Malaria: On a mission to block transmission
Life inside a human host is fraught with danger. Plasmodium falciparum malaria parasites need to be highly adapted to compete for resources and to take their chances with the immune system. The same degree of specialization is needed to survive inside the mosquito, and for the transitions from human to mosquito and vice versa. These seemingly incompatible needs are met by a life cycle that involves morphing into a series of different forms.
Under the microscope, a blood sample may reveal different stages of the parasite: asexual parasites that replicate and prolong the infection, and sexual stages called gametocytes, the form that transmits the infection from human to mosquito. Both male and female gametocytes arise when a small proportion of asexual parasites stop replication and commit to sexual development instead. After maturation they are either taken up by a feeding mosquito or die. Sex and transmission are inextricably linked. Fertilization occurs inside the mosquito gut within an hour of feeding and leads to several developmental phases inside the insect vector, after which parasites are ready to infect a new human host (Sinden, 2017).
Most licensed antimalarial drugs target the asexual forms of the parasite. These stages are the most abundant and cause the symptoms of malaria (Sinden, 2017; Nilsson et al., 2015). As the burden of disease has declined in many regions and the focus is shifting towards malaria elimination, the searchlight beam now falls onto other stages. On paper, gametocytes are a good target to break the circle of transmission: they are the only form capable of infecting mosquitoes and their low number creates a population bottleneck.
However, efforts to introduce new drugs and vaccines that target gametocytes and prevent malaria transmission face an obstacle: there is no method to directly evaluate their success in humans. Clinical studies in malaria patients can demonstrate the effect of drugs on asexual parasites, but often fail to capture the dynamics of gametocytes for simple reasons. Gametocytes generally occur at very low densities and are often missed by standard detection methods. They sequester away from the blood stream for eight to twelve days as they mature, and often are not seen at the time of illness. Consequently, the evaluation of drugs is mostly limited to unnatural conditions, such as measuring gametocyte densities in cell cultures or artificially feeding blood infected with gametocytes to mosquitoes.
Now, in eLife, Robert Sauerwein, Teun Bousema and colleagues – including Isaie Reuling as first author – report a promising method to fill this void (Reuling et al., 2018). The researchers – who are based at the Radboud University Medical Center and other universities and non-profit organizations in the UK, United States, Australia and the Netherlands – drew on recent scientific developments in sensitive molecular diagnostic methods, the stage-specificity of drugs and the resurgence of interest in human challenge studies (Farid et al., 2017; Stanisic et al., 2018; Stone et al., 2017).
Reuling et al. deliberately infected volunteers with P. falciparum using Anopheles mosquitoes. The ensuing asexual parasite densities were controlled and eventually cleared using different drugs while allowing the gametocytes to continue development under safe conditions. All of the volunteers yielded circulating gametocytes.
A crucial question is how well this method of generating gametocytes in humans would work to evaluate a compound that blocks transmission. Effects on gametocyte densities can be shown (Collins et al., 2018). However, such densities are only loosely related to transmission success (Churcher et al., 2013). The most relevant measure would be whether or not feeding mosquitoes become infected. Reuling et al. observed that the low gametocyte numbers in the volunteers only sporadically infected mosquitoes, and are aware that this renders conclusions about transmission-blocking activity impossible.
Changing certain elements of the study setup, for example using different drugs, treatment doses, P. falciparum laboratory strains or mosquito species, may overcome this issue. Another possibility could be to enhance gametocyte production by inducing sexual commitment. However, we are only beginning to understand the triggers for this process (Bechtsi and Waters, 2017). Recently, a human lipid was shown to inhibit sexual commitment (Brancucci et al., 2017). Interfering with the underlying regulatory pathway could be exploited in the future. But regardless of how these problems are tackled, questions of how well the system translates into real life settings would need to be addressed carefully.
A side-effect of piloting the method of controlled human infection is the insight into the secret lives of gametocytes. Previously, the most detailed studies of gametocyte dynamics had been made when deliberate infections were used to treat neurosyphilis patients at a time before antibiotics were available (Snounou and Pérignon, 2013). By using highly sensitive and sex-specific detection methods, Reuling et al. add to the pool of knowledge and provide evidence that mature male and female gametocytes first appear in the blood at different times, and that female gametocytes circulate in the blood for longer. The early appearance of mature gametocytes in the peripheral blood of the volunteers further suggests that a fraction of the first wave of parasites entering the blood stream can already be sexually committed.
A system to evaluate potential transmission-blocking strategies in vivo is hugely appealing. In future, an optimized setup that can achieve a higher proportion of infected mosquitoes may help to discover new ways to prevent malaria transmission. The work of Reuling et al. has opened a door.
