Mir204 and Mir211 suppress synovial inflammation and proliferation in rheumatoid arthritis by targeting Ssrp1
Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease characterized by synovial hyperplasia. Mir204 and Mir211 are homologous miRNAs with the same gene targeting spectrum. It is known that Mir204/211 play an important role in protecting osteoarthritis development; however, the roles of Mir204/211 in RA disease have not been determined. In the present study, we investigated the effects and molecular mechanisms of Mir204/211 on synovial inflammation and hyperproliferation in RA. The effects of Mir204/211 on the inflammation and abnormal proliferation in primary fibroblast-like synoviocytes (FLSs) were examined by Mir204/211 gain-of-function and loss-of-function approaches in vitro and in vivo. We identified the structure-specific recognition protein 1 (Ssrp1) as a downstream target gene of Mir204/211 based on the bioinformatics analysis. We overexpressed Ssrp1and Mir204/211 in FLS to determine the relationship between Ssrp1 and Mir204/211 and their effects on synovial hyperplasia. We created a collagen-induced arthritis (CIA) model in wild-type as well as Mir204/211 double knockout (dKO) mice to induce RA phenotype and administered adeno-associated virus (AAV)-mediated Ssrp1-shRNA (AAV-shSsrp1) by intra-articular injection into Mir204/211 dKO mice. We found that Mir204/211 attenuated excessive cell proliferation and synovial inflammation in RA. Ssrp1 was the downstream target gene of Mir204/211. Mir204/211 affected synovial proliferation and decelerated RA progression by targeting Ssrp1. CIA mice with Mir204/211 deficiency displayed enhanced synovial hyperplasia and inflammation. RA phenotypes observed in Mir204/211 deficient mice were significantly ameliorated by intra-articular delivery of AAV-shSsrp1, confirming the involvement of Mir204/211-Ssrp1signaling during RA development. In this study, we demonstrated that Mir204/211 antagonize synovial hyperplasia and inflammation in RA by regulation of Ssrp1. Mir204/211 may serve as novel agents to treat RA disease.
Editor's evaluation
This important study provides new understanding on the role of miR-204/-211 in the progression of rheumatoid arthritis and the underlying mechanisms. The evidence supporting the conclusions is convincing, with rigorous in vitro cell culture assays and in vivo mouse studies. The work will be of interest for skeletal biologists studying the pathogenesis of rheumatoid arthritis.
https://doi.org/10.7554/eLife.78085.sa0Introduction
Rheumatoid arthritis (RA) is a chronic systemic disease, mainly characterized by synovitis, synovial hyperplasia, pannus formation, and osteochondral destruction (Muraki et al., 2018; Shiraishi et al., 2017). Due to the joint dysfunction caused by RA, patients often have limited activities, and the quality of life is seriously reduced (Wang et al., 2020a). Although many effective drugs have been developed, the long treatment cycle of RA significantly increases the incidence of adverse drug reactions. Therefore, to provide the basis for individualized therapy, it is vital to elicit the molecular mechanisms of RA pathogenesis.
MicroRNAs (miRNAs) are small, non-coding RNA molecules consisting of 21–24 base pairs that specifically bind to target genes through the 3' untranslated region (3'-UTR) and control the expression of multiple genes at the post-transcriptional level (Zhang et al., 2019). In recent years, cumulative evidences have shown that miRNAs play crucial roles in the development of RA by regulating cell viability, apoptosis, and invasion (Lai et al., 2017; Stanczyk et al., 2008). In synovial tissue of patients with RA, miRNA expression was significantly altered compared with those of healthy individuals. The abnormal miRNAs promote the expression of proinflammatory cytokine and enzymes that erodes the cartilage matrix by interfering Wnt, NF-κB, and JAK/STAT signaling pathways (Han et al., 2022).
The Mir204 and Mir211 have similar nucleotide sequences and share the same seed sequence. There are only two nucleotide differences in humans and one nucleotide difference in mice. These structural similarity and homology between these two miRNAs allow them to have the same gene targeting spectrum (Lee et al., 2016). In breast cancer, Mir204 acts as a tumor suppressor. The expression of Mir204 induced apoptosis while the deletion of Mir204 gene led to abnormal cell invasion (Imam et al., 2012). MiRNA has also become an indispensable regulatory factor in bone pathophysiology (Asahara, 2016). We have previously reported that homologous miRNAs, Mir204 and Mir211, together protecting joint from osteoarthritis development. The loss of Mir204/211 led to the upregulation of matrix degradation enzymes in articular chondrocytes, resulting in articular cartilage destruction and synovial hyperplasia (Huang et al., 2019). Since synovial inflammation is now considered as the major pathology of RA and hyperplasia of fibroblast-like synoviocyte (FLS) displayed tumor-like behaviors that contribute to pannus growth, inflammation, and cartilage damage, here we aimed to investigate whether Mir204/211 play an indispensable role in RA synovial inflammation and hyperplasia (Winchester et al., 2015).
Structure-specific recognition protein 1 (SSRP1) is a subunit of the histone chaperone transcription (FACT) complex and is involved in almost all chromatin-related processes. As a transcription factor, Ssrp1 regulates multiple cell processes, including cell cycle regulation and DNA repair (Gao and Xiong, 2018; Wang et al., 2019). In addition, Ssrp1 expression was significantly upregulated in a variety of tumors, such as breast and ovarian cancer, and was strongly associated with poorer prognosis. The knockdown of Ssrp1 expression by siRNA technique depressed the proliferation of glioma cells (Liao et al., 2017). SSRP1 inhibitor, CBL0137, induced cell death by down-regulating gene expression in the NF-κB signaling pathway (Barone et al., 2017). Wu et al. suggested that Ssrp1 modulates P53 pathway and its downstream molecules, and Ssrp1 may be involved in the cell cycle progression through regulation of P53 pathway (Wu et al., 2019a). Although Ssrp1 has been shown to stimulate proliferation through cell cycle regulation in a variety of human cancers, the role, mechanism, and clinical significance of Ssrp1 in RA remain unclear.
Collagen-induced arthritis (CIA) shares similar characteristics with RA, including synovial hyperplasia and inflammatory articular cartilage destruction, and has been extensively used in RA research (Song et al., 2018; Bas et al., 2016). Multiple lines of evidence have shown that RA FLSs are the principal cells in participation in the initiation, development, and deterioration of joint inflammation in RA disease (Xiao et al., 2021). In this study, we determined the effects of Mir204/211 on the inflammatory response and proliferation of primary CIA FLS by overexpression or knockdown of Mir204/211, as well as the downstream mechanisms of Mir204/211 in mitigation of synovial inflammation and excessive proliferation. We determined the relationship between Mir204/211 and Ssrp1 and revealed their effects on CIA-induced synovial cell proliferation in Mir204/211 double knockout (dKO) mice. Our findings suggest that Mir204/211 suppressed synovial inflammation and proliferation in RA by targeting Ssrp1.
Results
Mir204/Mir211 downregulate in FLS of CIA mice
Using FLS of normal mice as the control group and U6 as the internal reference gene, we detected Mir204/Mir211 expression in FLS of CIA mice (Figure 1—figure supplement 1). As seen in Figure 1A, expression levels of Mir204 and Mir211 were significantly lower in FLS of CIA mice than those of normal mice (p<0.0001). These findings suggest that Mir204/Mir211 may play an important role in RA development.
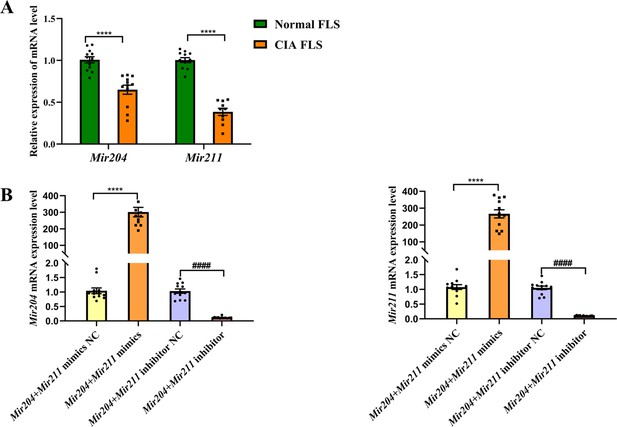
Mir204/Mir211 downregulate in fibroblast-like synoviocyte (FLS) of collagen-induced arthritis (CIA) mice.
