Repressing Ago2 mRNA translation by Trim71 maintains pluripotency through inhibiting let-7 microRNAs
Figures
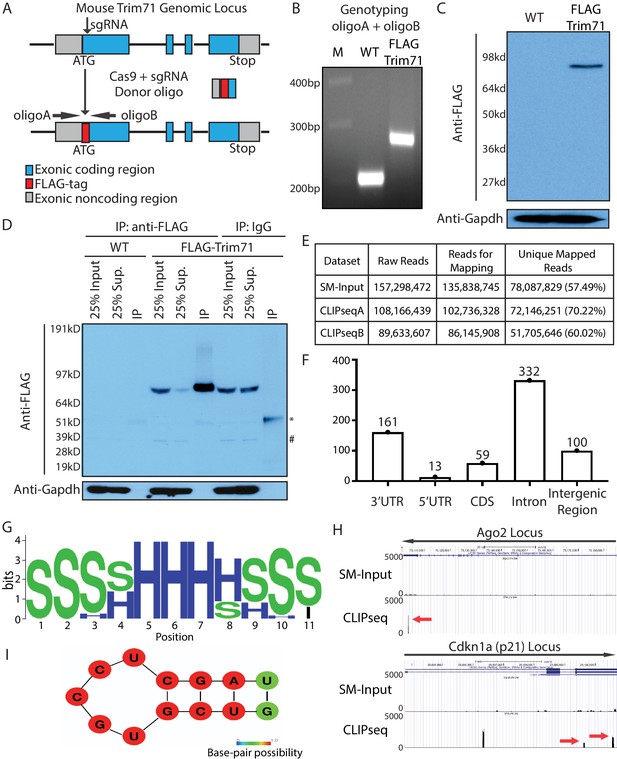
Transcriptome-wide identification of Trim71 target mRNAs in mouse embryonic stem cells (mESCs).
(A) Workflow for knock-in the FLAG-tag to the endogenous Trim71 locus in mESCs. (B) Genotyping of the FLAG-Trim71 mESCs using the two primers in (A). (C) Specific detection of the endogenous Trim71 via the FLAG-tag. Western blotting in the WT and the FLAG-Trim71 mESCs using an anti-FLAG monoclonal antibody. (D) Efficient and specific isolation of the endogenous Trim71. An anti-FLAG monoclonal antibody and mouse IgG were used to immunoprecipitate (IP) the endogenous Trim71 from the lysates of the WT and the FLAG-Trim71 mESCs. The inputs, supernatants (Sup.), and IP samples were subject to SDS-PAGE and western blotting using the indicated antibodies. * IgG heavy chain; # a non-specific band. (E) A table summarizing the number of reads from the Trim71 CLIP-seq experiments (F) Distribution of Trim71 binding regions in the mouse genome. (G) RNA secondary structures over-represented in the Trim71 binding regions within the 3’UTRs of mRNAs. (H) UCSC genome browser snapshots for the two Trim71 target mRNAs. The red arrows indicate the Trim71 binding regions in 3’UTRs. (I) Predicted RNA secondary structure in the Trim71 binding region in Ago2 mRNA’s 3’UTR.
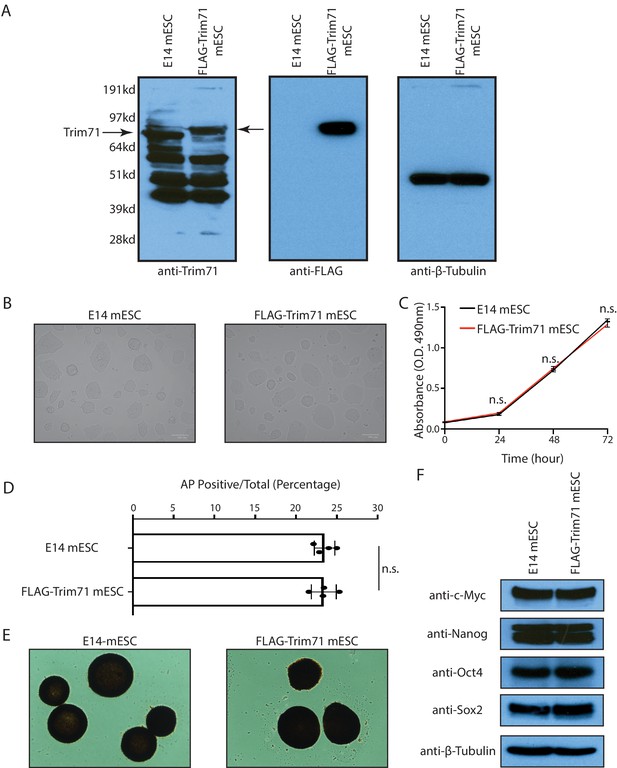
The FLAG-Trim71 mouse embryonic stem cells (mESCs) are phenotypically indistinguishable from the WT mESCs.
(A) Western blotting in the WT and the FLAG-Trim71 mESCs. (B) Cell morphology of the WT mESCs (E14) and the FLAG-Trim71 mESCs. (C) Growth curve of the WT and the FLAG-Trim71 mESCs determined by the CellTiter Assay. The results represent the means (± SD) of three independent experiments, and n.s. not significant (p>0.05) by the Student’s t-test. (D) Colony formation assay for the WT and the FLAG-Trim71 mESCs in the 2i + Lif medium. The results represent the means (± SD) of five independent experiments, and in each experiment, 100–200 colonies were evaluated for each type of cells. n.s. not significant (p>0.05) by the Student’s t-test. (E) Representative AP positive undifferentiated colonies from the WT and the FLAG-Trim71 mESCs. (F) Western blotting of pluripotency factors in the WT and the FLAG-Trim71 mESCs.
