The Natural History of Model Organisms: The unlimited potential of the great pond snail, Lymnaea stagnalis
- Article
- Figures and data
- Abstract
- Introduction
- Natural history of L. stagnalis
- A gold standard model organism for neuroscience
- Ecotoxicology and risk assessment in a changing global environment
- Combining evolution and natural history
- New opportunities from a growing multi-omics coverage
- Conclusion
- Data availability
- References
- Decision letter
- Author response
- Article and author information
- Metrics
Abstract
Only a limited number of animal species lend themselves to becoming model organisms in multiple biological disciplines: one of these is the great pond snail, Lymnaea stagnalis. Extensively used since the 1970s to study fundamental mechanisms in neurobiology, the value of this freshwater snail has been also recognised in fields as diverse as host–parasite interactions, ecotoxicology, evolution, genome editing and 'omics', and human disease modelling. While there is knowledge about the natural history of this species, what is currently lacking is an integration of findings from the laboratory and the field. With this in mind, this article aims to summarise the applicability of L. stagnalis and points out that this multipurpose model organism is an excellent, contemporary choice for addressing a large range of different biological questions, problems and phenomena.
Introduction
In ancient Greece, over 2,400 years ago, it was already recognised that by studying animals we could learn much about ourselves. Over the centuries since then, it has become clearer that some species are highly suitable in the fields of medical, basic and applied biological research (Ericsson et al., 2013). However, when considered carefully, there is perhaps only a limited set of animal species that are versatile enough to lend themselves to become model organisms in multiple biological disciplines (Frézal and Félix, 2015; Hilgers and Schwarzer, 2019; Markow, 2015; Phifer-Rixey and Nachman, 2015).
In the second half of the 20th century, one booming line of research has focused on molluscs. Neuroscientists such as the Nobel Prize winners Alan Hodgkin, Andrew Huxley and Eric Kandel recognised these animals’ potential as models for understanding basic neurobiological processes (Hodgkin and Huxley, 1952; Kupfermann and Kandel, 1969; Wachtel and Kandel, 1967). One particularly well-suited mollusc for this type of research is the freshwater pond snail, Lymnaea stagnalis, which has been used extensively since the 1970s to study the functioning of the nervous system from molecular signalling to behaviour.
The value of L. stagnalis also has been recognised in a wide range of applied biological fields. These include the study of host–parasite interactions, ecotoxicology, evolution, developmental biology, genome editing, 'omics' and human disease modelling. This extensive suitability stems from the most obvious advantages of L. stagnalis: its well-known anatomy, development (both of the embryonic and post-embryonic processes), and reproduction biology; its well-characterised central and peripheral nervous and neuroendocrine systems from key molecules to behavioural processes; and its readily accessible and mostly large neurons. There is also a growing body of available sequence data with an impending annotated genome and the option to use new technical approaches such as genome editing. Taking all of the above into consideration, these advantages simplify the study of different scientific topics integrated from the molecular to the population level.
This article is a tribute to over 50 years of research with L. stagnalis that has resulted in a considerable contribution to the understanding of general biological processes. Here, we present the essential background information on the natural history of this freshwater snail. We also provide an overview of the ground-breaking and recent information on different research fields using L. stagnalis (and snails in general). Our aim is to showcase L. stagnalis as a contemporary choice for addressing a wide range of biological questions, problems and phenomena, to inspire more researchers to use this invertebrate as a model organism, and to highlight how findings from the laboratory and the field could be better integrated.
Natural history of L. stagnalis
Initially described by Linnaeus in 1758 as Helix stagnalis, the species now known as L. stagnalis is generally referred to as the great pond snail (Panpulmonata; Hygrophila; Lymnaeidae). It is found throughout Northern America, Europe, and parts of Asia and Australia (Atli and Grosell, 2016; Zhang et al., 2018a; Figure 1). The snails inhabit stagnant and slowly running shallow waters rich in vegetation and are mainly herbivores, preferring algae, water plants and detritus (Lance et al., 2006). They are active all year round (even when there is a layer of ice on the water) but typically reproduce from spring to late autumn (Nakadera et al., 2015). They do not have a clear day-night rhythm, but display sleep-like behaviour (Stephenson and Lewis, 2011) and are more likely to lay eggs during daytime (Ter Maat et al., 2012). They are light to dark brown in colour and relatively large for pond snail species, with their spiral shells reaching lengths of up to 55 mm (Benjamin, 2008). In highly oxygenated water, they absorb oxygen directly across their body wall; but when dissolved oxygen levels drop, they switch to breathing via a lung accessed by a respiratory orifice called the pneumostome (Lukowiak et al., 1996).
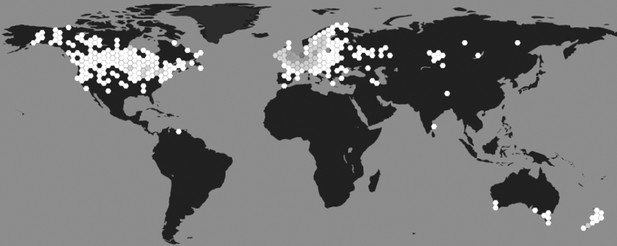
Geographical distribution of L. stagnalis.
Places where this species of snail has been reported to occur (hexagons), shaded based on population density (white indicates low density and dark grey indicates high density; source data from GBIF Secretariat, 2019).
L. stagnalis serves as the intermediate host for parasites including flatworms responsible for diseases such as fascioliasis (liver fluke and river rot) and cercarial dermatitis (swimmer’s itch) in humans (Adema et al., 1994; Davison and Blaxter, 2005; Ferté et al., 2005; Núñez et al., 1994; Skála et al., 2020). Laboratory and field studies showed that penetration of a parasite into a snail will initiate a chronic infection in which the parasite alters snail neurophysiology, metabolism, immunity, growth and reproduction (Kryukova et al., 2014; Langeloh and Seppälä, 2018; Vorontsova et al., 2019). These studies have also investigated how selection acts on immune defence traits (Langeloh et al., 2017). Investigations of the natural history of L. stagnalis, which focus on host-parasite associations, aid the development of novel control measures that reduce snail-mediated parasitic transmissions. Primary predators of juveniles and adults include leeches, crayfish and fish, some of which snails can detect via chemicals that the predators emit (Dalesman and Lukowiak, 2012).
The life cycle and reproductive biology of the species are well-characterised (Ivashkin et al., 2015; Koene, 2010; Mescheryakov, 1990; Morrill, 1982; Figure 2A). Embryos develop inside transparent eggs packaged in a translucent gelatinous mass allowing observers to follow their developmental stages in detail over the 11–12 days to hatching. The time from laid eggs to mature reproductive adults can be as short as two months depending on the temperature, photoperiod, feeding regime and mate availability at the location where they are being raised. In their natural habitat, they have been found to reach an age in excess of one year, but in the laboratory they live longer, up to two years (Janse et al., 1988; Nakadera et al., 2015). For laboratory breeding, a large and genetically diverse breeding stock is recommended as this will facilitate a well-standardised stock population without too much inbreeding. The largest and longest-maintained breeding facility is found at the Vrije Universiteit in Amsterdam, where L. stagnalis has been bred continuously for over 50 years (Nakadera et al., 2014).