(A) Low expression levels of Mir204/Mir211 in FLS of CIA mice. (B) qRT-PCR analysis of the relative Mir204/Mir211 expression in CIA FLS 48 hr after transfection. Please see Figure 1—source data 1. Data are pooled from at least three independent experiments and are presented as mean ± SEM. Data are analyzed using unpaired two-tailed Student t test (A) and one-way ANOVA (B). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 1—source data 1
Numerical data obtained during experiments represent in Figure 1.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig1-data1-v1.xlsx
According to PCR results in Figure 1B, the expression levels of Mir204 and Mir211 in CIA FLS transfected with two types of mimics were significantly higher compared with the NC mimics transfected group. Compared with the NC inhibitor group, the expression levels of Mir204 and Mir211 in CIA FLS transfected with both inhibitors were significantly decreased.
Mir204/Mir211 affect apoptosis and cell migration ability of CIA FLS
As shown in Figure 2A, compared with the NC mimics group, the overexpression of Mir204/Mir211 promoted the apoptosis of CIA FLS whereas knockdown of Mir204/211 inhibited the apoptosis rates of CIA FLS in comparison with the NC inhibitor group, suggesting that Mir204/Mir211 are potent regulators of FLS apoptosis.
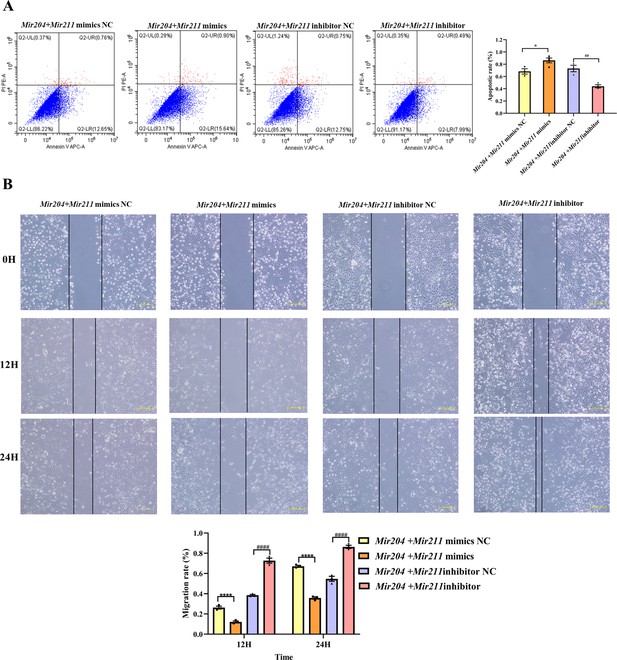
Effects of Mir204/Mir211 on apoptosis and cell migration ability of collagen-induced arthritis (CIA) fibroblast-like synoviocyte (FLS).
(A) Effects of Mir204/Mir211 on apoptosis of CIA FLS. (B) Effects of Mir204/Mir211 on cell migration ability of CIA FLS. Representative photomicrographs show a wound scratch assay at specific time points (0, 12, and 24 hr). Please see Figure 2—source data 1. Data are pooled from three independent experiments and are presented as mean ± SEM. Data are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 2—source data 1
Numerical data obtained during experiments represent in Figure 2.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig2-data1-v1.xlsx
As it can be seen from Figure 2B, there was no marked difference among the four groups of transfected CIA FLS at 0 hr. However, at 24 hr, co-transfection of Mir204/Mir211 markedly restrained the migration of CIA FLS in comparison with the NC mimics group (p<0.0001). Compared with the NC inhibitor group, knockdown of both Mir204/Mir211 greatly enhanced the migration ability of CIA FLS (p<0.0001). These results indicated that Mir204/Mir211 also play an important role in regulation of FLS migration.
Mir204/Mir211 ameliorate the inflammatory responses of CIA FLS
Both proinflammatory and anti-inflammatory cytokines play a vital role in synovial inflammation in RA. Therefore, the expression levels of pro-inflammatory mediators (Il1b, Il6, Il8, and Il23) and anti-inflammatory factors (Il4, Il10, and Tgfb1) were detected by qRT-PCR. As shown in Figure 3, the mRNA levels of Il1b, Il6, Il8, and Il23 were all decreased in FLS co-transfected with Mir204/Mir211 mimics, and mRNA levels of Il4, Il10, and Tgfb1 were all increased compared with FLS co-transfected with the Mir204/Mir211 mimics NC. Compared with FLS transfected with Mir204/Mir211 inhibitor NC, expression of pro-inflammatory cytokines was up-regulated, and expression of anti-inflammatory cytokines was down-regulated in FLS transfected with Mir204/Mir211 inhibitors. The data above corroborated the effects of Mir204/Mir211 on reducing synovial inflammation by down-regulation of pro-inflammatory cytokines and up-regulation of anti-inflammatory factors.
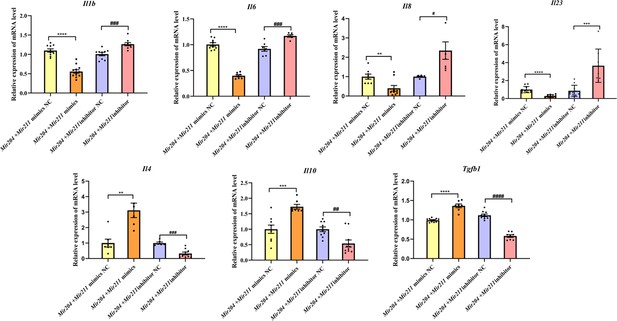
Effects of Mir204/Mir211 on the inflammatory responses of collagen-induced arthritis fibroblast-like synoviocyte.
All data are displayed as a value relative to those in the NC group. Please see Figure 3—source data 1. Data are pooled from at least three independent experiments and are presented as mean ± SEM. Data are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 3—source data 1
Numerical data obtained during experiments represent in Figure 3.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig3-data1-v1.xlsx
Mir204/Mir211 influence synovial inflammation by regulating NF-κB signaling pathway and p65 nuclear translocation
To further elucidate that Mir204/Mir211 attenuate the inflammation of CIA FLS, we performed western blot analysis of key molecules in the NF-κB signaling. The results showed that compared with FLS transfected with the NC mimics, expression of NF-κB p65 and IKK-α was decreased in CIA FLS co-transfected with miR-204/211, while expression of I-κBα protein was increased (Figure 4A). Compared with the FLS transfected with NC inhibitors, knockdown of both Mir204/Mir211 increased the protein levels of NF-κB p65 and IKK-α and decreased I-κBα protein levels. However, neither overexpression nor knockdown of Mir204/Mir211 had significant effects on IKK-β expression. These results suggest that Mir204/Mir211 might affect the CIA synovial inflammation by regulation of NF-κB signaling pathway.
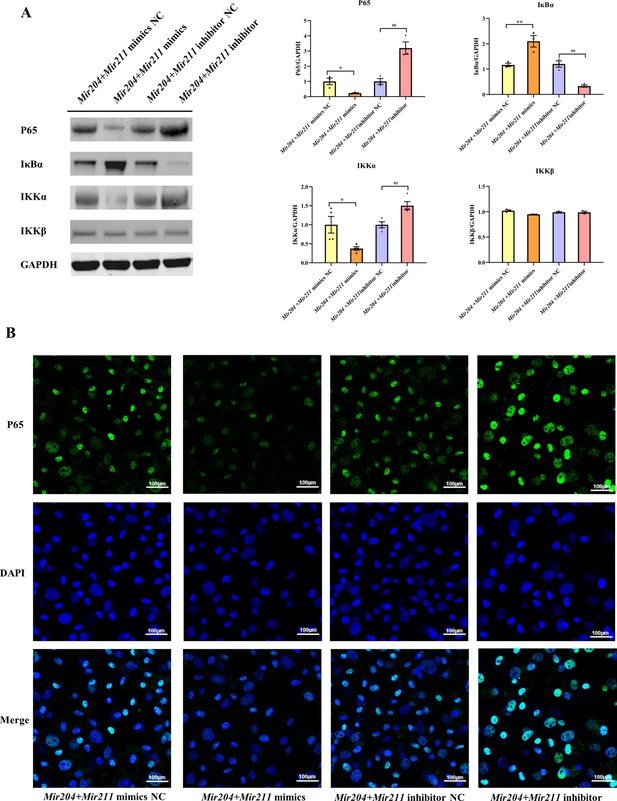
Effects of Mir204/Mir211 on NF-κB signaling pathway.