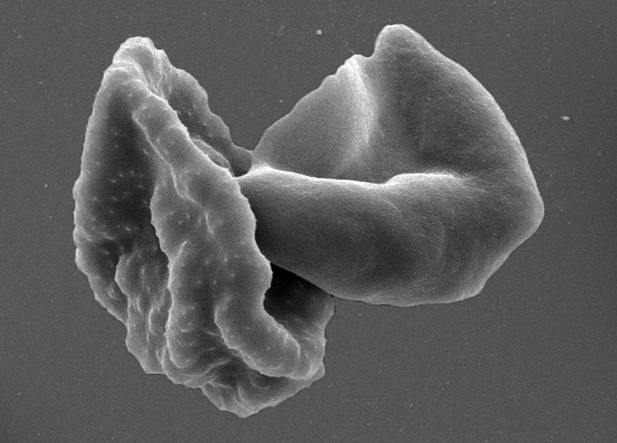
Red blood cells infected with Plasmodium falciparum parasites.
Malaria parasites have a complex life cycle and different forms of the parasites can be found in the blood. This scanning electron micrograph shows red blood cells infected with the parasite: an asexual stage on the left and a mature gametocyte on the right. Only the gametocytes can infect mosquitoes.
© Brand, 2018. Image courtesy of Françoise Brand, Swiss Tropical and Public Health Institute and University of Basel. Published under a CC BY 4.0 license.
References
-
Genomics and epigenetics of sexual commitment in PlasmodiumInternational Journal for Parasitology 47:425–434.https://doi.org/10.1016/j.ijpara.2017.03.002
-
A controlled human malaria infection model enabling evaluation of transmission-blocking interventionsJournal of Clinical Investigation 98012.https://doi.org/10.1172/JCI98012
-
Initiation of gametocytogenesis at very low parasite density in Plasmodium falciparum infectionThe Journal of Infectious Diseases 215:1167–1174.https://doi.org/10.1093/infdis/jix035
-
Targeting human transmission biology for malaria eliminationPLoS Pathogens 11:e1004871.https://doi.org/10.1371/journal.ppat.1004871
-
Malariotherapy--insanity at the service of malariologyAdvances in Parasitology 81:223–255.https://doi.org/10.1016/B978-0-12-407826-0.00006-0
-
Controlled human malaria infection: applications, advances, and challengesInfection and Immunity 86:e00479-17.https://doi.org/10.1128/IAI.00479-17
Article and author information
Author details
Acknowledgements
The authors thank Eilidh Carrington, Natalie Hofmann, Melissa Penny, Matthias Rottmann, Tom Smith and Till Voss for helpful discussions.
Publication history
Copyright
© 2018, Ross et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,337
- views
-
- 454
- downloads
-
- 3
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Genetics and Genomics
- Microbiology and Infectious Disease
Polyamines are biologically ubiquitous cations that bind to nucleic acids, ribosomes, and phospholipids and, thereby, modulate numerous processes, including surface motility in Escherichia coli. We characterized the metabolic pathways that contribute to polyamine-dependent control of surface motility in the commonly used strain W3110 and the transcriptome of a mutant lacking a putrescine synthetic pathway that was required for surface motility. Genetic analysis showed that surface motility required type 1 pili, the simultaneous presence of two independent putrescine anabolic pathways, and modulation by putrescine transport and catabolism. An immunological assay for FimA—the major pili subunit, reverse transcription quantitative PCR of fimA, and transmission electron microscopy confirmed that pili synthesis required putrescine. Comparative RNAseq analysis of a wild type and ΔspeB mutant which exhibits impaired pili synthesis showed that the latter had fewer transcripts for pili structural genes and for fimB which codes for the phase variation recombinase that orients the fim operon promoter in the ON phase, although loss of speB did not affect the promoter orientation. Results from the RNAseq analysis also suggested (a) changes in transcripts for several transcription factor genes that affect fim operon expression, (b) compensatory mechanisms for low putrescine which implies a putrescine homeostatic network, and (c) decreased transcripts of genes for oxidative energy metabolism and iron transport which a previous genetic analysis suggests may be sufficient to account for the pili defect in putrescine synthesis mutants. We conclude that pili synthesis requires putrescine and putrescine concentration is controlled by a complex homeostatic network that includes the genes of oxidative energy metabolism.
-
- Immunology and Inflammation
- Microbiology and Infectious Disease
The members of the Mycobacterium tuberculosis complex (MTBC) causing human tuberculosis comprise 10 phylogenetic lineages that differ in their geographical distribution. The human consequences of this phylogenetic diversity remain poorly understood. Here, we assessed the phenotypic properties at the host-pathogen interface of 14 clinical strains representing five major MTBC lineages. Using a human in vitro granuloma model combined with bacterial load assessment, microscopy, flow cytometry, and multiplexed-bead arrays, we observed considerable intra-lineage diversity. Yet, modern lineages were overall associated with increased growth rate and more pronounced granulomatous responses. MTBC lineages exhibited distinct propensities to accumulate triglyceride lipid droplets—a phenotype associated with dormancy—that was particularly pronounced in lineage 2 and reduced in lineage 3 strains. The most favorable granuloma responses were associated with strong CD4 and CD8 T cell activation as well as inflammatory responses mediated by CXCL9, granzyme B, and TNF. Both of which showed consistent negative correlation with bacterial proliferation across genetically distant MTBC strains of different lineages. Taken together, our data indicate that different virulence strategies and protective immune traits associate with MTBC genetic diversity at lineage and strain level.






