Alzheimer’s disease.
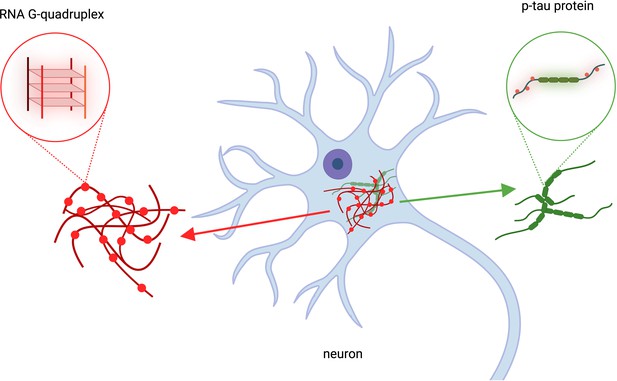
The role of RNA G-quadruplexes in Alzheimer’s Disease.
Kallweit et al. analyzed brain cells taken from deceased patients with Alzheimer’s disease. This revealed that RNA structures called RNA G-quadruplexes (red structures, left), which help regulate gene expression, accumulate in the same location as p-tau protein clusters (green, right). Clumps of protein p-tau and RNA G-quadruplexes overlapped in brain cells (blue structure in the center). This figure was created using BioRender.com.
The results of Kallweit et al. further highlight the potential of targeting RNA – including G-quadruplexes – to diagnose or treat neurodegenerative diseases (Abad-Yang et al., 2025). Given the high correlation with the severity of Alzheimer’s disease, RNA G-quadruplexes could be used as a biomarker for early Alzheimer’s disease development to help accelerate diagnoses. RNA targeting and degradation have been successfully employed in other conditions, which may one day be a reality for Alzheimer’s disease (Tong et al., 2024). In the future, small molecules that can target and dissolve RNA complexes may be the key to treating incurable neurodegenerative conditions like Alzheimer’s.
References
-
The potential of multimolecular G-quadruplex structures for targeted treatment of Amyotrophic Lateral SclerosisExpert Opinion on Therapeutic Targets 1:1–4.https://doi.org/10.1080/14728222.2025.2463361
-
Staging of Alzheimer’s disease-related neurofibrillary changesNeurobiology of Aging 16:00030-I.https://doi.org/10.1016/0197-4580(95)00030-I
-
APOE4 impact on soluble and insoluble tau pathology is mostly influenced by amyloid-beta 2Brain: A Journal of Neurology 1:awaf016.https://doi.org/10.1093/brain/awaf016/7958226
-
Widespread tau seeding activity at early Braak stagesActa Neuropathologica 133:91–100.https://doi.org/10.1007/s00401-016-1644-z
-
RNA binding proteins and the genesis of neurodegenerative diseasesAdvances in Experimental Medicine and Biology 822:11–15.https://doi.org/10.1007/978-3-319-08927-0_3
-
RNA induces unique tau strains and stabilizes Alzheimer’s disease seedsThe Journal of Biological Chemistry 298:102132.https://doi.org/10.1016/j.jbc.2022.102132
Article and author information
Author details
Publication history
Copyright
© 2025, Galli and Di Antonio
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 462
- views
-
- 47
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
The protein ligase Connectase can be used to fuse proteins to small molecules, solid carriers, or other proteins. Compared to other protein ligases, it offers greater substrate specificity, higher catalytic efficiency, and catalyzes no side reactions. However, its reaction is reversible, resulting in only 50% fusion product from two equally abundant educts. Here, we present a simple method to reliably obtain 100% fusion product in 1:1 conjugation reactions. This method is efficient for protein-protein or protein-peptide fusions at the N- or C-termini. It enables the generation of defined and completely labeled antibody conjugates with one fusion partner on each chain. The reaction requires short incubation times with small amounts of enzyme and is effective even at low substrate concentrations and at low temperatures. With these characteristics, it presents a valuable new tool for bioengineering.
-
- Biochemistry and Chemical Biology
N 6,2’-O-dimethyladenosine (m6Am) is a modified nucleotide located at the first transcribed position in mRNA and snRNA that is essential for diverse physiological processes. m6Am mapping methods assume each gene uses a single start nucleotide. However, gene transcription usually involves multiple start sites, generating numerous 5’ isoforms. Thus, gene-level annotations cannot capture the diversity of m6Am modification in the transcriptome. Here, we describe CROWN-seq, which simultaneously identifies transcription-start nucleotides and quantifies m6Am stoichiometry for each 5’ isoform that initiates with adenosine. Using CROWN-seq, we map the m6Am landscape in nine human cell lines. Our findings reveal that m6Am is nearly always a high stoichiometry modification, with only a small subset of cellular mRNAs showing lower m6Am stoichiometry. We find that m6Am is associated with increased transcript expression and provide evidence that m6Am may be linked to transcription initiation associated with specific promoter sequences and initiation mechanisms. These data suggest a potential new function for m6Am in influencing transcription.