(A) Western blot assays of the NF-κB pathway in transfected collagen-induced arthritis (CIA) fibroblast-like synoviocyte (FLS). (B) Immunofluorescence staining of p65 nuclear translocation in transfected CIA FLS. Please see Figure 4—source data 1. Data are pooled from at least three independent experiments and are presented as mean ± SEM. Data are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 4—source data 1
Numerical data obtained during experiments represent in Figure 4.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig4-data1-v1.xlsx
-
Figure 4—source data 2
Original western blot files for Figure 4.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig4-data2-v1.pptx
We also performed immunofluorescence (IF) staining to detect the nuclear translocation of p65 in transfected CIA FLS. As shown in Figure 4B, compared with the FLS transfected with NC mimics, the nuclear translocation of p65 in CIA FLS co-transfection with both Mir204 and Mir211 was inhibited. In contrast, compared with the FLS transfected with NC inhibitors, p65 nuclear translocation was significantly enhanced in CIA FLS with both Mir204 and Mir211 knockdown, indicating that Mir204/Mir211 inhibit NF-κB signaling by blocking p65 nuclear translocation.
Mir204/Mir211 influence proliferation of CIA FLS
Mir204 mimics and Mir211 mimics were co-transfected into CIA FLS, and the viability of CIA FLS was significantly decreased (Figure 5A, p<0.001). After transfection with Mir204 inhibitor and Mir211 inhibitor, the viability of CIA FLS was significantly increased (p<0.0001), suggesting that Mir204/Mir211 are important regulators of FLS proliferation during RA development. Staining of β-galactosidase was also performed in primary WT FLS and dKO FLS (Figure 5—figure supplement 1).
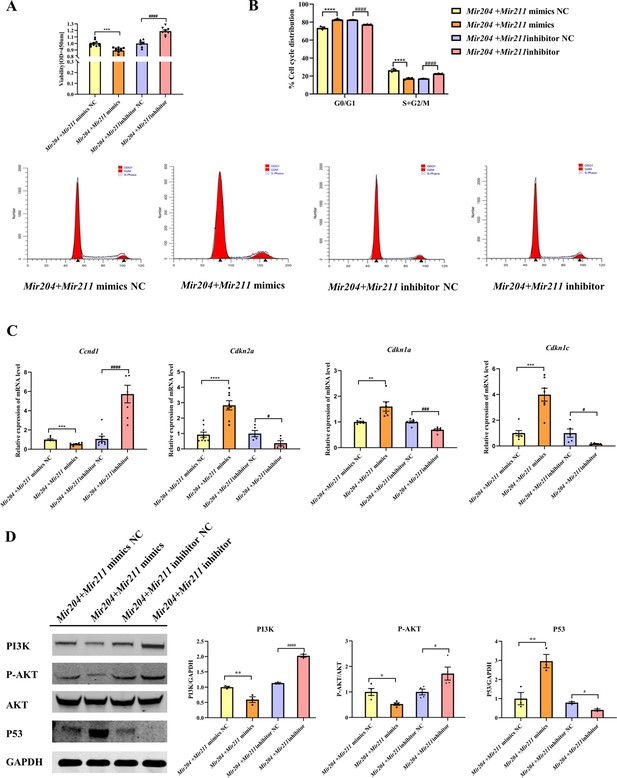
Effects of Mir204/Mir211 on proliferation of collagen-induced arthritis (CIA) fibroblast-like synoviocyte (FLS).
(A) CCK-8 analysis in four groups of transfected CIA FLS. (B) Mir204/Mir211 induce G0/G1 phase arrest of CIA FLS. (C) Expression levels of cell cycle regulatory molecule in transfected CIA FLS. (D) Western blot assays of the PI3K/AKT pathway in transfected CIA FLS. Please see Figure 5—source data 1. Data are pooled from at least three independent experiments and are presented as mean ± SEM. Data are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 5—source data 1
Numerical data obtained during experiments represent in Figure 5.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig5-data1-v1.xlsx
-
Figure 5—source data 2
Original western blot files for Figure 5.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig5-data2-v1.pptx
We also examined effects of Mir204/Mir211 on cell cycle progression in CIA FLS using flow cytometry and RT-qPCR assays. Compared with the NC mimics group, overexpression of both Mir204 and Mir211 increased the proportion of G0/G1 phase and reduced the proportion of S+G2/M phase (Figure 5B, p<0.0001). Compared with the NC inhibitor group, the percentage of G0/G1 phase cells decreased and the percentage of S+G2/M phase cells increased when Mir204 and Mir211 were inhibited (p<0.0001).
Next, we examined the mRNA expression of Ccnd1, Cdkn2a, Cdkn1a, and Cdkn1c to confirm the results of flow cytometry. Compared with the cells transfected with Mir204/Mir211 mimics NC, the expression of Ccnd1 in the cells transfected with Mir204/Mir211 mimics was decreased, while expression levels of Cdkn2a, Cdkn1a, and Cdkn1c were increased (Figure 5C). Compared with the cells transfected with Mir204/Mir211 inhibitor NC, Ccnd1 levels were increased in the cells transfected with Mir204/Mir211 inhibitors, while expression levels of Cdkn2a, Cdkn1a, and Cdkn1c were significantly decreased. These results revealed that Mir204/Mir211 affect cell cycle progression through regulation of Ccnd1, Cdkn2a, Cdkn1a, Cdkn1c expression.
Mir204/Mir211 affect synovial proliferation possibly by regulating PI3K/AKT signaling pathway
To investigate the mechanism of Mir204/Mir211 on CIA FLS proliferation, we determined changes in PI3K/AKT signaling pathway by western blotting. The results showed that compared with the Mir204 + Mir211 mimics NC group, the protein levels of PI3K and P-AKT were decreased while P53 expression was increased in the cells transfected with Mir204/Mir211 mimics (Figure 5D). Compared with the cells transfected with Mir204/Mir211 inhibitor NC, the protein expression of PI3K and P-AKT was up-regulated whereas P53 protein expression was decreased in the cells transfected with Mir204/Mir211 inhibitors. These results suggest that Mir204/Mir211 affect synovial proliferation possibly through regulation of PI3K/AKT signaling in CIA FLS.
Mir204/Mir211 target Ssrp1 in CIA FLS
We performed bioinformatic analysis using the databases, TargetScan, miRDB, miRGEN v.3, PicTar, and miRTARbase to predict the downstream target genes of Mir204/Mir211. 17 downstream target genes of Mir204 and 4 downstream target genes of Mir211 were identified through the cross check with 4 databases (TargetScan, miRDB, miRGEN v.3, and PicTar; Figure 6A). Through the second round of cross check with these databases, three same target genes (Ssrp1, M6pr, and Sox4) of Mir204/Mir211 were identified, and that Ssrp1 was the most likely downstream target gene of Mir204/Mir211. Meanwhile, TargetScan was used to predict the possible Mir204/Mir211 binding sites in the 3’-UTR of the Ssrp1 gene (Figure 6B).
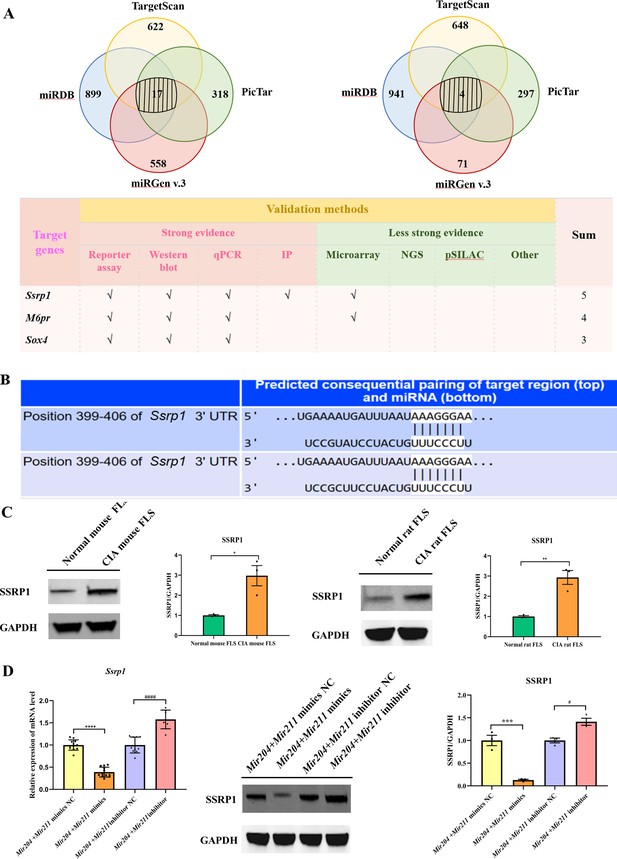
Mir204/Mir211 target structure-specific recognition protein 1 (Ssrp1) in collagen-induced arthritis (CIA) fibroblast-like synoviocyte (FLS).