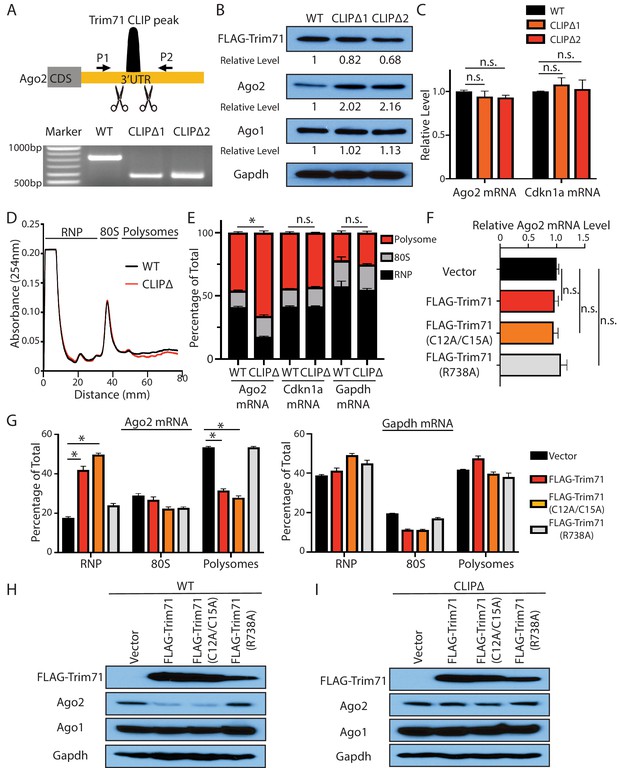
Trim71 represses Ago2 mRNA translation in mouse embryonic stem cells (mESCs).
(A) Deletion of Trim71 binding region in Ago2 mRNA’s 3’UTR. Genotyping PCR was performed using the indicated P1 and P2 primers. CLIPΔ1 and CLIPΔ2 are two independent clones from the genomic editing. (B) Western blotting in the WT, CLIPΔ1, and CLIPΔ2 mESCs. (C) qRT-PCR quantification of two Trim71 target mRNAs, Ago2 mRNA, and Cdkn1a mRNA, in the WT, CLIPΔ1, and CLIPΔ2 mESCs. 18S rRNA was used for normalization. (D) Polysome analysis in WT and CLIPΔ mESCs. (E) Inhibiting Trim71’s binding on Ago2 mRNA specifically upregulates its translation. The mRNA distribution in the RNP, the 80S, and the polysome fractions (shown in C) were quantified by qRT-PCR in the WT and the CLIPΔ mESCs, respectively. (F) Overexpression of Trim71 and its mutants does not change Ago2 mRNA level in the WT mESCs. The expression level of Ago2 mRNA in the WT mESCs with an empty vector, FLAG-Trim71, FLAG-Trim71(C12A/C15A), and FLAG-Trim71(R738A) was quantified by qRT-PCR. 18S rRNA was used for normalization. (G) Quantification of the indicated mRNA distributions in the RNP, 80S, and polysome fractions in the cell lysates from the WT mESCs expressing an empty vector, FLAG-Trim71, FLAG-Trim71(C12A/C15A), or FLAG-Trim71(R738A). (H) Western blotting in WT mESCs expressing an empty vector, FLAG-Trim71, a Trim71 ubiquitination mutant (C12A/C15A), and a Trim71 RNA-binding mutant (R738A). (I) Western blotting in CLIPΔ mESCs expressing an empty vector, FLAG-Trim71, a Trim71 ubiquitination mutant (C12A/C15A), and a Trim71 RNA-binding mutant (R738A). The qPCR results in (C) and (E–G) represent the means (± SD) of three independent experiments. *p<0.05, and n.s. not significant (p>0.05) by the Student’s t-test.
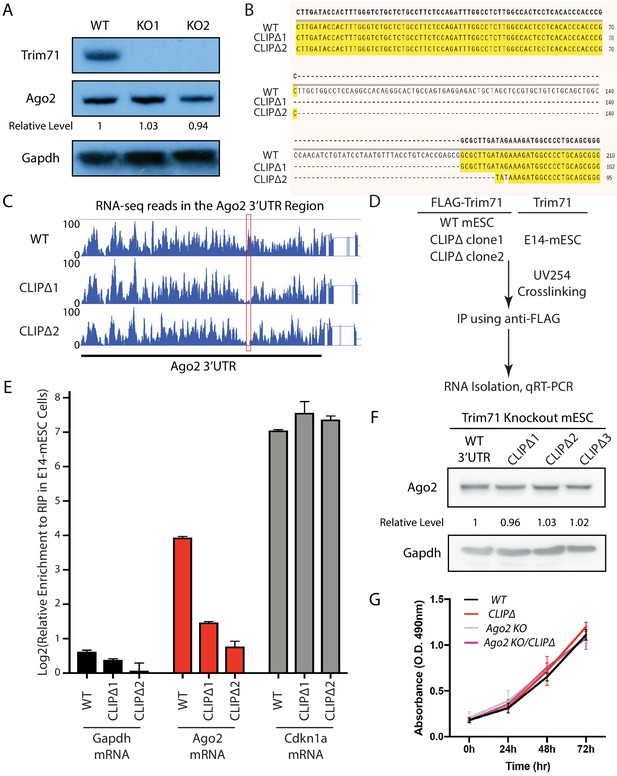
Specific disruption of the interaction between Trim71 and Ago2 mRNA.
(A) Trim71 was knocked out through CRISPR/Cas9-mediated genome editing. KO1 and KO2 are two independent knockout clones. (B) Sequence alignment of the Trim71 CLIP peak region from the WT, CLIPΔ1, and CLIPΔ2 mouse embryonic stem cells (mESCs). (C) RNA-seq reads in the 3’UTR of Ago2 from the WT, CLIPΔ1, and CLIPΔ2 mESCs. The red box indicates the Trim71 CLIP peak region. (D) Outline of the CLIP-qRT-PCR for examining Trim71-bound mRNAs. (E) Specific disruption of Trim71’s binding on Ago2 mRNA. qRT-PCR was performed for the indicated mRNAs isolated from the anti-FLAG IPs in the E14 mESCs, the WT, CLIPΔ1, and CLIPΔ2 mESCs. The mRNA signals from the E14 mESCs were set as 1 for relative comparison. The results represent the means (± SD) of three independent experiments. (F) The Trim71-binding site in the 3’UTR of Ago2 mRNA was deleted through CRISPR/Cas9-mediated genome editing in the Trim71 knockout mESCs. Ago2 level was examined by western blot using Gapdh for normalization in determining the relative expression levels of Ago2. (G) WT, CLIPΔ, Ago2 KO, and CLIPΔ/Ago2 KO mESCs proliferate at similar rates. The results represent the means (± SD) of three independent experiments. n.s. not significant (p>0.05) by the Student’s t-test.