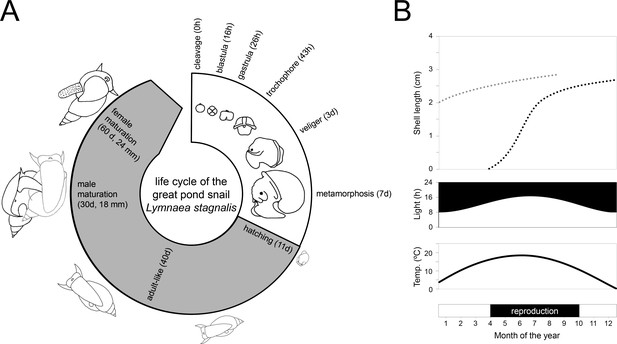
Life cycle and wild reproductive habit of L. stagnalis.
(A) The embryonic development in the egg from zygote to hatching (over 11–12 days) is depicted in the white area of the life cycle and consists of six main stages: cleavage, blastula, gastrula, trochophore, veliger and metamorphosis (Source data from Ivashkin et al., 2015). The grey area of the life cycle depicts growth and development after hatching. Although L. stagnalis is a simultaneous hermaphrodite, the male reproductive organs are functional before the female ones (Koene and Ter Maat, 2004): specimens reach male and female maturation on average at an age of 30 and 60 days, respectively (based on Koene, 2010). (B) In the wild, generations only partly overlap, as depicted by the two dotted growth curves (top; based on Nakadera et al., 2015). Individuals that are born during spring and summer, overwinter as adults (light grey dotted line) after which they overlap with the adult generation of the next year (black dotted line). The external conditions such as light and temperature (middle), which strongly influence when egg laying occurs (bottom), are depicted for the situation in a typical temperate zone.
Its well-characterised embryonic and post-embryonic processes have promoted extensive use of L. stagnalis in the field of developmental biology. This snail has helped us to understand the mechanisms underlying shell formation (Hohagen and Jackson, 2013), the transfer of non-genetic information to the developing embryos (Ivashkin et al., 2015), and resource allocation during development (Koene and Ter Maat, 2004). Moreover, studies with L. stagnalis has also helped develop and evaluate models in physiology, such as the “dynamic energy budget” model (Zonneveld and Kooijman, 1989; Zimmer et al., 2014).
This snail is a simultaneous hermaphrodite, meaning that mature individuals express a functional male and female reproductive system at the same time within one body. Despite having two functional sexes, specimens copulate unilaterally; one individual plays the male role and the other the female role within one mating interaction (Hoffer et al., 2017). There is no obligate alternation of sexual roles, but when both individuals of a mating pair are motivated to mate in the male role they can perform a second copulation with the same partner in the opposite sexual role (Koene, 2010; Ter Maat et al., 2012; Van Duivenboden and Maat, 1985). An integrated laboratory and field study showed that, in the wild, most individuals are born during the spring and summer seasons and generations partly overlap because the latter cohorts overwinter and overlap with mature individuals of the new spring cohort (Figure 2B; Nakadera et al., 2015). The same study showed that both age and size significantly affected the sex role decision under laboratory conditions. This species is quite fecund in the laboratory: snails from the mass culture in Amsterdam produce a large number of offspring all year round (Nakadera et al., 2014); however, an initial field study found a more moderate fecundity rate in natural populations (Nakadera et al., 2017). In the laboratory, specimens produce on average 2–3 egg masses per week each containing 100–150 eggs, depending on the body size of the individual (Nakadera et al., 2014). The hatching rate under laboratory conditions is generally above 90% (Hoffer et al., 2017). Based on laboratory, semi-field and field studies, explicit inbreeding or self-fertilisation depression for this species have been found to be absent (Coutellec and Lagadic, 2006; Escobar et al., 2011; Koene et al., 2008; Puurtinen et al., 2007) or very unlikely (Coutellec and Caquet, 2011), however the reasons for this remain unclear (Box 1). Nevertheless, eggs are preferentially outcrossed with sperm from mating partners, which can be stored for two months, and individuals only use their own 'autosperm' when this 'allosperm' is not available (Nakadera et al., 2014).
Box 1.
Outstanding questions about the natural history of the great pond snail.
Why is inbreeding depression less strong in L. stagnalis than in related freshwater snail species?
How different are long-term laboratory-bred strains from natural populations as a result of different selection pressures influencing development, mating propensity, self-fertilisation, learning and/or changes in sensitivity due to changing biotic and abiotic factors?
How can the knowledge about host-parasite interaction be applied to control the spread of parasites in the natural habitat?
How phenotypically plastic or evolutionarily adaptable is this species to changes in biotic and abiotic conditions in its habitat (e.g., temperature, light and/or chemical pollution, and resulting changes in ecosystem composition)?
Why are sinistral individuals not found more often in natural populations and what does that mean for the natural selection pressures on this chiral morph?
Are the detection and avoidance of positive and negative stimuli only present in the laboratory or is this learned behaviour also exhibited under field conditions (e.g., predicting presence of food, mating partners and/or predators)?
How can the knowledge about the regulatory mechanisms underlying reproduction be better used to understand the evolution and flexibility of the hermaphroditic lifestyle?
A gold standard model organism for neuroscience
The squid Loligo forbesii and sea hare Aplysia californica were the first molluscan models for examining neuronal processes. L. stagnalis emerged shortly afterwards, and was described as “a reductionistic, yet sophisticated model to address fundamental questions in learning and memory” (Rivi et al., 2020). Molluscs were used extensively in the field of neurobiology in the 20th century, typically because their central nervous systems were, in most cases, more accessible than those of vertebrate animals. Technical developments since then mean many such experiments can now be performed on vertebrates as well, yet we would argue that invertebrates still have substantial advantages for our understanding of the central nervous system.
The relatively simple central nervous system of L. stagnalis is organised in a ring of 11 interconnected ganglia (Figure 3A,B) with ~25,000 neurons. The neurons are mostly large (30–150 µm in diameter) and their bright, orange-coloured cell bodies are located on the surface of the ganglia (Figure 3B; Kemenes and Benjamin, 2009). Thus they are readily accessible for experimental purposes, simplifying investigations of neural clusters, circuits and even single neurons, which can be reliably identified for functional examination using a variety of approaches such as electrophysiological, molecular and analytical techniques (Crossley et al., 2018; de Hoog et al., 2019; El Filali et al., 2015; Harris et al., 2012; Kemenes et al., 2011; Lu et al., 2016; Samu et al., 2012; Wagatsuma et al., 2005; Zhang et al., 2018b).
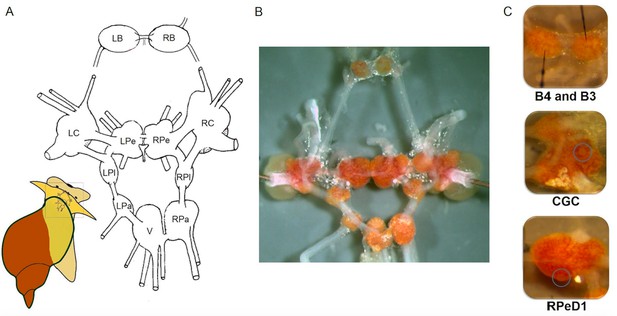
The central nervous system and identified single neurons of L. stagnalis.