(A) Diagrams describing the process of predicting target genes of Mir204/Mir211 (B) Diagram of putative Mir204/Mir211 binding sequence in Ssrp1 3’ untranslated region (UTR) from TargetScan. (C) High expression levels of SSRP1 in CIA mice and CIA rats. (D) Gene and protein expressions of SSRP1 in four groups of transfected CIA FLS. Please see Figure 6—source data 1. Data are pooled from at least three independent experiments and are presented as mean ± SEM. Data are analyzed using unpaired two-tailed Student t test (C) and one-way ANOVA (D). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 6—source data 1
Numerical data obtained during experiments represent in Figure 6.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig6-data1-v1.xlsx
-
Figure 6—source data 2
Original western blot files for Figure 6.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig6-data2-v1.pptx
Expression of SSRP1 protein levels in FLS of CIA rats and CIA mice was significantly higher than those of normal rats and mice (Figure 6C), indicating that SSRP1 was highly expressed in CIA. Moreover, compared with the cells transfected with Mir204/Mir211 mimics NC, the mRNA and protein levels of SSRP1 were significantly decreased in the cells transfected with Mir204/Mir211 mimics (Figure 6D). Compared with the cells transfected with Mir204/Mir211 inhibitor NC, expression of Ssrp1 mRNA and protein levels was significantly increased in the cells transfected with Mir204/Mir211 inhibitors. These results demonstrated that Ssrp1 may serve as a downstream target of Mir204/Mir211 in CIA FLS.
Mir204/Mir211 affect cell proliferation by targeting Ssrp1
As shown in Figure 7A and Figure 7—figure supplement 1, compared with the control group, the expression of SSRP1 protein in the Mir204 + Mir211 mimics group was markedly decreased. In comparison with the Mir204 + Mir211 mimics group, the expression SSRP1 protein in the Mir204 +Mir211 mimics + SSRP1 group was significantly increased. These results suggest that Ssrp1 may act as a downstream target of Mir204/Mir211.
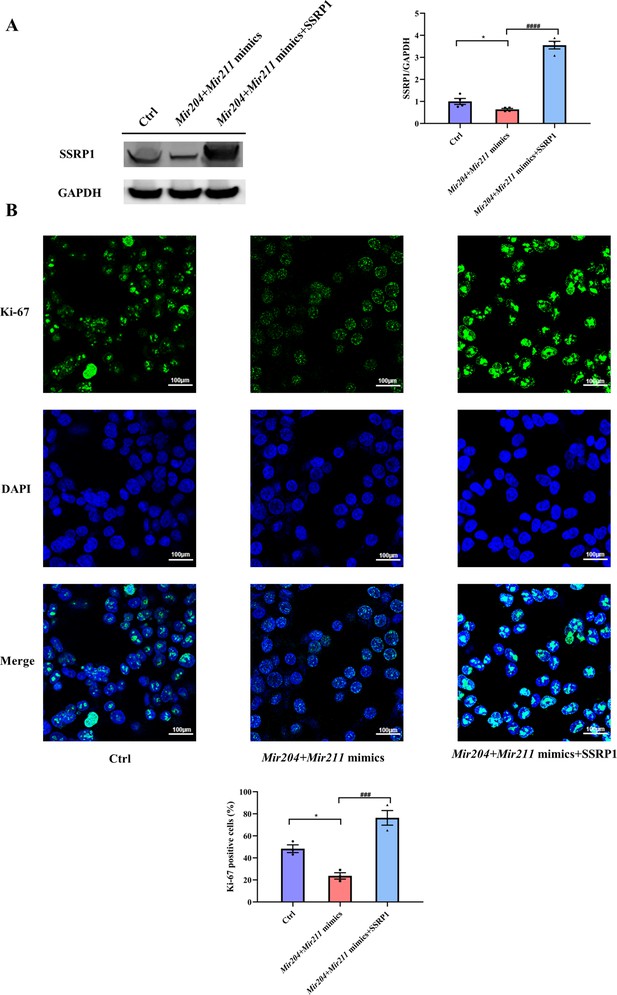
Effects of Mir204/Mir211 on cell proliferation by targeting structure-specific recognition protein 1 (Ssrp1).
(A) Overexpression of Mir204/Mir211 decreases SSRP1 expression. (B) Mir204/Mir211 decrease Ki-67 levels by targeting Ssrp1. Please see Figure 7—source data 1. Data are pooled from four independent experiments and are presented as mean ± SEM. Data are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 7—source data 1
Numerical data obtained during experiments represent in Figure 7.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig7-data1-v1.xlsx
-
Figure 7—source data 2
Original western blot files for Figure 7.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig7-data2-v1.pptx
Effects of Mir204/Mir211 and SSRP1 on the expression of Ki-67 were examined in 293T cells by IF staining. Compared with the Ctrl group, the number of Ki-67 positive cells was significantly decreased in the Mir204/Mir211 mimics group; in contrast, the number of Ki-67 positive cells was significantly increased in the Mir204/Mir211 mimics + SSRP1 group in comparison with the Mir204/Mir211 mimics group (Figure 7B). These results suggest that Mir204/Mir211 inhibit cell proliferation by regulation of Ssrp1.
High expression level of SSRP1 in CIA Mir204/Mir211 dKO mice
Compared with WT mice with CIA induction, Mir204/Mir211 dKO mice with CIA induction showed much more severe paw swelling and increased arthritis score (Figure 8A). Compared with CIA WT mice with mild arthritis, CIA Mir204/Mir211 dKO mice displayed severe arthritis, as manifested by synovial hyperplasia along with angiogenesis and cartilage destruction (Figure 8B), suggesting that loss of Mir204/Mir211 deteriorates RA and Mir204/Mir211 may play a protective role in RA development. Staining of tartrate resistant acid phosphatase (TRAP) and IHC staining (MMP-13) of knee joints were also performed (Figure 8—figure supplement 1).
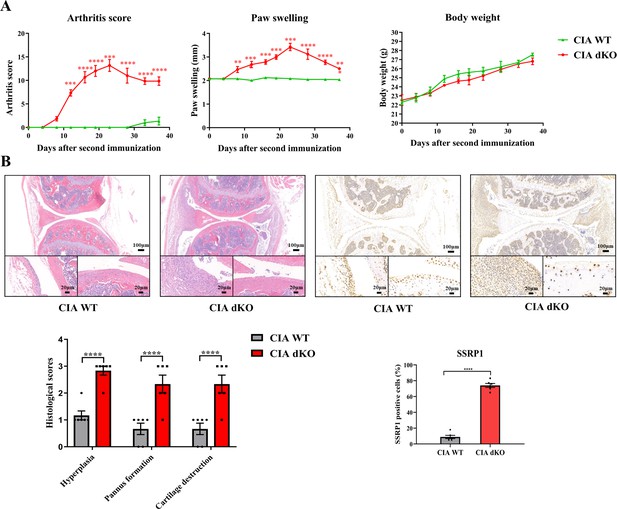
High expression level of structure-specific recognition protein 1 (SSRP1) in collagen-induced arthritis (CIA) Mir204/Mir211 double knockout (dKO) mice.
(A) Severity of arthritis in CIA wild-type (WT) and CIA dKO mice. The arthritis scores, paw swelling, and body weight are documented by two independent blinded observers since the day of the second booster twice a week (n=6). At 37 days after the second immunization, all mice are sacrificed. Knee joints of all mice are collected for the following experiments. (B) Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) analyses of knee joints in CIA WT and CIA dKO mice. Representative photomicrographs of knee joint sections in synovium and cartilage stained with H&E and IHC (SSRP1) are displayed (n=6). Please see Figure 8—source data 1. Data are presented as mean ± SEMand analyzed using one-way ANOVA (A) and unpaired two-tailed Student t test (B). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 8—source data 1
Numerical data obtained during experiments represent in Figure 8.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig8-data1-v1.xlsx
Results of immunohistochemical (IHC) staining showed that SSRP1 positive cells were significantly increased in synovial tissues of CIA dKO mice compared with those of CIA WT mice (Figure 8B). These results suggest that Ssrp1 may be regulated by Mir204/Mir211 and is involved in RA development.