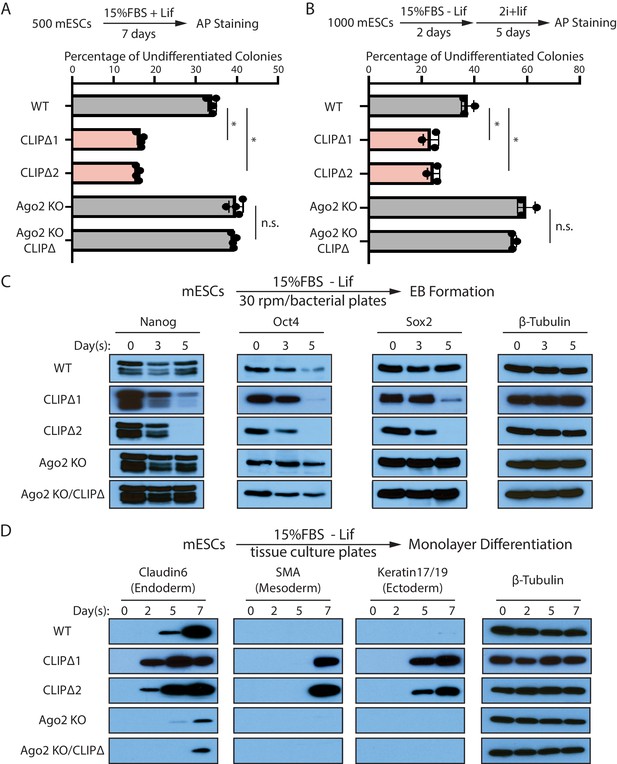
Trim71-mediated repression of Ago2 mRNA translation is required for maintaining pluripotency.
(A) Colony formation assay for mouse embryonic stem cells (mESCs). The mESCs were cultured in 15%FBS + Lif for 7 days, and the resultant colonies were fixed and stained for AP. (B) Exit pluripotency assay for mESCs. The mESCs were induced to exit pluripotency in medium without Lif for 2 days and then switched to 2i+Lif medium for 5 days. The resultant colonies were fixed and stained for AP. In (A) and (B), the colony morphology and AP intensity were evaluated through microscopy. 100–200 colonies were examined each time to determine the percentage of undifferentiated colonies. The results represent the means (± SD) of four independent experiments. *p<0.05, and n.s. not significant (p>0.05) by the Student’s t-test. (C) Western blotting of pluripotency factors during EB formation. (D) Western blotting of markers of lineage-committed cells during mESC monolayer differentiation.
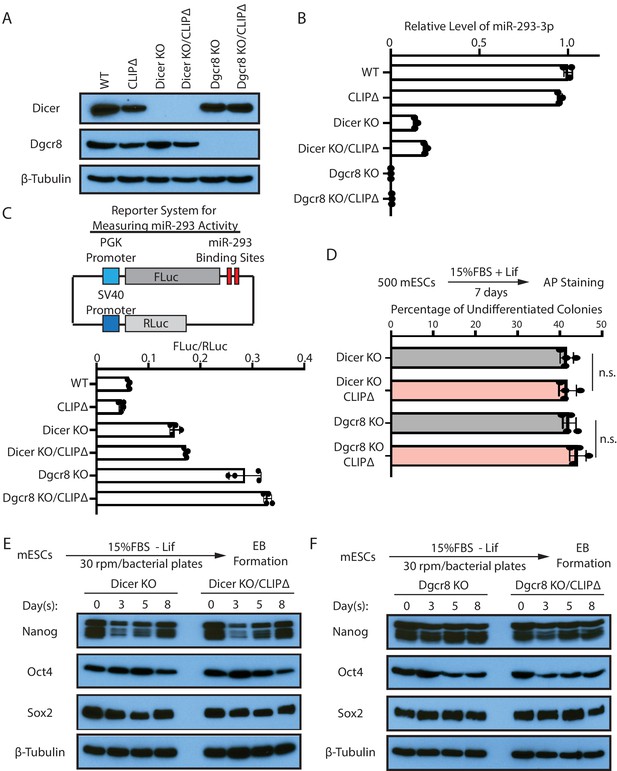
The stemness defects caused by the loss of Trim71-mediated repression of Ago2 mRNA translation is dependent on the miRNA pathway.
(A) Western blotting in the WT, CLIPΔ, Dicer KO, and Dgcr8 KO mouse embryonic stem cells (mESCs). (B) qRT-PCR quantification of mature miR-293 in the WT, CLIPΔ, Dicer KO, and Dgcr8 KO mESCs. (C) Reporter assays for measuring miR-293 activity. Two miR-293 binding sites are in the 3’UTR of the FLuc mRNA, and the RLuc mRNA from the same plasmid is used as an internal control. The ratio of FLuc to RLuc indicates the efficiency of miR293-mediated repression. (D) Colony formation assays for the Dicer KO, Dicer KO/CLIPΔ, Dgcr8 KO, Dgcr8 KO/CLIPΔ mESCs. The results represent the means (± SD) of four independent experiments, and in each experiment, 100–200 colonies were evaluated for each type of cells. n.s. not significant (p>0.05) by the Student’s t-test. (E) Western blotting of pluripotency factors during EB formation from the Dicer KO and Dicer KO/CLIPΔ mESCs. (F) Western blotting of pluripotency factors during EB formation from the Dgcr8 KO and Dgcr8 KO/CLIPΔ mESCs.
