Heredity: The gene family that cheats Mendel
Many of the traits an individual has, from eye color to the risk of having certain diseases, are passed from parents to their children via their genes. In diploid organisms, such as humans, most cells contain two copies – or alleles – of every gene. The exceptions to this rule are gametes (that is, sperm and egg cells), which contain just one allele. According to Mendel’s famous law of segregation, half of the gametes will carry one allele for a given gene, and the other half will carry the other allele. Thus, both of the mother’s alleles have an equal chance of being passed on to her children, and likewise for the father’s alleles.
However, some alleles defy Mendel’s law and can increase their chances of being transmitted to the next generation by killing gametes that do not share the same alleles (Burt and Trivers, 2006). Genes harboring alleles that behave in this way have been identified in plants, fungi and animals – including humans – and are called by various names, including selfish drivers, gamete killers and spore killers.
There are many types of selfish drivers and much remains unclear about how they work, though they can generally be distinguished by the way they destroy other cells. In the ‘poison-antidote’ model, the selfish driver produces a poison that destroys all gametes unless an antidote is there to protect them from the effects of the poison (Figure 1A and B). In the ‘killer-target’ model, the selfish driver produces a poison that kills gametes that carry a specific target (Figure 1C).
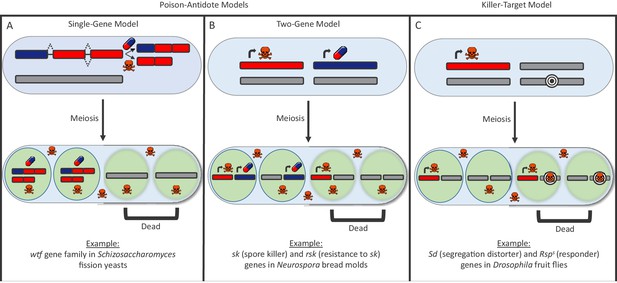
The poison-antidote and killer-target models of selfish drivers.
In both models, a particular allele has an increased chance of being passed on to the next generation because it produces a toxin to kill gametes that do not carry it. (A, B) In the poison-antidote model, cells produce a toxin (shown as skull-and-crossbones) that can be neutralized by an antidote (shown as a pill); the alleles that do not code for either are shown in gray. In the single-gene model (A) the same gene codes for both the poison and the antidote through alternative transcription. Nuckolls et al. show that the gene wtf4 is a selfish driver in Schizosaccharomyces yeasts. Hu et al. show that two other genes in the wtf family (cw9 and cw27) are also selfish drivers. In the two-gene model (B) different genes produce the poison and antidote, as in the fungus Neurospora (Hammond et al., 2012). (C) In the killer-target model, the toxin only destroys cells that contain alleles with a specific target marker (shown here by concentric black circles). This is the case in Drosophila, where the segregation distortion (Sd) allele acts by killing gametes that contain a sensitive Responder (Rsps) marker (Larracuente and Presgraves, 2012).
Aiming to understand how selfish drivers have evolved and work, two research groups – one led by Sarah Zanders and Harmit Malik, the other by Li-Lin Du – turned to two species of fission yeast, Schizosaccharomyces kambucha and S. pombe. These two species are genetically nearly identical, and some researchers do not even consider them as separate species (Rhind et al., 2011), but hybrids between the two are often sterile. In previous studies, Zanders and co-workers discovered that S. kambucha has at least three selfish drivers that cause infertility in the hybrids (Zanders et al., 2014).
To unravel the genetic identity of these selfish drivers in yeast, Zanders, Malik and co-workers at the Stowers Institute for Medical Research, the Fred Hutchinson Cancer Research Center and the University of Kansas Medical Center – including Nicole Nuckolls and Maria Angelica Bravo Núñez as joint first authors – isolated a region on a chromosome that caused selfish drive (Nuckolls et al., 2017). Within this region, they found a selfish-driver gene called wtf4 – a member of a large and cheekily-named gene family – which creates both a poison and an antidote.
To explore the underlying mechanisms in more detail, Nuckolls et al. created fluorescent versions of the poison and the antidote and mapped their location inside and around the gametes. These elegant experiments showed that wtf4’s poison can leave their originating cells and cross into surrounding cells while the antidote remains trapped inside the cells that produce it.
In an independent study, Du and co-workers at the National Institute of Biological Sciences in Beijing – including Wen Hu as first author – identified two other genes from the wtf gene family, named cw9 and cw27, as selfish drivers that also employ the poison-antidote model in crosses between different strains of S. pombe (Hu et al., 2017). They found that mutant diploid strains missing both copies of either cw9 or cw27 survived more than strains missing only one copy of the gene, indicating that both genes are selfish drivers. When they created diploid mutants missing a copy of cw9 and cw27, the yeast strains survived even less compared to strains missing a copy of only one of the two genes. This suggests that the two genes do not rescue each other and that they act independently to drive survival.
By identifying several genes within the same family that can kill cells that are different, and by exploring their mode of action, the work of these two groups enriches our understanding of the genes that break Mendel’s acclaimed genetic law. Future work in this area will help us to understand the impact of selfish elements on genetic diversity and may lead to a deeper understanding of how these mechanisms affect conditions such as infertility in species as diverse as plants, fungi and animals – including humans.
References
-
BookGenes in Conflict: The Biology of Selfish Genetic ElementsHarvard University Press.https://doi.org/10.4159/9780674029118
Article and author information
Author details
Publication history
Copyright
© 2017, Shropshire et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 4,772
- views
-
- 356
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Genetics and Genomics
Spore killers in fungi are selfish genetic elements that distort Mendelian segregation in their favor. It remains unclear how many species harbor them and how diverse their mechanisms are. Here, we discover two spore killers from a natural isolate of the fission yeast Schizosaccharomyces pombe. Both killers belong to the previously uncharacterized wtf gene family with 25 members in the reference genome. These two killers act in strain-background-independent and genome-location-independent manners to perturb the maturation of spores not inheriting them. Spores carrying one killer are protected from its killing effect but not that of the other killer. The killing and protecting activities can be uncoupled by mutation. The numbers and sequences of wtf genes vary considerably between S. pombe isolates, indicating rapid divergence. We propose that wtf genes contribute to the extensive intraspecific reproductive isolation in S. pombe, and represent ideal models for understanding how segregation-distorting elements act and evolve.