Ssrp1 knockdown exerts anti-arthritis effect in CIA Mir204/Mir211 dKO mice
All animal experiments in this part of study were performed in Mir204/Mir211 dKO mice. Adeno-associated virus (AAV)-shRNA Ctrl or AAV-shSsrp1 was administered to dKO mice with or without CIA induction. The RA phenotype of paw swelling, erythema, joint deformity, and increased arthritis scores was found in CIA dKO mice as compared with the dKO mice without CIA induction (Figure 9A). In addition, compared with the CIA dKO mice administered with the AAV-shRNA Ctrl, the severity of arthritis phenotype was significantly attenuated in CIA dKO mice administered with AAV-shSsrp1. Results of hematoxylin and eosin (H&E), TRAP, and IHC staining of knee joint sections showed that synovial inflammation and articular cartilage destruction were significantly alleviated in CIA dKO mice administered with AAV-shSsrp1, further demonstrating that Mir204/Mir211 may play an anti-arthritis effect by modulation of Ssrp1 (Figure 9B, Figure 9—figure supplement 1).
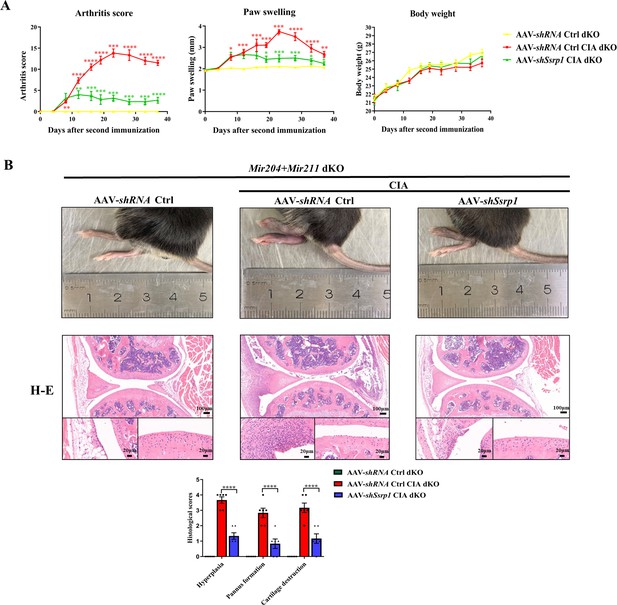
Structure-specific recognition protein 1 (Ssrp1) knockdown exerts anti-arthritis effect in collagen-induced arthritis (CIA) Mir204/Mir211 double knockout (dKO) mice.
(A) Ssrp1 knockdown ameliorates swelling degrees of hind paws in CIA Mir204/Mir211 dKO mice. Representative photomicrographs of knee joint sections in synovium and cartilage stained with hematoxylin and eosin (H&E) are displayed (n=6). (B) H&E staining of knee joints in Mir204/Mir211 dKO mice (n=6). Please see Figure 9—source data 1. Data are presented as mean ± SEM and are analyzed using one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; and #p<0.05; ##p<0.01, ###p<0.001, ####p<0.0001.
-
Figure 9—source data 1
Numerical data obtained during experiments represent in Figure 9.
- https://cdn.elifesciences.org/articles/78085/elife-78085-fig9-data1-v1.xlsx
Discussion
RA is a chronic inflammatory disease, and genetic and environmental factors are involved during the disease initiation and progression. It is characterized by synovial inflammation leading to abnormal synovial hyperplasia, which eventually invades cartilage and leads to joint destruction (Li et al., 2018). The structural similarity and homology between Mir204 and Mir211 allow them to have the same gene targeting spectrum (Lee et al., 2016). Since the roles of Mir204 and Mir211 in RA are still unclear, here we will discuss the key role of Mir204/211 in RA from two aspects, synovial inflammation and abnormal synovial proliferation. It has been reported that Mir204 is down-regulated in synovial tissues of RA patients, and Mir204 regulates RA FLS survival by regulating STAT3 protein (Xiao et al., 2021; Li et al., 2018). Consistent with these findings, we found that expression levels of Mir204 and Mir211 in FLS of CIA mice were significantly reduced compared with those of normal mice. As demonstrated in this study that Mir204/211 suppress RA progression and play a protective role in RA, in our in vitro cell culture studies, we transfected CIA FLS with Mir204 mimics (or Mir204 inhibitor) and Mir211 mimics (or Mir211 inhibitor) simultaneously, to verify the important roles of Mir204/211 in RA.
In in vitro cell culture experiments, simultaneous overexpression of Mir204/211 was found to alleviate synovial inflammation-associated cell phenotypes in RA. We also performed cell scratching assay and cell apoptosis assay using flow cytometry method. The results suggest that simultaneous overexpression of Mir204/211 inhibited the migration of CIA FLS and promoted their apoptosis whereas simultaneous knockdown of Mir204/211 caused opposite phenomena. In RA, inflammatory FLS is the main source of inflammatory cytokines, and these pro-inflammatory mediators will further aggravate synovial inflammation and lead to the RA deterioration (Wu et al., 2016; Wu et al., 2019a; Hong et al., 2018). Yuan et al. reported that the expressions of pro-inflammatory cytokines, such as Il1b, Il6, and Il23, were all up-regulated in synovial tissues of CIA mice (Yuan et al., 2019). While other cytokines, such as Il4, Il10, and Tgfb1, played an implicit part in the anti-inflammatory response (Fabbrini and Magkos, 2015). In our assays, simultaneous overexpression of Mir204/211 decreased the expression of inflammatory cytokines (Il1b, Il6, Il8, and Il23) in CIA FLS and promoted the synthesis of anti-inflammatory cytokines (Il4, Il10, and Tgfb1), while knockdown of both Mir204 and Mir211 resulted in absolute opposite outcomes. Our results demonstrated that Mir204/211 treatment suppressed the abnormal migration and apoptotic behavior of RA FLS, as well as inflammatory factor-mediated disease deterioration.
NF-κB is a crucial transcription factors family and acts as a pro-inflammatory mediator to induce the production of cytokines, chemokines, and cell adhesion molecules in inflammatory cells (Umezawa et al., 2000; Imbert and Peyron, 2017; Chen et al., 2021). The translocation of p65 promotes the generation of numerous inflammatory factors (e.g. Il1b, Il6, Il23) and matrix metalloproteinases (MMPs) (Baldwin, 2012; Morgan and Liu, 2011; Yang et al., 2017). The expressions of these inflammatory factors and MMPs will eventually lead to synovial inflammation and joint destruction, thereby becoming one of the indispensable pathogenic factors of RA (Xu et al., 2018). We found decreased expression levels of P65 and IKKα, increased I-κBα protein expression in the Mir204 + Mir211 mimics group, while there were promoted P65 and IKKα protein expressions and declined I-κBα expression level in the Mir204 + Mir211 inhibitor group. Simultaneous overexpression or knockdown of Mir204/211 had no significant effect on the protein expression of IKKβ, and it seemed Mir204/211 might regulate the activity of NF-κB signaling pathway through modulation of IKKα. Later, IF assays also confirmed the results of western blotting. Above all, we found that Mir204/211 attenuated inflammation-mediated exacerbation by regulation of NF-κB p65 translocation in RA.
In terms of cell proliferation, CCK-8 analysis and flow cytometry were both conducted. Cell cycle regulatory proteins include cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (CKIs). Ccnd1 and Cdkn1c (CKI) both regulate the cell cycle through regulation of the G1/S phase (Wang et al., 2020b). Both Cdkn2a and Cdkn1a genes are crucial modulators in the regulation of cell senescence and are CDK inhibitors that lead to cell cycle stagnation in the G1 phase (Fernandez et al., 2015; Zhang et al., 2018). Simultaneous overexpression of Mir204/211 blocked cell cycle in G0/G1 phase, notably reduced Ccnd1 expression, and greatly increased Cdkn2a, Cdkn1a, and Cdkn1c mRNA expression, whereas simultaneous knockdown of Mir204/211 led to a significant increase in Ccnd1 expression and a significant decrease in Cdkn2a, Cdkn1a, and Cdkn1c expressions. Mir204/211 influenced the cell cycle by regulating Ccnd1, Cdkn2a, Cdkn1a, and Cdkn1c expression, thus inhibited cell proliferation.