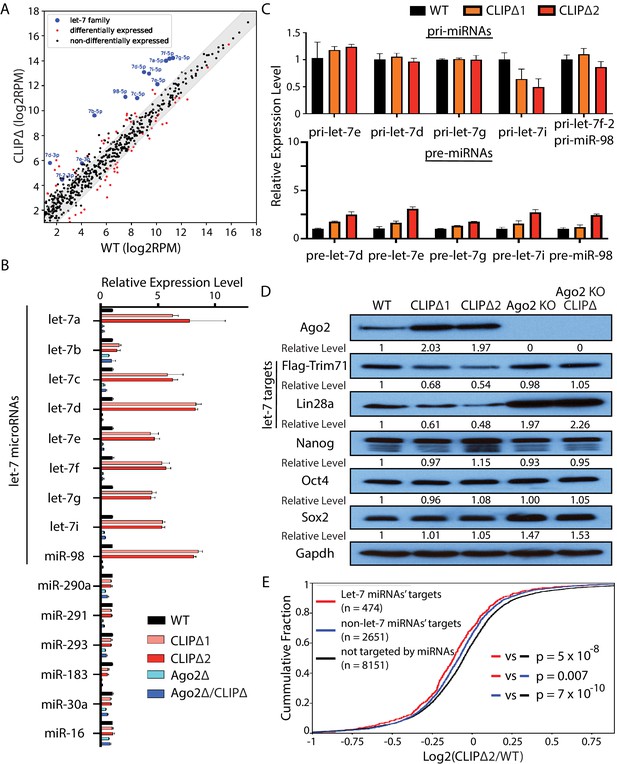
Loss of Trim71-mediated repression of Ago2 mRNA translation results in significant post-transcriptional increase of let-7 miRNAs.
(A) Comparison of global miRNA expression in WT and CLIPΔ mouse embryonic stem cells (mESCs). The results are the average of four independent small RNA-seqs in the WT and the CLIPΔ mESCs. Blue dots: let-7 miRNAs; red dot: differentially expressed miRNAs; black dots: non-differentially expressed miRNAs. (B) qRT-PCR on let-7 miRNAs and non-let-7 miRNAs. For each miRNA, the expression level in WT cells was set as 1 for relative comparison. U6 RNA was used for normalization. (C) qRT-PCR on the let-7 pri-miRNAs and pre-miRNAs. For pri-miRNAs and pre-miRNAs, the expression level in the WT cells was set as 1 for relative comparison. 18S rRNA and U6 RNA were used for pri-miRNA and pre-miRNA normalization, respectively. The results in (B) and (C) are from three independent replicates. (D) Western blotting of Ago2, conserved let-7 targets, and non-let-7 targets. Gapdh was used for normalization in calculating the relative expression levels. (E) Cumulative distributions of expression level changes of let-7 targets, miRNA targets without let-7 binding sites, and mRNAs not targeted by miRNAs in WT and CLIPΔ mESCs.
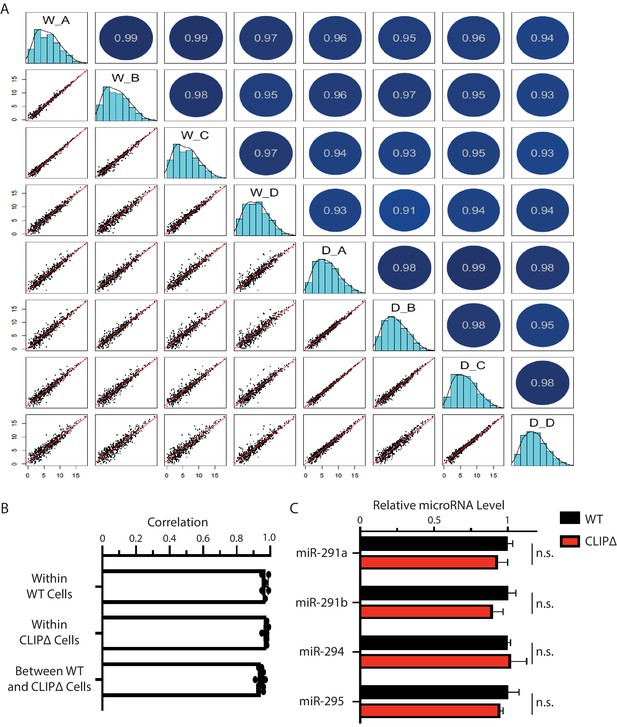
The loss of Trim71-mediated repression of Ago2 mRNA translation does not alter global miRNA in mouse embryonic stem cells (mESCs).
(A) The relative miRNA expression pattern is highly similar between the WT and the CLIPΔ mESCs. Scatter plots show pairwise comparisons of the miRNA levels (log2 reads per million mapped reads, RPM) for annotated miRNAs from the four biological replicates. (B) Correlations of global miRNA expression patterns in the WT and the CLIPΔ mESCs determined by small RNA-seq from the four sets of biological replicates. (C) The relative levels of miRNAs. U6 RNA was used for normalization. The results represent the means (± SD) of three independent experiments. n.s. not significant (p>0.05) by the Student’s t-test.
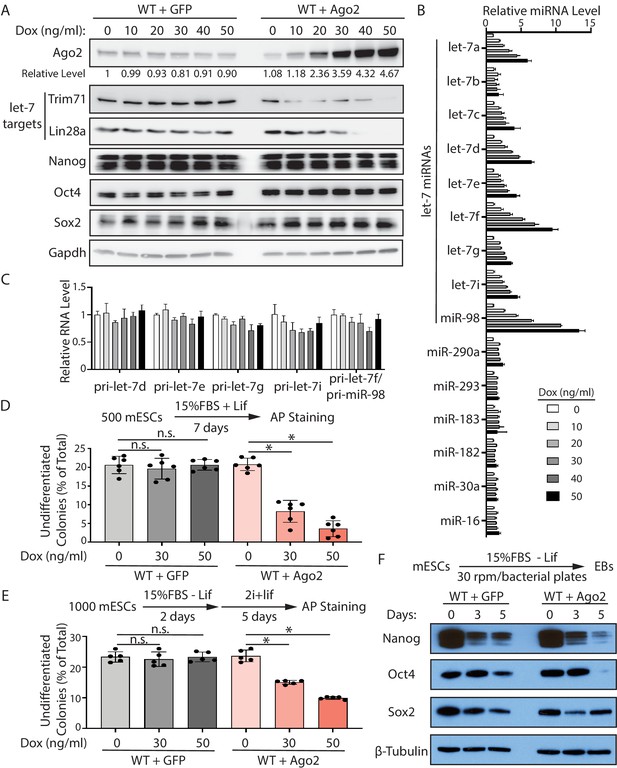
Increased Ago2 leads to significant increase of let-7 miRNAs and accelerated differentiation in mouse embryonic stem cells (mESCs).