(A) Schematic map (dorsal view) of the isolated whole central nervous system that is formed of the paired (left and right) buccal (LB, RB), cerebral (LC, RC), pedal (LPe, RPe), pleural (LPl, RPl), parietal (LPa, RPa) and unpaired visceral (V) ganglia. (B) Isolated central nervous system showing the arrangement of the 11 interconnected ganglia. Brightly pigmented orange-coloured neurons are localised on the surfaces of the ganglia. (C) Identified single neurons: B4 (left), B3 (right; motor neurons responsible for the implementation of feeding), CGC (interneuron in cerebral ganglia modulating the feeding and learning) and RPeD1 (interneuron in pedal ganglia regulating the respiration and heartbeat).
Individual neurons (Figure 3C; Benjamin and Crossley, 2020) and their synaptic connectivity were identified as parts of circuits controlling behaviours (Audesirk et al., 1985; Benjamin, 2012; McCrohan and Benjamin, 1980a; McCrohan and Benjamin, 1980b; Syed and Winlow, 1991; Syed et al., 1990). Combining this knowledge with an understanding of the molecular mechanisms, often from laboratory studies, has helped produce an integrated picture of the processes underlying learning and memory, such as consolidation, reconsolidation, extinction and forgetting. The molecular pathways involved in memory formation in L. stagnalis were recently identified, providing further evidence the mechanisms of learning and memory consolidation are conserved across phylogenetic groups in a variety of learning paradigms, including non-associative or associative learning, and operant or classical conditioning (Benjamin and Kemenes, 2013; Fulton et al., 2005; Josselyn and Nguyen, 2005; Kemenes and Benjamin, 2009; Kemenes et al., 2002; Marra et al., 2013; Michel et al., 2008; Nikitin et al., 2008; Park et al., 1998; Pirger et al., 2010; Pirger et al., 2014a; Pirger et al., 2014b; Ribeiro et al., 2003; Rivi et al., 2020; Sadamoto et al., 1998; Sadamoto et al., 2010; Schacher et al., 1988; Vigil and Giese, 2018; Wan et al., 2010). Recently studies have also revealed differences in learning ability at the behavioural level between situations in the laboratory and the field (e.g., Dalesman and Lukowiak, 2012; Dalesman et al., 2015; Dalesman, 2018).
The well-characterised proximate processes at the molecular, cellular and circuit levels mean studying this simple nervous system has the potential to provide insights into how snails can respond appropriately to environmental challenges (e.g., climatic change or pharmacologically active compounds). Also, since their behaviours are generated by reflexive and central pattern generator networks similar to those of vertebrates (Katz and Hooper, 2007), results from snails offer insights into the fundamental processes important for these animals too.
Finally, recent developments have enabled this species to be used as a model for understanding the basis of neurodegenerative diseases. Comparative analyses have yielded several homologs to human genes linked to ageing and neurodegenerative diseases in A. californica and this species has proved well suited for studying these processes (Moroz et al., 2006; Moroz and Kohn, 2010). Similar molecular sequences have been identified in L. stagnalis (Fodor et al., 2020b). With the appropriate genetic background, its accessible central nervous system and relatively long and well-characterised life span mean L. stagnalis is highly suitable for studying the biological mechanisms of ageing, age-related memory loss and neurodegenerative diseases, such as Parkinson’s and Alzheimer’s diseases (Arundell et al., 2006; de Weerd et al., 2017; Ford et al., 2017; Hermann et al., 2007; Hermann et al., 2020; Maasz et al., 2017; Patel et al., 2006; Pirger et al., 2014b; Scutt et al., 2015; Vehovszky et al., 2007; Yeoman and Faragher, 2001; Yeoman et al., 2008).
Ecotoxicology and risk assessment in a changing global environment
It has become clear that in the globalised world, climate change, light pollution, micro- and nanoplastics, and pharmacologically active compounds all pose a challenge to animal life. These challenges affect the availability of suitable habitats and reduce the quality of the land, lakes and rivers. They also change the environmental composition of pathogens, parasites, competitors and invaders. Understanding how global ecosystems are adapting to pollution is a complex problem; it requires researchers to monitor natural populations and conduct laboratory studies to discover the bases of adaptations or the lack thereof (Markow, 2015).
Molluscs the second most diverse animal group and considered to be excellent indicators of ecosystem health. For example, L. stagnalis is a sensitive and reliable species for such studies (Amorim et al., 2019), in a large part because of its well-characterised developmental and reproductive biology as described above. The major targets in this field of study have been metal-risk assessment (Crémazy et al., 2018; Pyatt et al., 1997; Vlaeminck et al., 2019), the effects of pesticides (Coutellec et al., 2008; Lance et al., 2016; Tufi et al., 2015; Vehovszky et al., 2015), nanotoxicology (Hudson et al., 2019; Stoiber et al., 2015), the development of toxicokinetic models (Baudrot et al., 2018), immunocompetence analyses (Boisseaux et al., 2018; Gust et al., 2013b), and global warming risk assessment (Leicht et al., 2017; Leicht and Seppälä, 2019; Teskey et al., 2012). Studies on L. stagnalis have measured toxicological values such as mortality concentrations (e.g., LC50) and impairment of reproduction (e.g., EC50) but also sub-lethal and more sensitive endpoints such as reproductive success, growth, cellular and molecular biomarkers that may be coupled with behavioural responses (Amorim et al., 2019).
L. stagnalis has also been recognised as a useful organism to examine the effects of pharmacologically active compounds and micro- and nanoplastics on aquatic organisms (Amorim et al., 2019; Charles et al., 2016; Ducrot et al., 2014; Gust et al., 2013a; Horton et al., 2020; Pirger et al., 2018; Zrinyi et al., 2017). However, it must be pointed out that researchers need to be critical of the generalisability of results while performing such experiments since there are differences between the endocrine system of molluscs and vertebrates; molluscs, for example, do not have functional oestrogen receptors (Eick and Thornton, 2011; Lagadic et al., 2007; Scott, 2012). It is also important to recognise that molluscs are not suitable for some types of ecotoxicological studies and they cannot always substitute for fish.
Notably, L. stagnalis is the first aquatic non-arthropod invertebrate model organism to be recognised in environmental risk assessments. The developed standard reproduction test was officially approved by the national coordinators of the Organisation for Economic Cooperation and Development (OECD, 2016) thus paving the way for investigating ecotoxicological effects in more detail. Such information will contribute to a more complete picture of the mode of action of potentially toxic substances and other environmental factors and provide assessments of risk for individual species of different types and wider ecosystems.