PI3K/AKT is a classical signaling pathway participating in cell proliferation, migration, survival, and angiogenesis. Recent studies have shown that activation of PI3K/AKT signaling promotes the migration of RA FLS and plays an indispensable role in the pathogenesis of RA (Wang et al., 2020cWang et al., 2020a; Jiao et al., 2018). PI3K regulates cell proliferation, apoptosis, and metabolism. Lin et al. reported increased expression level of PI3K in RA synovial tissue, and the elevated PI3K promoted AKT phosphorylation and activated the PI3K/AKT signaling pathway. Activated AKT phosphorylates a variety of proteins and mediates cell proliferation (Lin et al., 2019). Western blotting results revealed that expression of PI3K and P-AKT protein was suppressed in the Mir204 + Mir211 mimics group, while upregulations of PI3K and P-AKT were observed in the Mir204 + Mir211 inhibitor group, revealing that Mir204/211 inhibited aberrant proliferation of CIA FLS by regulating the PI3K/AKT signaling pathway.
Based on the results of bio-informative analysis, PCR assay, and western blot analysis, we found that Ssrp1 was the downstream target gene of Mir204/211. We proposed that the abnormal expression of Ssrp1 in CIA may be caused by the dysregulation of the upstream miRNAs, Mir204 and Mir211. Trp53 is a vital tumor suppressor gene, which plays a role in initiating cell death and leads to the inhibition of Cdkn1a in cancer cells (Zhang et al., 2021). Wu et al. reported that NF-κB and P53 pathways could be regulated by FACT complex, and activation of NF-κB pathway and inhibition of P53 pathway might play the regulatory role of Ssrp1 in colorectal cancer (Wu et al., 2019b). Ding et al. found that in hepatocellular carcinoma, high expression of Ssrp1 activated the NF-κB pathway and suppressed the P53 pathway. Cyclins, such as Cdkn1a and Cdkn1b, are downstream signaling factors and can be regulated by Trp53. The expression level of Ccnd1 could also be negatively regulated by Cdkn1a (Vogelstein et al., 2000). Woksepp et al. demonstrated that knockdown of Ssrp1 by siRNA enhanced the phosphorylation of P53 and resulted in P53 activation, indicating that Ssrp1 had an inhibitory effect on Trp53. Over-activated NF-κB pathway is a common cause of the inhibition of P53 pathway in tumors, and P53 pathway is negatively regulated by NF-κB pathway (Gasparian et al., 2011). Liao et al. reported that low expression of Ssrp1 depressed p65 expression and a series of proliferation-related genes (such as Ccnd1) (Liao et al., 2017). Furthermore, Wang et al. also found that Ssrp1 promoted cell proliferation and apoptosis by activating AKT pathway (Wang et al., 2019). Thus, we hypothesized that Ssrp1 might regulate cell proliferation in RA by cell cycle.
Ki-67 is a widely used proliferation marker, highly expressed in cyclonic cells and significantly down-regulated in dormant cells. Ki-67 is an important proliferation marker for various cancer grades (Sun and Kaufman, 2018). Ki-67 is well recognized to identify aberrant synovial cells, which display a characteristic of excessive proliferation. Pessler et al. have quantified a strong correlation between Ki-67 expression and the histological degree of synovitis (Pessler et al., 2008). Through cell transfection and IF assay, we observed significantly declined expression of Ki-67 in cells with overexpression of Mir204/211 and prominently promoted Ki-67 expression in cells with overexpression of Mir204/211 and SSRP1, revealing that Mir204/211 suppressed cell proliferation by manipulating Ssrp1 expression.
To further test our hypothesis, we performed the in vivo experiments in the following studies. Severe arthritis, as manifested by synovial hyperplasia along with angiogenesis and cartilage destruction was more significant in mice of the CIA dKO group as compared with mice in the CIA WT group. IHC staining of SSRP1 showed evidently increased SSRP1 positive cells in the knee joint sections of CIA dKO mice as compared with those in CIA WT mice, confirming that Ssrp1 was the downstream target gene of Mir204/211, and the expression of Ssrp1 was elevated in RA. Lastly, we found that Ssrp1 knockdown exerts an anti-arthritis effect in CIA dKO mice by regulating proliferation and inflammation. CIA dKO mice received AAV-shSsrp1 displayed alleviated paw swelling, arthritis scores, synovial inflammation, and articular cartilage destruction compared with the AAV-shRNA Ctrl CIA dKO mice. Our study revealed that Ssrp1knockdown exerted an anti-arthritis effect in RA by suppressing cell proliferation and inflammation.
Mir204/211 suppress RA progression by regulating both inflammation and cell proliferation, and Mir204/211 affect cell proliferation and inflammation in RA by regulating downstream target gene Ssrp1 (Figure 10).
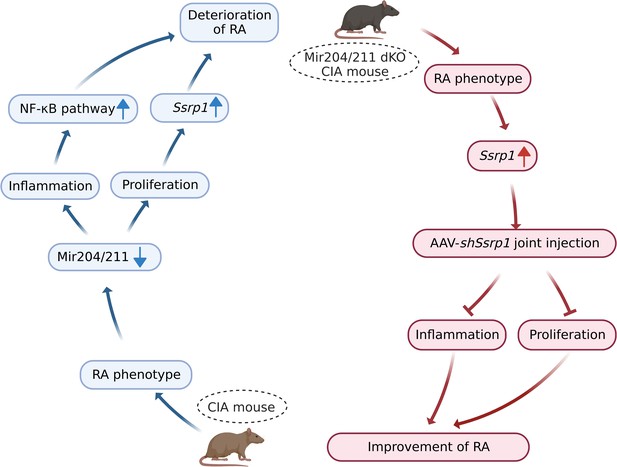
Graphical summary of how Mir204/211 suppresses synovial inflammation and proliferation in rheumatoid arthritis (RA) by targeting structure-specific recognition protein 1 (Ssrp1).
Primary fibroblast-like synoviocytes (FLSs) derived from collagen-induced arthritis (CIA) mice display decreased expression of Mir204/211. On the one hand, upon being challenged by IL-1β, FLS from CIA mice with Mir204/211 overexpression inhibits the production of inflammatory cytokines and promotes the production of large amounts of anti-inflammatory factors by regulating NF-κB pathway. On the other hand, Mir204/211 overexpression induces declined cell proliferation by targeting Ssrp1. Moreover, Mir204/211 dKO CIA mice displays RA phenotype and increased SSRP1 expression whereas AAV-shSsrp1 joint injection significantly reverses this phenomenon from the aspects of inflammation and proliferation. Consequently, we assume that Mir204/211 may influence cell proliferation and inflammation in RA by regulating downstream target gene Ssrp1.
Conclusion
RA is a chronic disease characterized by proliferation and infiltration of FLS. Mir204/211 could delay the progression of RA by regulating both inflammation and cell proliferation. Ssrp1 is the downstream target gene of Mir204/211, and Mir204/211 affect cell proliferation and retard RA progression by regulating Ssrp1. Ssrp1 plays a critical role in RA development. Further exploration is needed to investigate the interaction between Mir204/211 and Ssrp1 in RA.
Materials and methods
Animals
Male DBA/1 J mice, male C57 mice, and male Wistar rats (aged 6–8 weeks) were purchased from the Shanghai SLAC Animal Center (Shanghai, China). Germline deletions of the Mir204/211 by breeding Mir204flox and Mir211flox mice with CMV-Cre mice were generated and reported in our previous studies (Huang et al., 2019). All male mice and rats were placed in a specific pathogen free facility and acclimatized to environment before induction of CIA model. The animal protocol for this study has been approved by the Ethics Committee of the Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (ID HKDL[2018]344).
Induction of CIA model
To establish CIA model in DBA/1 J mice, complete Freund’s Adjuvant (CFA, 4 mg/mL) was emulsified with bovine collagen Ⅱ (1:1). On day –21, DBA/1 J mice were immunized by multiple subcutaneous injections of collagen emulsion around the base of the tail, and a booster of the same dose of emulsion was administrated in the same site to further induce immunization on day 0 (the day of the second booster administration). On day 15, paw swelling and arthritis scores were assessed to confirm whether the CIA mouse model was successfully constructed. CIA rat model was induced with emulsification of bovine collagen Ⅱ (2 mg/mL) and incomplete Freund’s Adjuvant at ratio of 1:1 around the base of the rat tail on day –7 and day 0.