(A) Western blotting in mESCs with dox-inducible expression of Ago2. Gapdh was used for normalization in calculating the relative expression of Ago2. (B) Relative levels of miRNAs in mESCs with dox-inducible expression of Ago2. U6 RNA was used for normalization. (C) Relative levels of pri-miRNAs in mESCs with dox-inducible expression of Ago2. 18S rRNA was used for normalization. In (B) and (C), the miRNA and pri-miRNA expression levels in mESCs without dox treatment were set as 1 for determining relative levels. The results are from four biological replicates. (D) Colony formation assay for mESCs with dox-inducible expression of either GFP or Ago2. (E) Exit pluripotency assay for mESCs with dox-inducible expression of either GFP or Ago2. The results in (D) and (E) represent the means (± SD) of six independent experiments. *p<0.05, and n.s. not significant (p>0.05) by the Student’s t-test. (F) Western blot analysis on pluripotency factors during EB formation from the GFP-expressing mESCs and Ago2-expressing mESCs (treated with 50 ng/ml dox).
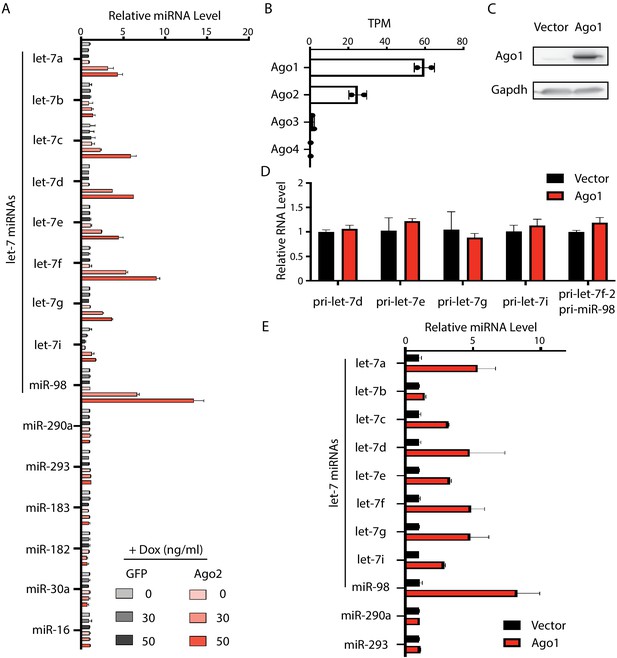
Increased Ago proteins in mouse embryonic stem cells (mESCs) result in a specific increase of let-7 miRNAs.
(A) Relative expression levels of miRNAs in the mESCs with dox-inducible expression of either GFP or Ago2. U6 RNA was used for normalization. (B) mRNA expression levels of Ago1, Ago2, Ago3, and Ago4 in mESCs. TPM, transcripts per kilobase million reads. (C) Western blot of mESCs with inducible exogeneous Ago1 expression. (D) qRT-PCR on the let-7 pri-miRNAs. The expression level in the WT cells was set as 1 for relative comparison. 18S rRNA was used for normalization. (E) qRT-PCR on let-7 miRNAs and non-let-7 miRNAs. For each miRNA, the expression level in WT cells was set as 1 for relative comparison. U6 RNA was used for normalization. The qPCR results in (A) and (D and E) represent the means (± SD) of three independent experiments.
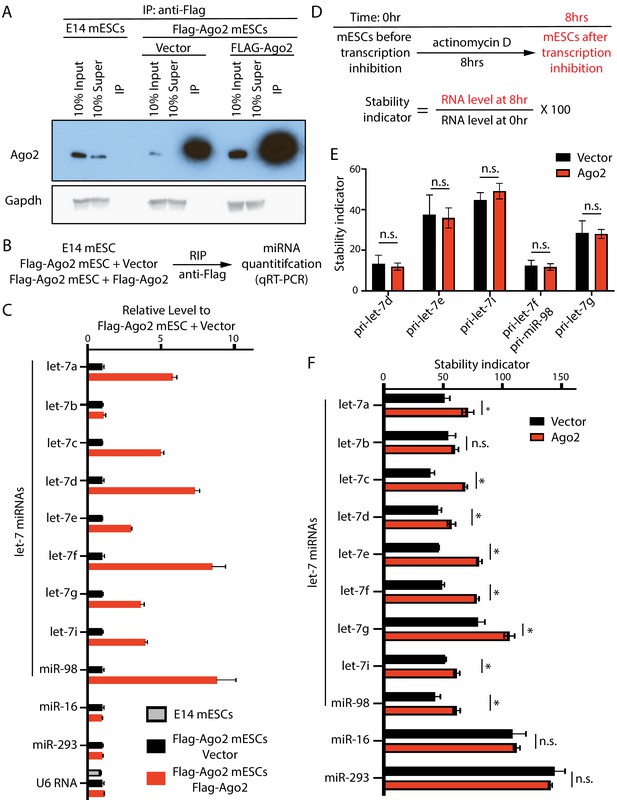
The increased let-7 miRNAs are bound and stabilized by the elevated Ago2 in mouse embryonic stem cells (mESCs).
(A) Specific isolation of both endogenous Ago2 and dox-induced FLAG-Ago2 from mESCs with a FLAG-tag knock-in at the N-terminus of Ago2 locus. Super: supernatant; IP: immunoprecipitated sample. (B) Outline of the RNA immunoprecipitation (RIP) experiment. (C) qRT-PCR quantification of the Ago2-bound RNAs. (D) Outline of the actinomycin-D(5 µg/ml)-mediated transcriptional shut-off experiment to measure RNA stability in mESCs. (E) Stabilities of let-7 pri-miRNAs are not sensitive to Ago2 levels. (F) Increased Ago2 specifically stabilizes let-7 miRNAs in mESCs. The results from (C), (E), and (F) represent the means (± SD) of three independent experiments. *p<0.05, and n.s. not significant (p>0.05) by the Student’s t-test.