Combining evolution and natural history
Within the field of evolutionary biology, L. stagnalis has helped us to understand the evolution of several phenomena. Left-right asymmetry is a general evolutionary phenomenon seen across a variety of species, including humans where the congenital condition situs inversus results in the mirrored position and shape of the heart and liver (Blum et al., 2014; Oliverio et al., 2010; Palmer, 2009; Palmer, 2016). The coil or chirality of snail shells is one of the more spectacular outward manifestations of this asymmetry. Snails found in nature can have shells that coil either to the right or left, with most species being right-coiling. Specimens of L. stagnalis that coil in the opposite left-winding, or sinistral, direction are rare and often categorised as 'unlucky' because their different chirality makes it difficult for them to mate the more usual, right-winding individuals (Davison et al., 2009). Left-winding snails are also less able to learn in a mate-choice context (Koene and Cosijn, 2012). The existence of the two different morphs has made this species ideal for studying chiromorphogenesis, i.e. the first step of left-right symmetry breaking. Genes and signalling pathways that are responsible for snail coiling have been identified (Abe et al., 2014; Davison et al., 2016; Kuroda, 2014; Kuroda, 2015; Kuroda et al., 2016), and similar signalling pathways are required for vertebrate chiromorphogenesis as well (Kuroda, 2015). Studies on L. stagnalis can give important insights into the evolution of body plans in other phyla, and may have wider medical implications, including an understanding of situs inversus.
L. stagnalis has also played a crucial role in studies into the evolution of hermaphroditism and its consequences for sexual selection. This area of research relies heavily on a solid understanding of the natural history of this species. Selection of sexual traits that affect mating success was previously considered not to act in simultaneous hermaphrodites (Charnov, 1979; Darwin, 1871; Greeff and Michiels, 1999; Morgan, 1994). However, recent research, including work with L. stagnalis, has contradicted this earlier conclusion (Anthes et al., 2010; Baur, 1998; Chase, 2007; Janicke et al., 2016; Michiels, 1998; Nakadera and Koene, 2013). Unilateral mating of L. stagnalis offered a unique opportunity to test whether sexual selection acts independently on the two sexual roles of a simultaneous hermaphrodite (Anthes et al., 2010; Arnold, 1994; Hoffer et al., 2017). Recent experiments have also revealed that male and female reproductive strategies can be optimised independently in this species. This was done by measuring sexual selection gradients (also called Bateman gradients), which reveal the relationship between the number of matings and the reproductive success of the sexual functions (Anthes et al., 2010). Experiments with L. stagnalis showed that this mating system seems largely male-driven, and that the sexual selection gradients are consistently positive for the male function but change over time to benefit the opposite sex (Anthes et al., 2010; Hoffer et al., 2017; Janicke et al., 2016; Pélissié et al., 2012). These pioneering works, which measured and quantified the processes of sexual selection and their underlying mechanisms, thus incorporated this hermaphrodite into the general Darwin-Bateman paradigm that had so far mainly been tested on separate-sexed species. They also described both the evolutionary potential and limitations of hermaphrodite animals and revealed important practical applications for the conservation of wildlife.
New opportunities from a growing multi-omics coverage
From about 1980, continued attention was given to the physiological characterisation of L. stagnalis, but more recent research has focussed on an 'omics' approach to better understand the underlying molecular processes (Santama et al., 1993; Santama et al., 1995a; Santama et al., 1995b; Santama and Benjamin, 2000). Due to its pre-eminence as a model system in neuroscience, early molecular studies tended to focus on the central nervous system (Feng et al., 2009; Johnson and Davison, 2019). The favourable anatomical features enabled the accumulation of peptidomic data from the mass spectrometry of single neurons (Perry et al., 1999; Worster et al., 1998), making the neuropeptidergic system the most intensely studied part of the central nervous system (Buckett et al., 1990; Perry et al., 1998). Taking advantage of a variety of platforms available for nucleotide sequencing: Sanger (Davison and Blaxter, 2005; Sadamoto et al., 2004; Swart et al., 2019), Illumina (Korneev et al., 2018; Sadamoto et al., 2012; Stewart et al., 2016), BGISEQ (Jehn et al., 2018) and Oxford Nanopore (Fodor et al., 2020a), many sequencing methodologies have been successfully applied to this species.
Extensive genomic, transcriptomic and peptidomic data for L. stagnalis are available in the NCBI database. Four major transcriptome datasets were established by sequencing mRNA from the central nervous system (Bouétard et al., 2012; Davison and Blaxter, 2005; Feng et al., 2009; Sadamoto et al., 2012), and then used to identify genes and proteins, thus providing a solid genetic background for L. stagnalis. Furthermore, an unannotated draft genome is already available and a collaborative effort is underway to produce an annotated genome (Johnson and Davison, 2019) which would largely solve the problem of the lack of molecular information that has so far inhibited research in the L. stagnalis model system (Rivi et al., 2020). Approximately 100 (neuro)peptides have been identified so far (Benjamin and Kemenes, 2020), encoded by genes involved in various regulatory processes (Table 1). These findings contributed to a global understanding of the natural history of L. stagnalis by characterising the molecular and cellular processes underlying chirality, reproduction, immune processes, host-parasite interaction, and acute and chronic adaptive responses to toxic substances in the environment.
List of some of the most important (neuro)peptides identified in L. stagnalis.
| Molecule | Abbreviation | Function | Accession number | Reference |
|---|---|---|---|---|
| caudodorsal cell hormones | CDCH | reproduction | P06308 | Vreugdenhil et al., 1988 |
| FMRFamides | FMRF | reproduction, cardiac control | P19802 | Linacre et al., 1990 |
| conopressin | - | reproduction | AAB35220 | Van Kesteren et al., 1995 |
| neuropeptide Y | NPY | reproduction, development | CAB63265 | De Jong-Brink et al., 1999 |
| actin-related diaphanous genes (1, 2) | dia 1, dia 2 | development, chirality | KX387869, KX387870 KX387871, KX387872 | Kuroda et al., 2016 |
| insulin-related peptides (I, II, III, V, VII) | MIPs | development | CAA41989; P25289; AAB28954; AAA09966; AAB46831 | Smit et al., 1991; Smit et al., 1992; Smit et al., 1993b; Smit et al., 1996; Smit et al., 1998 |
| sodium stimulating hormone | SIS | ion and water control | P42579 | Smit et al., 1993a |
| small cardioactive peptide | SCP | feeding, cardiac control | AAC99318 | Perry et al., 1999 |
| myomodulin | MIP | feeding, cardiac control | CAA65635 | Kellett et al., 1996 |
| pituitary adenylate cyclase-activating polypeptide-like molecule | PACAP-like | learning and memory | - | Pirger et al., 2010 |
| cAMP response element-binding proteins (1, 2) | CREB 1 CREB 2 | learning and memory | AB041522; AB083656 | Sadamoto et al., 2004 |
| glutathione reductase and peroxidase | Gred Gpx | metabolic detoxification | FJ418794, FJ418796 | Bouétard et al., 2014 |
| catalase | CAT | metabolic detoxification | FJ418795 | Bouétard et al., 2014 |
| superoxide dismutase | SOD | metabolic detoxification | AY332385 | Zelck et al., 2005 |
| heat-shock protein | HSP70 | stress response | DQ206432 | Fei et al., 2007 |
| molluscan defence molecule | MDM | immune system | AAC47132 | Hoek et al., 1996 |
| allograft inflammatory factor-1 | AIF-1 | immune system | DQ278446 | van Kesteren et al., 2006 |
Furthermore, the CRISPR/Cas9 genome editing method has recently been applied to molluscs (Henry and Lyons, 2016; Perry and Henry, 2015). In L. stagnalis, it was used to knock out the gene responsible for coiling direction during development, leading to a better understanding of chirality in the life of the two morphs (Abe and Kuroda, 2019). The establishment of genome editing in L. stagnalis opens up significant opportunities for functional genomics to investigate the role of specific genes, for example, in snail developmental, toxicology and immunobiological studies.