To induce CIA model in Mir204/211 dKO male mice (C57 background) and their WT littermates, 6.67 mg/mL CFA were emulsified with chicken collagen Ⅱ (1:1). On day –14, 8-week-old mice were immunized with injections of chicken collagen Ⅱ emulsion around the tail. On day 0, the same emulsion was injected again in the same place to strengthen immunity and establish CIA model.
Isolation and culture of FLS
FLSs were isolated from normal and CIA DBA/1 J mice. CIA mice which showed severe paw swelling and an arthritis score of 16 were scarified on day 15 after the second immunization. The individual bones of the hind paws were isolated and dissected. Dissected bones with synovial tissue were digested in Dulbecco Modified Eagle Media (DMEM) containing 1 mg/mL collagenase Ⅳ and 0.1 mg/mL dexydiboyydease Ⅰ for 1 hr. Suspended and filtered through a nylon mesh, synovial cells were washed three times in DMEM medium containing 10% heat inactivated fetal bovine serum and then incubated overnight. The culture media were changed every other day, and FLSs used in this study were at passage 2–6.
Quantitative qPCR of miRNA
MiRNA from FLS was extracted according to the instructions of miRcute miRNA Isolation Kit (TIANGEN, Beijing) and reversely transcribed with the miRNA first strand cDNA synthesis (Sangon Biotech, Shanghai). The expression levels of Mir204 and Mir211 were detected, respectively, by quantitative PCR with the miRNA fluorescence quantitative PCR kit (Sangon Biotech, Shanghai). The expressions of U6 were used as internal reference, and the experiment was repeated at least three times (Table 1).
PCR primer sequences.
| Gene | Primer sequences |
|---|---|
| mmu-Mir204 forward primer | 5 '- GGGCTTCCCTTTGTCATCCTAT –3' |
| mmu-Mir211 forward primer | 5 ' - GGGCTTCCCTTTGTCATCCTT –3' |
| Universal U6 Forward primer | 5 ' - GCAAATTCGTGAAGCGTTCCATA –3' |
| Universal PCR reverse primer | 5 ' - AACGAGACGACGACAGAC –3' |
Cell transfection and intra-articular injection of AAV
NC (Negative control), NC inhibitor, Mir204 and Mir211 mimics, Mir204 and Mir211 inhibitor, and AAV-mediated Ssrp1 short hairpin RNA (shRNA) (AAV-shSsrp1) and AAV-shRNA Ctrl were synthesized by Genomeditech (Shanghai, China). Empty pcDNA3.1 vector and pcDNA3.1-Ssrp1 plasmid were purchased from Youze Biotechnology (Changsha, China).
CIA FLSs were seed in six-well plates at the density of 5×105 and randomly divided into Mir204 + Mir211 mimics NC group, Mir204 + Mir211 mimics group, Mir204 + Mir211 inhibitor NC group, and Mir204 + Mir211 inhibitor group. CIA FLSs were transfected with Lipofectamine 3000 (Invitrogen, America) and 100 nM NC/Mir204 + Mir211 mimics/NC inhibitor/Mir204 + Mir211 inhibitor for 48 hr, respectively, according to the protocols. To maintain the inflammatory state and invasive activity of CIA FLS, the medium was discarded and replaced with DMEM containing 10 ng/mL IL-1β for another 24 hr.
293T cells were transfected and divided into control group (transfected with scramble miRNA), Mir204 + Mir211 mimics group (transfected with Mir204 + Mir211 mimics), and Mir204 + Mir211 mimics + SSRP1 group (transfected with Mir204 + Mir211 mimics and pcDNA3.1-Ssrp1 plasmid simultaneously). 48 hr after transfection, cells in the three groups were given 10 ng/mL IL-1β for another 24 hr, and the total proteins of the cells were extracted 72 hr after transfection.
Mice in the AVV-shSsrp1 CIA group underwent joint injections of 1×1012 AAV-shSsrp1 particles in a 10 μL volume and the same dose of AAV expressing shRNA Ctrl were administered into the knee joints of mice in the AAV-shRNA Ctrl and AAV-shRNA Ctrl CIA groups.
Apoptosis analysis and cell migration assay
For Annexin V-APC/PI apoptosis analysis, CIA FLSs in the four groups were transfected, respectively, and collected 72 hr after transfection. After washing twice with PBS, CIA FLSs were resuspended in the binding buffer and stained with Annexin V and propidium iodide (PI) immediately (BD, CA, USA). Cells were collected to detect apoptosis rates.
A straight cell-free scratch was created in transfected CIA FLS with a 20-μL pipette tip. Photographs at specific time points (0, 12, and 24 hr) were documented in four groups to observe the healing of FLS scratches. Wound repair was assessed by ImageJ software (USA).
Total RNA isolation and qPCR
Total RNA of transfected CIA FLS in four groups was extracted with Trizol reagent (Invitrogen). The cDNA synthesis kit (Takara, Tokyo, Japan) was adopted for reverse transcription of RNA. According to the instructions of the fluorescent quantitative PCR kit (Takara, Japan), real-time PCR was conducted. With Actb gene as internal reference, the expression levels of genes in Mir204 + Mir211 mimics NC group and Mir204 + Mir211 inhibitor NC group were set as 1, respectively, and the relative expressions of genes in Mir204 + Mir211 mimics group and Mir204 + Mir211 inhibitor group were detected (Table 2). The experiment was repeated independently for three times, with three replicates for each sample.
PCR primer sequences.
| Name | Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|---|
| β-Actin | Actb | 5'-GTGACGTTGACATCCGTAAAGA-3' | 5'-GCCGGACTCATCGTACTCC-3' |
| IL-1β | Il1b | 5'-GAAATGCCACCTTTTGACAGTG-3' | 5'-TGGATGCTCTCATCAGGACAG-3' |
| IL-4 | Il4 | 5'-GGTCTCAACCCCCAGCTAGT-3' | 5'-GCCGATGATCTCTCTCAAGTGAT-3' |
| IL-6 | Il6 | 5'-CTGCAAGAGACTTCCATCCAG-3' | 5'-AGTGGTATAGACAGGTCTGTTGG-3' |
| IL-8 | Il8 | 5'-TCGAGACCATTTACTGCAACAG-3' | 5'-CATTGCCGGTGGAAATTCCTT-3' |
| IL-10 | Il10 | 5'-GCTGGACAACATACTGCTAACC-3' | 5'-ATTTCCGATAAGGCTTGGCAA-3' |
| IL-23 | Il23 | 5'-CAGCAGCTCTCTCGGAATCTC-3' | 5'-TGGATACGGGGCACATTATTTTT-3' |
| TGF-β1 | Tgfb1 | 5'-CCACCTGCAAGACCATCGAC-3' | 5'-CTGGCGAGCCTTAGTTTGGAC-3' |
| Cyclin D1 | Ccnd1 | 5'-GCGTACCCTGACACCAATCTC-3' | 5'-ACTTGAAGTAAGATACGGAGGGC-3' |
| p16 | Cdkn2a | 5'-CGCAGGTTCTTGGTCACTGT-3' | 5'-TGTTCACGAAAGCCAGAGCG-3' |
| p21 | Cdkn1a | 5'-CCTGGTGATGTCCGACCTG-3' | 5'-CCATGAGCGCATCGCAATC-3' |
| p57 | Cdkn1c | 5'-GCAGGACGAGAATCAAGAGCA-3' | 5'-GCTTGGCGAAGAAGTCGTT-3' |
| SSRP1 | Ssrp1 | 5'-CAGAGACATTGGAGTTCAACGA-3' | 5'-GACGGCTCAATCGAAGCCTC-3' |
Western blotting
Total proteins were extracted with sodium dodecyl sulfate (SDS) reagent (Beyotime, Shanghai), and eBlot L1 Protein Transfer System (GenScript Corporation, China) was used for protein electrophoresis. SDS-PAGE gel electrophoresis, protein transfer, and immunohybridization were performed to determine changes in expression levels of target genes (Table 3). Finally, the target bands were scanned by a fluorescence scanning imager, and the data were quantitatively analyzed.