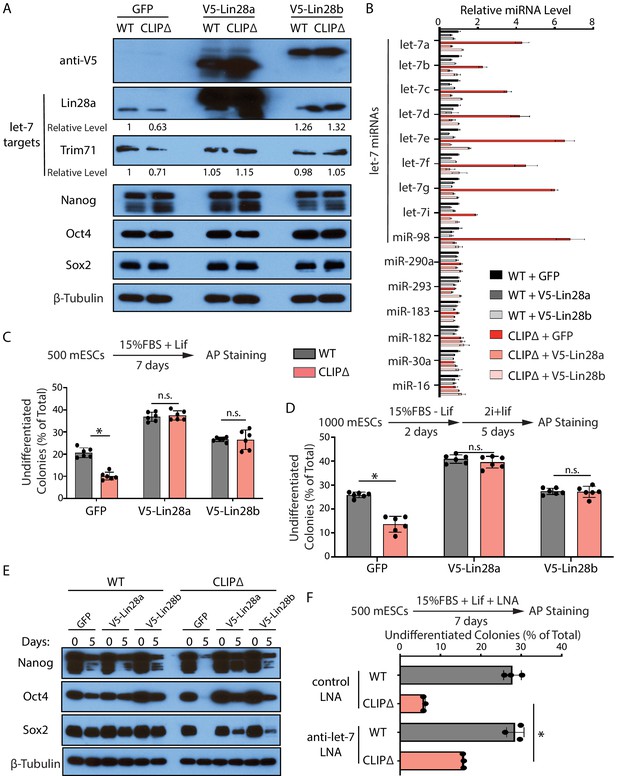
Inhibiting let-7 miRNAs blocks the stemness defects caused by the loss of Trim71-mediated repression of Ago2 mRNA translation.
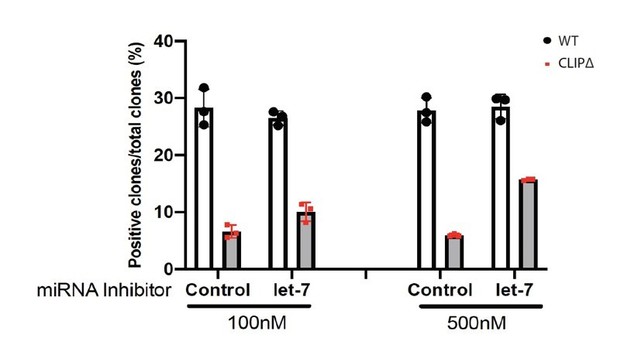
(A) Western blotting in WT and CLIPΔ mouse embryonic stem cells (mESCs) expressing GFP, V5-Lin28a, or Lin28b. Beta-tubulin was used for normalization in determining the relative expression level of let-7 targets Lin28a and Trim71. (B) Relative levels of miRNAs. U6 RNA was used for normalization. The results represent the means (± SD) of four biological replicates. (C) Colony formation assay for WT and CLIPΔ mESCs expressing GFP, V5-Lin28a, or Lin28b. (D) Exit pluripotency assay for WT and CLIPΔ mESCs expressing GFP, V5-Lin28a, or Lin28b. The results in (C) and (D) represent the means (± SD) of six independent experiments. (E) Western blotting of pluripotency factors during EB formation at Day 0 and Day 5 of WT and CLIPΔ mESCs expressing GFP, V5-Lin28a, or Lin28b. (F) Colony formation assay for WT and CLIPΔ mESCs cultured in the presence of 500 nM anti-let-7 LNA or a control LNA. The results represent three independent experiments. *p<0.05, and n.s. not significant (p>0.05) by the Student’s t-test.
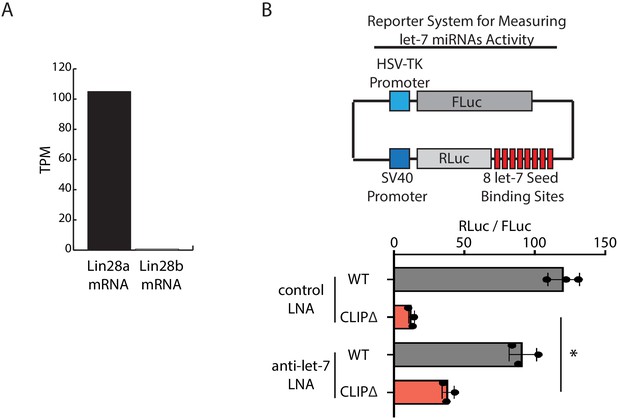
Inhibition of let-7 miRNAs in mouse embryonic stem cells (mESCs).
(A) Lin28a and Lin28b mRNA levels, determined as TPM (transcripts per million reads) from RNA-seq in the WT mESCs. (B) Inhibition of let-7 activities by LNAs in mESCs. The dual luciferase reporter system for measuring let-7 miRNA activities was shown in the top panel. The let-7 activities, determined as RLuc/FLuc, were examined in the indicated mESCs transfected with the dual luciferase reporter and the indicated LNAs. The results represent the means (± SD) of three independent experiments. *p<0.01 by the Student’s t-test.
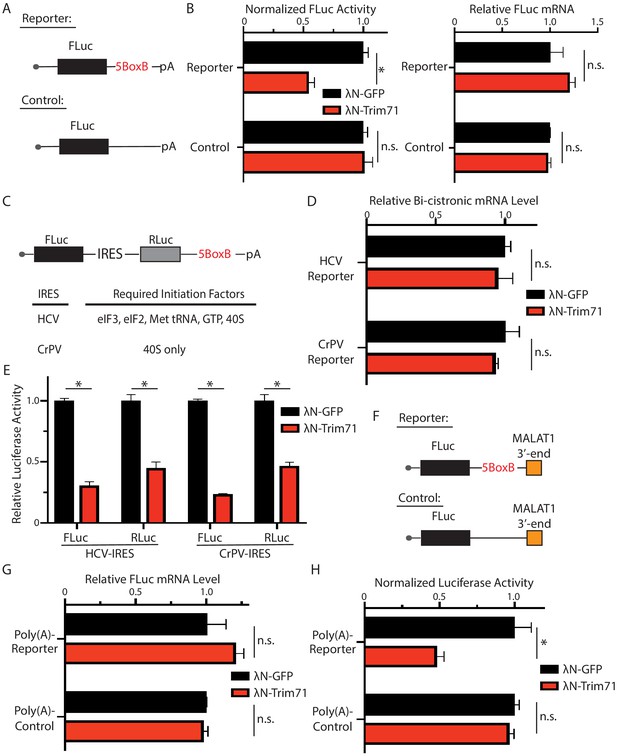
Trim71 represses mRNA translation at post-initiation step(s) in mouse embryonic stem cells (mESCs).