Conclusion
Research on model organisms has been essential to developing the current understanding of how life works. The unique features of L. stagnalis make it an excellent experimental system to complement the classic invertebrate (C. elegans, D. melanogaster) and vertebrate (D. rerio, M. musculus) models. Research utilising this species is expected to lead to future breakthroughs in a number of scientific fields, especially in neuroscience and evolutionary biology. For example, as a simultaneously hermaphroditic outcrossing species, it presents the opportunity to test the generality of hypotheses that are mainly based on non-hermaphroditic or self-fertilising models. There is considerable information about the natural history of L. stagnalis compared to some other model species, but we feel some areas of research using L. stagnalis – in particular neurobiology and ecotoxicology – would benefit by extending more of their studies out of the laboratory and into the field. We believe that a deeper integration of information from field studies with input from laboratory findings – such as applying experimental designs and approaches developed in the laboratory to populations in the wild – will provide future opportunities for further innovation (Box 2). Such efforts could address the unanswered questions regarding this model organism (see Box 1). Significantly, emerging recent technical approaches such as pocket-sized sequencing devices, especially with their impending breakthrough also in protein sequencing, start allowing researchers to perform more experiments in the field such as following molecular mechanisms of learning.
Box 2.
How can findings at different biological levels be integrated to better understand this species’ natural history?
It needs to be established at what level L. stagnalis can function as a model for medical research such as neurodegenerative disease and be a substitute for standard vertebrate models. This requires a better understanding of how such functions affect this species in its natural habitat.
The new molecular techniques and available 'omics' data provide an incentive for research that aims to understand the mechanisms underlying natural history processes such as sex allocation, simultaneous hermaphroditism, reproductive success, chirality and learning.
The knowledge about learning and decision-making in the laboratory needs to be extended to field populations to promote future developments in, for example, neural network-inspired robotics.
Data availability
All data generated during the preparation of this review are included in the manuscript.
References
-
Spiral cleavages determine the left-right body plan by regulating nodal pathway in Monomorphic Gastropods, Physa acutaThe International Journal of Developmental Biology 58:513–520.https://doi.org/10.1387/ijdb.140087rk
-
Lymnaea stagnalis as a freshwater model invertebrate for ecotoxicological studiesScience of the Total Environment 669:11–28.https://doi.org/10.1016/j.scitotenv.2019.03.035
-
Bateman gradients in hermaphrodites: an extended approach to quantify sexual selectionThe American Naturalist 176:249–263.https://doi.org/10.1086/655218
-
Bateman's Principles and the Measurement of Sexual Selection in Plants and AnimalsThe American Naturalist 144:S126–S149.https://doi.org/10.1086/285656
-
Evidence for genetic influences on neurotransmitter content of identified neurones of Lymnaea stagnalisComparative Biochemistry and Physiology Part C: Comparative Pharmacology 81:57–60.https://doi.org/10.1016/0742-8413(85)90091-X
-
New insights to compare and choose TKTD models for survival based on an interlaboratory study for Lymnaea stagnalis exposed to cdEnvironmental Science & Technology 52:1582–1590.https://doi.org/10.1021/acs.est.7b05464
-
BookSperm competition in molluscsIn: TRBaAP M, editors. Sperm Competition and Sexual Selection. London: Academic Press. pp. 826–827.https://doi.org/10.1016/B978-012100543-6/50033-7
-
BookGastropod Feeding Systems: Evolution of Neural Circuits That Generate Diverse BehaviorsOxford Research Encyclopedia of Neuroscience.https://doi.org/10.1093/acrefore/9780190264086.013.285
-
BookPeptidergic systems in the pond snail Lymnaea: From genes to hormones and behaviorIn: Saleuddin A. B. L, Orchard I, editors. Advances in Invertebrate (Neuro) Endocrinology. Apple Academic Press. pp. 213–254.https://doi.org/10.1201/9781003029854-7
-
The evolution and conservation of left-right patterning mechanismsDevelopment 141:1603–1613.https://doi.org/10.1242/dev.100560
-
Immunocompetence analysis of the aquatic snail Lymnaea stagnalis exposed to urban wastewatersEnvironmental Science and Pollution Research 25:16720–16728.https://doi.org/10.1007/s11356-018-1790-z
-
Regulation of heartbeat in Lymnaea by motoneurons containing FMRFamide-like peptidesJournal of Neurophysiology 63:1426–1435.https://doi.org/10.1152/jn.1990.63.6.1426
-
Optimizing the design of a reproduction toxicity test with the pond snail Lymnaea stagnalisRegulatory Toxicology and Pharmacology 81:47–56.https://doi.org/10.1016/j.yrtph.2016.07.012
-
The function of dart shooting in helicid snails*American Malacological Bulletin 23:183–189.https://doi.org/10.4003/0740-2783-23.1.183
-
Heterosis and inbreeding depression in bottlenecked populations: a test in the hermaphroditic freshwater snail Lymnaea stagnalisJournal of Evolutionary Biology 24:2248–2257.https://doi.org/10.1111/j.1420-9101.2011.02355.x
-
Chronic toxicity of binary mixtures of six metals (Ag, cd, cu, ni, pb, and zn) to the great pond snail Lymnaea stagnalisEnvironmental Science & Technology 52:5979–5988.https://doi.org/10.1021/acs.est.7b06554
-
A central control circuit for encoding perceived food valueScience Advances 4:eaau9180.https://doi.org/10.1126/sciadv.aau9180
-
Habitat stability, predation risk and 'memory syndromes'Scientific Reports 5:10538.https://doi.org/10.1038/srep10538
-
Habitat and social context affect memory phenotype, exploration and covariance among these traitsPhilosophical Transactions of the Royal Society B: Biological Sciences 373:20170291.https://doi.org/10.1098/rstb.2017.0291
-
Retinoid receptor-based signaling plays a role in voltage-dependent inhibition of invertebrate voltage-gated Ca2+ channelsJournal of Biological Chemistry 294:10076–10093.https://doi.org/10.1074/jbc.RA118.006444
-
Parasites flicking the NPY gene on the host's switchboard: why NPY?The FASEB Journal 13:1972–1984.https://doi.org/10.1096/fasebj.13.14.1972
-
Linking the 'why' and 'how' of ageing: evidence for somatotropic control of long-term memory function in the pond snail Lymnaea stagnalisThe Journal of Experimental Biology 220:4088–4094.https://doi.org/10.1242/jeb.167395
-
Development and validation of an OECD reproductive toxicity test guideline with the pond snail Lymnaea stagnalis (Mollusca, gastropoda)Regulatory Toxicology and Pharmacology 70:605–614.https://doi.org/10.1016/j.yrtph.2014.09.004
-
Evolution of steroid receptors from an estrogen-sensitive ancestral receptorMolecular and Cellular Endocrinology 334:31–38.https://doi.org/10.1016/j.mce.2010.09.003
-
A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioningEuropean Journal of Neuroscience 21:1347–1358.https://doi.org/10.1111/j.1460-9568.2005.03970.x
-
Sperm digestion and reciprocal sperm transfer can drive hermaphrodite sex allocation to equalityThe American Naturalist 153:421–430.https://doi.org/10.1086/303184
-
Molluscan models: crepidula fornicataCurrent Opinion in Genetics & Development 39:138–148.https://doi.org/10.1016/j.gde.2016.05.021
-
Impairment of long-term associative memory in aging snails (Lymnaea stagnalis)Behavioral Neuroscience 121:1400–1414.https://doi.org/10.1037/0735-7044.121.6.1400
-
Physiological and pharmacological characterization of a molluscan neuronal efflux transporter; evidence for age-related transporter impairmentThe Journal of Experimental Biology 223:jeb213785.https://doi.org/10.1242/jeb.213785
-
A new Ig-superfamily member, molluscan defence molecule (MDM) from Lymnaea stagnalis, is down-regulated during parasitosisEuropean Journal of Immunology 26:939–944.https://doi.org/10.1002/eji.1830260433
-
Species-Specific (Hyalella azteca and lymnea stagnalis) Dietary accumulation of gold Nano-particles associated with periphytonBulletin of Environmental Contamination and Toxicology 103:255–260.https://doi.org/10.1007/s00128-019-02620-2
-
Darwinian sex roles confirmed across the animal kingdomScience Advances 2:e1500983.https://doi.org/10.1126/sciadv.1500983
-
Survival characteristics of the mollusc Lymnaea stagnalis under constant culture conditions: effects of aging and diseaseMechanisms of Ageing and Development 42:263–274.https://doi.org/10.1016/0047-6374(88)90052-8
-
CREB, synapses and memory disorders: past progress and future challengesCurrent Drug Targets. CNS and Neurological Disorders 4:481–497.https://doi.org/10.2174/156800705774322058
-
BookInvertebrate central pattern generatorsIn: Greenspan G. N. R. J, editors. Invertebrate Neurobiology. Cold Spring Harbor Press. pp. 251–280.https://doi.org/10.1038/451019c
-
Myomodulin gene of Lymnaea: structure, expression, and analysis of neuropeptidesThe Journal of Neuroscience 16:4949–4957.
-
Critical time-window for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioningThe Journal of Neuroscience 22:1414–1425.https://doi.org/10.1523/JNEUROSCI.22-04-01414.2002
-
Dynamic clamp with StdpC softwareNature Protocols 6:405–417.https://doi.org/10.1038/nprot.2010.200
-
Mate choice is not affected by mating history in the simultaneously hermaphroditic snail Lymnaea stagnalisJournal of Molluscan Studies 74:331–335.https://doi.org/10.1093/mollus/eyn020
-
Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: mechanisms and evolutionFrontiers in Behavioral Neuroscience 4:167.https://doi.org/10.3389/fnbeh.2010.00167
-
Twisted sex in an hermaphrodite: mirror-image mating behaviour is not learnedJournal of Molluscan Studies 78:308–311.https://doi.org/10.1093/mollus/eys016
-
Energy budgets in the simultaneously hermaphroditic pond snail, Lymnaea stagnalis: a trade-off between growth and reproduction during developmentBelgian Journal of Zoology 134:41–45.
-
How a single gene twists a snailIntegrative and Comparative Biology 54:677–687.https://doi.org/10.1093/icb/icu096
-
A twisting story: how a single gene twists a snail? mechanogeneticsQuarterly Reviews of Biophysics 48:445–452.https://doi.org/10.1017/S0033583515000098
-
High sensitivity of spontaneous spike frequency to sodium leak current in a Lymnaea pacemaker neuronThe European Journal of Neuroscience 44:3011–3022.https://doi.org/10.1111/ejn.13426
-
Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalisThe Journal of Experimental Biology 199:683–691.
-
Susceptibility of memory consolidation during lapses in recallNature Communications 4:1578.https://doi.org/10.1038/ncomms2591
-
Synaptic relationships of the cerebral giant cells with motoneurones in the feeding system of Lymnaea stagnalisThe Journal of Experimental Biology 85:169–186.
-
Patterns of activity and axonal projections of the cerebral giant cells of the snail, Lymnaea stagnalisThe Journal of Experimental Biology 85:149–168.
-
BookThe common pond snail Lymnaea stagnalisIn: Detlaf D. A, Vassetzky S. G, editors. Animal Species for Developmental Studies,. Plenum Press. pp. 69–132.https://doi.org/10.1007/978-1-4615-3654-3
-
BookMating conflicts and sperm competition in simultaneous hermaphroditesIn: Trbap M, editors. Sperm Competition and Sexual Selection. Academic Press. pp. 219–254.https://doi.org/10.1016/B978-0-12-100543-6.X5022-3
-
Models of sexual selection in hermaphrodites, especially plantsThe American Naturalist 144:S100–S125.https://doi.org/10.1086/285655
-
BookDevelopmental biology of the freshwater invertebratesIn: Harrison F. W, Cowden R. R, editors. Development of the Pulmonate Gastropod, Lymnaea. Alan R. Liss. pp. 399–483.
-
Duration of sperm storage in the simultaneous hermaphrodite Lymnaea stagnalisJournal of Molluscan Studies 80:1–7.https://doi.org/10.1093/mollus/eyt049
-
Multiple mating in natural populations of a simultaneous hermaphrodite, Lymnaea stagnalisJournal of Molluscan Studies 83:56–62.https://doi.org/10.1093/mollus/eyw043
-
Reproductive strategies in hermaphroditic gastropods: conceptual and empirical approachesCanadian Journal of Zoology 91:367–381.https://doi.org/10.1139/cjz-2012-0272
-
Shells and heart: are human laterality and chirality of snails controlled by the same maternal genes?American Journal of Medical Genetics Part A 152A:2419–2425.https://doi.org/10.1002/ajmg.a.33655
-
What determines direction of asymmetry: genes, environment or chance?Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150417.https://doi.org/10.1098/rstb.2015.0417
-
Neural modulation of gut motility by myomodulin peptides and acetylcholine in the snail LymnaeaJournal of Neurophysiology 79:2460–2474.https://doi.org/10.1152/jn.1998.79.5.2460
-
Reversal of age-related learning deficiency by the vertebrate PACAP and IGF-1 in a novel invertebrate model of aging: the pond snail (Lymnaea stagnalis)The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 69:1331–1338.https://doi.org/10.1093/gerona/glu068
-
Predominance of outcrossing in Lymnaea stagnalis despite low apparent fitness costs of self-fertilizationJournal of Evolutionary Biology 20:901–912.https://doi.org/10.1111/j.1420-9101.2007.01312.x
-
Short Communication—Distribution of metals and accumulation of lead by different tissues in the freshwater snail Lymnaea stagnalis (L.)Environmental Toxicology and Chemistry 16:1393–1395.https://doi.org/10.1002/etc.5620160710
-
Lymnaea stagnalis as model for translational neuroscience research: from pond to benchNeuroscience & Biobehavioral Reviews 108:602–616.https://doi.org/10.1016/j.neubiorev.2019.11.020
-
Learning-Dependent gene expression of CREB1 isoforms in the molluscan brainFrontiers in Behavioral Neuroscience 4:25.https://doi.org/10.3389/fnbeh.2010.00025
-
Single electrode dynamic clamp with StdpCJournal of Neuroscience Methods 211:11–21.https://doi.org/10.1016/j.jneumeth.2012.08.003
-
Gene expression and function of FMRFamide-related neuropeptides in the snail LymnaeaMicroscopy Research and Technique 49:547–556.https://doi.org/10.1002/1097-0029(20000615)49:6<547::AID-JEMT5>3.0.CO;2-Y
-
Snail defence responses to parasite infection: the Lymnaea stagnalis-Trichobilharzia szidati modelDevelopmental & Comparative Immunology 102:103464.https://doi.org/10.1016/j.dci.2019.103464
-
Characterization of a cDNA clone encoding molluscan insulin-related peptide II of Lymnaea stagnalisEuropean Journal of Biochemistry 199:699–703.https://doi.org/10.1111/j.1432-1033.1991.tb16173.x
-
Cdna cloning of the sodium-influx-stimulating peptide in the mollusc, Lymnaea stagnalisEuropean Journal of Biochemistry 215:397–400.https://doi.org/10.1111/j.1432-1033.1993.tb18046.x
-
Behavioural evidence for a sleep-like quiescent state in a pulmonate mollusc, Lymnaea stagnalis (Linnaeus)Journal of Experimental Biology 214:747–756.https://doi.org/10.1242/jeb.050591
-
A "Love" Dart Allohormone Identified in the Mucous Glands of Hermaphroditic Land SnailsJournal of Biological Chemistry 291:7938–7950.https://doi.org/10.1074/jbc.M115.704395
-
Coordination of locomotor and cardiorespiratory networks of Lymnaea stagnalis by a pair of identified interneuronesThe Journal of Experimental Biology 158:37–62.