(A) FLuc reporters for the tethering assay. (B) The FLuc activity and mRNA level determined in the tethering assay. (C) The IRES-containing bicistronic reporters. (D) mRNA levels from the IRES-containing reporters. (E) Luciferase activities from the IRES-containing reporters. (F) The poly(A) minus FLuc reporters. (G) mRNA levels from the poly(A) minus reporters. (H) FLuc activities from the poly(A) minus reporters. The results represent the means (± SD) of three independent experiments. *p<0.05, and ns. not significant (p>0.05) by the Student’s t-test.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Mouse monoclonal anti-FLAG M2 | Sigma-Aldrich | Cat# F1804 | WB (1:5000) IP |
| Antibody | Normal mouse IgG | Santa Cruz Biotechnology | Cat# sc-2025 | IP |
| Antibody | Mouse monoclonal anti-GAPDH (6C5) | Santa Cruz Biotechnology | Cat# sc-32233 | WB (1:5000) |
| Antibody | Rabbit monoclonal anti-beta-Tubulin | Selleckchem | Cat# A5032 | WB (1:5000) |
| Antibody | Rabbit monoclonal anti-Ago1 (D84G10) | Cell Signaling Technology | Cat# 5053 | WB (1:1000) |
| Antibody | Rabbit monoclonal anti-Ago2 (C34C6) | Cell Signaling Technology | Cat# 2897 | WB (1:1000) |
| Antibody | Mouse monoclonal anti-Oct-4 | BD Transduction Laboratories | Cat# 611202 | WB (1:5000) |
| Antibody | Rabbit monoclonal anti-Nanog (D2A3) | Cell Signaling Technology | Cat# 8822 | WB (1:3000) |
| Antibody | Rabbit monoclonal anti-Sox2 (D9B8N) | Cell Signaling Technology | Cat# 23064 | WB (1:3000) |
| Antibody | Rabbit monoclonal anti-Keratin 17/19 (D32D9) | Cell Signaling Technology | Cat# 3984 | WB (1:1000) |
| Antibody | Rabbit monoclonal anti-a-SMA (D4K9N) | Cell Signaling Technology | Cat# 19245 | WB (1:1000) |
| Antibody | Mouse monoclonal anti-Claudin-6 (A-4) | Santa Cruz Biotechnology | Cat# sc-393671 | WB (1:1000) |
| Antibody | Rabbit polyclonal anti-Dicer | Sigma-Aldrich | Cat# SAB4200087 | WB (1:3000) |
| Antibody | Rabbit monoclonal anti-DGCR8 | Abcam | Cat# ab191875 | WB (1:3000) |
| Antibody | Rabbit polyclonal anti-V5 Tag | Bethyl | Cat# A190-120A | WB (1:5000) |
| Antibody | Rabbit monoclonal anti-Lin28A (D1A1A) | Cell Signaling Technology | Cat# 8641 | WB (1:5000) |
| Antibody | Sheep polyclonal anti-Trim71 | R and D Systems | Cat# AF5104 | WB (1:1000) |
| Antibody | Goat Anti-Rabbit IgG (H L)-HRP Conjugate | Bio-Rad | Cat# 170–6515 | WB (1:5000) |
| Antibody | Goat Anti-Mouse IgG (H L)-HRP Conjugate | Bio-Rad | Cat# 170–6516 | WB (1:5000) |
| Antibody | Donkey anti-Sheep IgG-HRP Conjugate | R and D Systems | Cat# HAF016 | WB (1:2000) |
| Chemical compound, drug | DMEM/F-12 | Gibco | Cat# 12500096 | |
| Chemical compound, drug | FBS | Millipore | Cat# ES-009-B | |
| Chemical compound, drug | mLIF | Millipore | Cat# ESG1107 | |
| Chemical compound, drug | PD0325901 | APExBio | Cat# A3013 | |
| Chemical compound, drug | CHIR99021 | APExBio | Cat# A3011 | |
| Chemical compound, drug | N2 | Millipore | Cat# SCM012 | |
| Chemical compound, drug | B27 | Thermo Fisher Scientific | Cat# 17504044 | |
| Chemical compound, drug | MEM NEAA | Gibco | Cat# 11140–50 | |
| Chemical compound, drug | Penicillin–Streptomycin | Gibco | Cat# 11548876 | |
| Chemical compound, drug | L-glutamine | Sigma-Aldrich | Cat# G7513 | |
| Chemical compound, drug | β-mercaptoethanol | Sigma-Aldrich | Cat# M3148 | |
| Chemical compound, drug | Accutase | Millipore | Cat# SF006 | |
| Chemical compound, drug | Fugene6 | Promega | Cat# E2691 | |
| Chemical compound, drug | Puromycin | Sigma-Aldrich | Cat# P9620 | |
| Chemical compound, drug | Doxycycline | Sigma-Aldrich | Cat# D9891 | |
| Chemical compound, drug | Protease inhibitors | Bimake | Cat# B14001 | |
| Chemical compound, drug | Gelatin | Sigma-Aldrich | Cat# G1890 | |
| Chemical compound, drug | One Step-RNA Reagent | Bio Basic | Cat# BS410A | |
| Chemical compound, drug | DNase 1 | NEB | Cat# M0303L | |
| Chemical compound, drug | RNase1 | Ambion | Cat# AM2295 | |
| Chemical compound, drug | SUPERaseIn RNase Inhibitor | Ambion | Cat# AM2696 | |
| Chemical compound, drug | SuperScript II Reverse Transcriptase | Invitrogen | Cat# 18064014 | |
| Chemical compound, drug | SsoAdvanced Universal SYBR Green Supermix | Bio-Rad | Cat# 1725270 | |
| Chemical compound, drug | Q5 High-Fidelity DNA Polymerase | NEB | Cat# M0491L | |
| Chemical compound, drug | Let-7 LNA | Qiagen | Cat# YFI0450006 | |
| Chemical compound, drug | Control LNA | Qiagen | Cat# 339137 | |
| Chemical compound, drug | Actinomycin D | Thermo Fisher Scientific | Cat# 11805017 | |
| Commercial assay or kit | Alkaline Phosphatase Assay Kit | System Biosciences | Cat# AP100R-1 | |
| Commercial assay or kit | Gibson Assembly Master Mix | NEB | Cat# E2611L | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | Cat# E1960 | |
| Commercial assay or kit | CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) | Promega | Cat# G3582 | |
| Commercial assay or kit | Dynabeads M-270 Epoxy | Invitrogen | Cat# 14301 | |
| Commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Cat# 23225 | |
| Commercial assay or kit | Mir-X miRNA First Strand Synthesis Kit | Takara | Cat# 638313 | |