-
The effect of light on induced egg laying in the simultaneous hermaphrodite Lymnaea stagnalisJournal of Molluscan Studies 78:262–267.https://doi.org/10.1093/mollus/eys008
-
What's hot: the enhancing effects of thermal stress on long-term memory formation in Lymnaea stagnalisJournal of Experimental Biology 215:4322–4329.https://doi.org/10.1242/jeb.075960
-
Metabolomics to explore Imidacloprid-Induced toxicity in the central nervous system of the freshwater snail Lymnaea stagnalisEnvironmental Science & Technology 49:14529–14536.https://doi.org/10.1021/acs.est.5b03282
-
Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowthJournal of Neuroscience 26:152–157.https://doi.org/10.1523/JNEUROSCI.4164-05.2006
-
Calcium/calmodulin-dependent kinase II and memory destabilization: a new role in memory maintenanceJournal of Neurochemistry 147:12–23.https://doi.org/10.1111/jnc.14454
-
The use of mechanistic population models in metal risk assessment: combined effects of copper and food source on Lymnaea stagnalis populationsEnvironmental Toxicology and Chemistry 38:1104–1119.https://doi.org/10.1002/etc.4391
-
Determination of the exact copy numbers of particular mRNAs in a single cell by quantitative real-time RT-PCRJournal of Experimental Biology 208:2389–2398.https://doi.org/10.1242/jeb.01625
-
Matrix-assisted laser desorption/ionization time of flight mass spectrometric analysis of the pattern of peptide expression in single neurons resulting from alternative mRNA splicing of the FMRFamide geneEuropean Journal of Neuroscience 10:3498–3507.https://doi.org/10.1046/j.1460-9568.1998.00361.x
-
Superoxide dismutase expression and H2O2 production by hemocytes of the trematode intermediate host Lymnaea stagnalis (Gastropoda)Developmental & Comparative Immunology 29:305–314.https://doi.org/10.1016/j.dci.2004.09.002
-
Metabolic acceleration in the pond snail Lymnaea stagnalis?Journal of Sea Research 94:84–91.https://doi.org/10.1016/j.seares.2014.07.006
-
Application of a dynamic energy budget model to Lymnaea stagnalis (L.)Functional Ecology 3:269–278.https://doi.org/10.2307/2389365
Decision letter
-
Stuart RF KingReviewing Editor; eLife, United Kingdom
-
Peter RodgersSenior Editor; eLife, United Kingdom
-
Iker IrisarriReviewer; Museo Nacional de Ciencias Naturales, Spain
-
Elena E VoronezhskayaReviewer
-
Marie-Agnes CoutellecReviewer
In the interests of transparency, eLife publishes the most substantive revision requests and the accompanying author responses.
Thank you for submitting your article "The Natural History of Model Organisms: The unlimited potential of the great pond snail, Lymnaea stagnalis" for consideration by eLife. Your Feature Article has been reviewed by three peer reviewers, and the evaluation has been overseen by an Associate Features Editor (Stuart King). All individuals involved in the review of your submission have agreed to reveal their identity: Iker Irisarri (Reviewer #1); Elena Voronezhskaya (Reviewer #2); Marie-Agnes Coutellec (Reviewer #3).
After a consultation with the reviewers, the editor has drafted this decision to help you prepare a revised submission. We appreciate that the COVID-19 pandemic is disrupting many aspects of everyday life, so please take as long as you need to work on these revisions.
The editor may also contact you separately about some editorial issues that you will need to address.
Summary:
This essay is being considered as part of a series of articles on "The Natural History of Model Organisms": https://elifesciences.org/collections/8de90445/the-natural-history-of-model-organisms. Each article should explain how our knowledge of the natural history of a model organism has informed recent advances in biology, and how understanding its natural history can influence/advance future studies.
This article highlights the main advantages of the freshwater snail (Lymnaea stagnalis) as a model organism for research in various scientific domains. The authors recall the specific features that have led this species to be used as an invertebrate model for the past 50 years and identify pending issues in these domains. Broadening the scope of previous reviews on this organism, details of L. stagnalis's natural habitat and life history are also presented in a clear and compact format.
Overall, the reviewers felt the authors had done a good job of summarizing a broad body literature on the topic and had judiciously selected information to provide an engaging account of this model mollusc. This article may interest a wide scientific community across various fields, and particularly any researcher wishing to start using L. stagnalis as a de novo model.
Below are a number of details that should be attended to prior to publication.
Essential revisions:
1) Strengthen connection to primary literature:
There is a tendency throughout the article to cite previous reviews. It would be more useful for readers – and for those researchers actively working with this model organism – if the citations were instead to the original research as much as possible.
In particular, the reviewers suggest citing the relevant primary literature for some of the basic information: e.g., the species distribution (currently Amorim et al., 2019), breathing physiology (currently Benjamin, 2008), diet (currently Lance et al., 2008), or reproductive period (currently Nakadera et al., 2015).
2) Benefits as a model organism:
The Introduction notes that: "The value of L. stagnalis also has been recognized in a surprisingly-wide range of applied biological fields". The Introduction would also benefit if the main features that make L. staganalis a valuable model could be quickly summarised in a single sentence. In general, giving the evidence to support the assertions as to the value of this model helps to avoid parts of the text being perceived as dogmatism. Related to this, and while there was no doubt that the species is suited to test many hypotheses, there was a feeling among the reviewers that softening some claims and doing more to acknowledge that other models may be more relevant or have other specific advantages (depending on the issue or the state of knowledge at the start of the project) would be beneficial.