| Commercial assay or kit | NEBNext Ultra Directional RNA Library Prep Kit | Illumina | Cat# E7420S | |
| Commercial assay or kit | NEBNext Multiplex Small RNA Library Prep Set | Illumina | Cat# E7300S | |
| Cell line (M. musculus) | ES-E14TG2a mESC | ATCC | CRL-1821 | |
| Cell line (M. musculus) | FLAG-Trim71 mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 CLIP∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 Ago2∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 Dgcr8∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 Dicer∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 CLIP∆ Ago2∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 CLIP∆ Dgcr8∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71 CLIP∆ Dicer∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Ago2 mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71∆ mESC | this paper | ||
| Cell line (M. musculus) | FLAG-Trim71∆ CLIP∆ mESC | this paper | ||
| Recombinant DNA reagent | PiggyBac-based dox-inducible expression vector | this paper | pWH406 | |
| Recombinant DNA reagent | Inducible mouse FLAG-Trim71 expressing vector | this paper | pWH826 | |
| Recombinant DNA reagent | Inducible mouse FLAG-Trim71-C12AC15A expressing vector | this paper | pWH831 | |
| Recombinant DNA reagent | Inducible mouse FLAG-Trim71-R738A expressing vector | this paper | pWH840 | |
| Recombinant DNA reagent | Inducible mouse Ago2 expressing vector | this paper | pWH1070 | |
| Recombinant DNA reagent | Inducible GFP expressing vector | this paper | pWH1055 | |
| Recombinant DNA reagent | Inducible mouse V5-Lin28A expressing vector | this paper | pWH1081 | |
| Recombinant DNA reagent | Inducible mouse V5- Lin28B expressing vector | this paper | pWH1082 | |
| Recombinant DNA reagent | sgRNA and Cas9 expressing vector (pX458) pWH464 | Addgene | Cat# 48138 | |
| Recombinant DNA reagent | Super PiggyBac Transposase expressing vector (pWH252) | System Biosciences | Cat# PB210PA-1 | |
| Recombinant DNA reagent | The Luciferase reporter for measuring miR-293 activity | this paper | pWH854 | |
| Recombinant DNA reagent | FLuc-5BoxB reporter | PMID:28635594 | pWH290 | |
| Recombinant DNA reagent | The control reporter for the FLuc-5BoxB | PMID:28635594 | pWH291 | |
| Recombinant DNA reagent | lambdaN-GFP expressing plasmid | PMID:28635594 | pWH294 | |
| Recombinant DNA reagent | lambdaN-Trim71 expressing plasmid | this paper | pWH815 | |
| Recombinant DNA reagent | HCV-IRES bicistronic reporter | PMID:28635594 | pWH530 | |
| Recombinant DNA reagent | CrPV-IRES bicistronic reporter | PMID:28635594 | pWH531 | |
| Recombinant DNA reagent | FLuc-5BoxB-Malat1 reporter | PMID:28635594 | pWH569 | |
| Recombinant DNA reagent | FLuc-Malat1 reporter | PMID:28635594 | pWH570 | |
| Software, algorithm | FastQC v0.11.4 | Andrews S. 2010 | https://www.bioinformatics.babraham.ac.uk/projects/download.html | |
| Software, algorithm | Bowtie v1.1.2 | PMID:19261174 | http://bowtie-bio.sourceforge.net/index.shtml | |
| Software, algorithm | STAR v2.5.0 | PMID:23104886 | https://github.com/alexdobin/STAR; Lorenz et al., 2011 | |
| Software, algorithm | Piranha v1.2.1 | PMID:23024010 | http://smithlabresearch.org/software/piranha/ | |
| Software, algorithm | iCount v2.0.1 | Lovci et al., 2013 | https://icount.readthedocs.io/en/latest/ | |
| Software, algorithm | CLIPper v1.1 | Lovci et al., 2013 | https://github.com/YeoLab/clipper/wiki/CLIPper-Home; Lovci et al., 2013 | |
| Software, algorithm | CTK package v1.0.9 | PMID:27797762 | https://zhanglab.c2b2.columbia.edu/index.php/CTK_Documentation | |
| Software, algorithm | BEDtools v2.25.0 | PMID:20110278 | https://bedtools.readthedocs.io/en/latest/ | |
| Software, algorithm | SAMtools v0.1.19 | PMID:19505943 | http://samtools.sourceforge.net/ | |
| Software, algorithm | RNAfold v2.1.5 | PMID:22115189 | https://www.tbi.univie.ac.at/RNA/ RNAfold.1.html | |
| Software, algorithm | WebLogo v3.6.0 | PMID:15173120 | http://weblogo.threeplusone.com/ | |
| Software, algorithm | HISAT2 v2.1.0 | PMID:31375807 | https://daehwankimlab.github.io/hisat2/ | |
| Software, algorithm | HTSeq v0.11.1 | PMID:25260700 | https://htseq.readthedocs.io/en/release_0.11.1 | |
| Software, algorithm | R package EdgeR v3.26.8 | PMID:19910308 | https://bioconductor.org/packages/release/bioc/html/edgeR.html | |
| Software, algorithm | TargetScan v7.2 | PMID:26267216 | http://www.targetscan.org/vert_72/ |
Additional files
-
Supplementary file 1
Trim71-binding regions in its target mRNA identified by the CLIP-seq.
- https://cdn.elifesciences.org/articles/66288/elife-66288-supp1-v2.xlsx
-
Supplementary file 2
miRNAs detected in the WT and the CLIPΔ mESCs.
The first tab lists the differentially expressed miRNAs, and the second tab lists the non-differentially expressed miRNAs. The expression level is indicated by reads per million (RPM).
- https://cdn.elifesciences.org/articles/66288/elife-66288-supp2-v2.xlsx
-
Supplementary file 3
Antibodies, plasmids, and oligonucleotides used in this study.
- https://cdn.elifesciences.org/articles/66288/elife-66288-supp3-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66288/elife-66288-transrepform-v2.docx