3) Highlight connections between field and laboratory studies:
The reviewers also noted that most of the article reports on laboratory experiments, which likely reflects an existing bias in the literature. Yet given that the aim of this article (and the wider collection) is to provide a context of natural history, more reporting on studies in natural settings would be preferable. In the conclusions, the authors noted: "[A deeper integration of 'field' information with input from laboratory] will allow for novel experimental designs and approaches developed in the laboratory to be applied to field populations and/or in the field". Other explicit examples of how the integration between laboratory and field experiments would be the key to success would strengthen the article. This would also be one way that the authors could help to address the "lack of integration between laboratory and field experiments" that they recognize as a major missing element in L. stagnalis research.
4) Highlight contributions to diverse fields:Lymnaea has had a long history in the field of developmental biology, and, in the field of physiology, where it was also one of the first model species to which the dynamic energy budget (DEB) theory was applied (see Zonneveld, C., & Kooijman, S. A. L. M. (1989). Application of a dynamic energy budget model to Lymnaea stagnalis (L.). Functional Ecology, 269-278). It would be good if these details could be briefly mentioned at the relevant points in the text.
https://doi.org/10.7554/eLife.56962.sa1Author response
Essential revisions:
1) Strengthen connection to primary literature:
There is a tendency throughout the article to cite previous reviews. It would be more useful for readers – and for those researchers actively working with this model organism – if the citations were instead to the original research as much as possible.
In particular, the reviewers suggest citing the relevant primary literature for some of the basic information: e.g., the species distribution (currently Amorim et al., 2019), breathing physiology (currently Benjamin, 2008), diet (currently Lance et al., 2008), or reproductive period (currently Nakadera et al., 2015).
Unfortunately, in some places, the relevant primary literatures had to be removed from the text and had to be substituted with a review paper to comply with the word count. However, we agree with the reviewers and made some changes in the relevant parts. To note, Nakadera et al., 2015 is not a review, but a research study having integrated the situation of the lab and field, and so we kept this paper.
2) Benefits as a model organism:
The Introduction notes that: "The value of L. stagnalis also has been recognized in a surprisingly-wide range of applied biological fields". The Introduction would also benefit if the main features that make L. stagnalis a valuable model could be quickly summarised in a single sentence. In general, giving the evidence to support the assertions as to the value of this model helps to avoid parts of the text being perceived as dogmatism. Related to this, and while there was no doubt that the species is suited to test many hypotheses, there was a feeling among the reviewers that softening some claims and doing more to acknowledge that other models may be more relevant or have other specific advantages (depending on the issue or the state of knowledge at the start of the project) would be beneficial.
We agree with the reviewers and added the asked summary to the Introduction about the main features that make L. stagnalis a valuable model species in a wide range of biological fields. Moreover, we added some parts to the main text (Neuroscience and Ecotoxicology sections) that compare the relevance and usefulness of L. stagnalis to other models (mice, fish) for some types of studies.
3) Highlight connections between field and laboratory studies:
The reviewers also noted that most of the article reports on laboratory experiments, which likely reflects an existing bias in the literature. Yet given that the aim of this article (and the wider collection) is to provide a context of natural history, more reporting on studies in natural settings would be preferable. In the conclusions, the authors noted: "[ A deeper integration of 'field' information with input from laboratory] will allow for novel experimental designs and approaches developed in the laboratory to be applied to field populations and/or in the field". Other explicit examples of how the integration between laboratory and field experiments would be the key to success would strengthen the article. This would also be one way that the authors could help to address the "lack of integration between laboratory and field experiments" that they recognize as a major missing element in L. stagnalis research.
We are grateful to the reviewers for pointing this out. We made an effort to include and discuss additional field studies in the manuscript and in some places we made a comparison between the findings of the laboratory and field. Furthermore, we modified and supplemented the Conclusion.
4) Highlight contributions to diverse fields:
Lymnaea has had a long history in the field of developmental biology, and, in the field of physiology, where it was also one of the first model species to which the dynamic energy budget (DEB) theory was applied (see Zonneveld, C., & Kooijman, S. A. L. M. (1989). Application of a dynamic energy budget model to Lymnaea stagnalis (L.). Functional Ecology, 269-278). It would be good if these details could be briefly mentioned at the relevant points in the text.
There was a short paragraph in the original text about the contribution of L. stagnalis to the field of developmental biology, however, it was removed to comply with the word count. At the same time, we agree with the reviewers and added the suggested paragraph to the main text (Introduction and Natural history sections).
https://doi.org/10.7554/eLife.56962.sa2Article and author information
Author details
Funding
National Brain Research Project (2017-1.2.1-NKP-2017-00002)
- Zsolt Pirger
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Publication history
- Received:
- Accepted:
- Version of Record published:
Copyright
© 2020, Fodor et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 6,900
- views
-
- 483
- downloads
-
- 94
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Developmental Biology
Hair follicle development is initiated by reciprocal molecular interactions between the placode-forming epithelium and the underlying mesenchyme. Cell fate transformation in dermal fibroblasts generates a cell niche for placode induction by activation of signaling pathways WNT, EDA, and FGF in the epithelium. These successive paracrine epithelial signals initiate dermal condensation in the underlying mesenchyme. Although epithelial signaling from the placode to mesenchyme is better described, little is known about primary mesenchymal signals resulting in placode induction. Using genetic approach in mice, we show that Meis2 expression in cells derived from the neural crest is critical for whisker formation and also for branching of trigeminal nerves. While whisker formation is independent of the trigeminal sensory innervation, MEIS2 in mesenchymal dermal cells orchestrates the initial steps of epithelial placode formation and subsequent dermal condensation. MEIS2 regulates the expression of transcription factor Foxd1, which is typical of pre-dermal condensation. However, deletion of Foxd1 does not affect whisker development. Overall, our data suggest an early role of mesenchymal MEIS2 during whisker formation and provide evidence that whiskers can normally develop in the absence of sensory innervation or Foxd1 expression.
-
- Developmental Biology
The evolutionarily conserved Hippo (Hpo) pathway has been shown to impact early development and tumorigenesis by governing cell proliferation and apoptosis. However, its post-developmental roles are relatively unexplored. Here, we demonstrate its roles in post-mitotic cells by showing that defective Hpo signaling accelerates age-associated structural and functional decline of neurons in Caenorhabditis elegans. Loss of wts-1/LATS, the core kinase of the Hpo pathway, resulted in premature deformation of touch neurons and impaired touch responses in a yap-1/YAP-dependent manner, the downstream transcriptional co-activator of LATS. Decreased movement as well as microtubule destabilization by treatment with colchicine or disruption of microtubule-stabilizing genes alleviated the neuronal deformation of wts-1 mutants. Colchicine exerted neuroprotective effects even during normal aging. In addition, the deficiency of a microtubule-severing enzyme spas-1 also led to precocious structural deformation. These results consistently suggest that hyper-stabilized microtubules in both wts-1-deficient neurons and normally aged neurons are detrimental to the maintenance of neuronal structural integrity. In summary, Hpo pathway governs the structural and functional maintenance of differentiated neurons by modulating microtubule stability, raising the possibility that the microtubule stability of fully developed neurons could be a promising target to delay neuronal aging. Our study provides potential therapeutic approaches to combat age- or disease-related neurodegeneration.