Target proteins detected by western blotting.
| Gene | Protein | Company | Molecular weight (kDa) | Dilution ratio |
|---|---|---|---|---|
| Rela | NF-κB p65 | Cell Signaling Technology | 65 | 1:1000 |
| Nfkbia | I-κBα | Cell Signaling Technology | 39 | 1:1000 |
| Chuk | IKKα | Cell Signaling Technology | 85 | 1:1000 |
| Ikbkb | IKKβ | Cell Signaling Technology | 87 | 1:1000 |
| Pik3r1 | PI3K | Cell Signaling Technology | 85 | 1:1000 |
| Akt1 | P-AKT | Cell Signaling Technology | 60 | 1:1000 |
| Akt1 | AKT | Cell Signaling Technology | 60 | 1:1000 |
| Trp53 | P53 | Cell Signaling Technology | 53 | 1:1000 |
| Ssrp1 | SSRP1 | Cell Signaling Technology | 81 | 1:1000 |
| Gapdh | GAPDH | Cell Signaling Technology | 37 | 1:1000 |
Immunofluorescence assay
Translocation of p65 and expression of Ki-67 were confirmed by IF assay. After being fixed in paraformaldehyde for 15 min, cells were permeabilized with 0.3% Triton X-100 for 10 min, blocked with goat serum for 1 hr, and incubated with primary antibody overnight. Next, cells were washed and incubated with corresponding secondary antibody in the dark for 1 hr. 4’,6-Diamidino-2’-phenylindole (DAPI,Beyotime, Shanghai) was adopted to counterstain the nuclei, and the fluorescent signals were immediately detected by laser confocal microscopy.
CCK-8 assay and flow cytometric assays of cell cycle
At 72 hr after transfection, the media in the four groups were replaced with 1 mL complete medium containing 10% CCK-8 reagent and incubated at 37°C for 4 hr. The absorbance of each well at 450 nm was read using a microplate meter. The experiment was repeated independently for three times, with three replicates for each sample.
The effects of Mir204/211 on CIA FLS cell cycle were evaluated according to cell cycle detection kit (Beyotime, Shanghai). Transfected cells in four groups were collected 72 hr after transfection. Cell cycle assays were conducted with PI staining reagent in a flow cytometer (BD, CA).
Prediction of downstream target genes of Mir204/211
The TargetScan (http://www.targetscan.org), miRDB (http://miRdb.org/ miRDB/ index.html), miRGEN v.3 (https://www.microrna.gr/mirgen), PicTar (https://pictar.mdc-berlin.de/), and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/) were used to predict the downstream target genes of miR-204 and miR-211, respectively. Through the intersection of the four databases (The TargetScan, miRDB, miRGEN v.3 and PicTar), the downstream target genes of Mir204 and Mir211 were predicted. Through the re-intersection of the predicted downstream target genes of Mir204 and Mir211, the same target genes of Mir204 and Mir211 were obtained. The probability of each predicted target gene was verified by the databases miRTarbase and miRGen v.3 again. Meanwhile, TargetScan was used to predict the possible binding sites between target genes and Mir204/211.
Behavioral evaluation, immunohistochemistry, and histological analysis
Body weight, paw swelling, and arthritis scores of each mouse were measured twice a week since day 0. To evaluate the severity of arthritis, the swelling of the forelimbs and hindlimbs of each mouse was assessed using the AI. A score of 0–4 for each paw and 0–16 for each mouse was calculated twice a week, where 0 represents normal and 4 represents severe swelling and joint deformity.
Mice were sacrificed 37 days after the second immunization. Knee joints were retained and fixed in 4% paraformaldehyde for paraffin embedding and pathological observation.
Knee joints of hind legs were decalcified in 10% EDTA and embedded in paraffin. 5-μm thick sections were obtained and underwent H&E staining for histological analysis. The pathological changes were assessed by two blinded observers from aspects of hyperplasia, pannus formation, and cartilage destruction, where 0 indicated normal cell structure without inflammation, and 3 exhibited excessive inflammation with pannus formation and severe articular cartilage damage.
IHC staining was performed on knee joint tissues. Sections were dewaxed in xylene, hydrated with gradient alcohol, incubated overnight with the corresponding primary antibody at 4°C. The primary antibody was then discarded, incubated with the secondary antibody at room temperature for 30 min, and stained with Mayer hematoxylin. The number and percentage of positive-stained cells were observed under the microscope.
Statistical analysis
All experiment data were expressed as mean ± SEM, and data analyses were conducted with SPSS20.0. One-way ANOVA followed by the Tukey Post-Hoc test was adopted for multiple comparisons of statistical differences, and Students’ t-test was performed for comparisons between two groups. A p-value less than 0.05 was regarded as statistical significance.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting file.
References
-
Current status and strategy of microRNA research for cartilage development and osteoarthritis pathogenesisJournal of Bone Metabolism 23:121–127.https://doi.org/10.11005/jbm.2016.23.3.121
-
Pain in rheumatoid arthritis: models and mechanismsPain Management 6:265–284.https://doi.org/10.2217/pmt.16.4
-
Mir-223 inhibits the proliferation, invasion and EMT of nasopharyngeal carcinoma cells by targeting SSRP1International Journal of Clinical and Experimental Pathology 11:4374–4384.
-
Curaxins: anticancer compounds that simultaneously suppress NF-κB and activate p53 by targeting factScience Translational Medicine 3:95ra74.https://doi.org/10.1126/scitranslmed.3002530
-
Functional interactions between lncrnas/circrnas and mirnasFrontiers in Immunology 13:810317.https://doi.org/10.3389/fimmu.2022.810317
-
Bcl-Xl and Mcl-1 upregulation by calreticulin promotes apoptosis resistance of fibroblast-like synoviocytes via activation of PI3K/Akt and STAT3 pathways in rheumatoid arthritisClinical and Experimental Rheumatology 36:841–849.
-
Hsa_circ_0001859 regulates ATF2 expression by functioning as an mir-204/211 sponge in human rheumatoid arthritisJournal of Immunology Research 2018:9412387.https://doi.org/10.1155/2018/9412387
-
Blocking of YY1 reduce neutrophil infiltration by inhibiting IL-8 production via the PI3K-AKT-mTOR signaling pathway in rheumatoid arthritisClinical and Experimental Immunology 195:226–236.https://doi.org/10.1111/cei.13218
-
Crosstalk of reactive oxygen species and NF-κB signalingCell Research 21:103–115.https://doi.org/10.1038/cr.2010.178
-
Monitoring of peripheral blood cluster of differentiation 4+ adenosine triphosphate activity and CYP3A5 genotype to determine the pharmacokinetics, clinical effects and complications of tacrolimus in patients with autoimmune diseasesExperimental and Therapeutic Medicine 15:532–538.https://doi.org/10.3892/etm.2017.5364
-
Subintimal Ki-67 as a synovial tissue biomarker for inflammatory arthropathiesAnnals of the Rheumatic Diseases 67:162–167.https://doi.org/10.1136/ard.2007.071670
-
Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritisArthritis and Rheumatism 58:1001–1009.https://doi.org/10.1002/art.23386
-
Ki-67: more than a proliferation markerChromosoma 127:175–186.https://doi.org/10.1007/s00412-018-0659-8
-
Naturally occurring and synthetic inhibitors of NF-kappab functionsAnti-Cancer Drug Design 15:239–244.https://doi.org/10.2174/138955706777934937
-
Ssrp1 influences colorectal cancer cell growth and apoptosis via the Akt pathwayInternational Journal of Medical Sciences 16:1573–1582.https://doi.org/10.7150/ijms.38439
-
Paeoniflorin-6’-O-benzene sulfonate down-regulates CXCR4-Gβγ-PI3K/AKT mediated migration in fibroblast-like synoviocytes of rheumatoid arthritis by inhibiting GRK2 translocationBiochemical and Biophysical Research Communications 526:805–812.https://doi.org/10.1016/j.bbrc.2020.03.164
-
CypB-CD147 signaling is involved in crosstalk between cartilage and FLS in collagen-induced arthritisMediators of Inflammation 2020:6473858.https://doi.org/10.1155/2020/6473858
-
Inflammation suppression by dexamethasone via inhibition of CD147-mediated NF-κB pathway in collagen-induced arthritis ratsMolecular and Cellular Biochemistry 473:63–76.https://doi.org/10.1007/s11010-020-03808-5
-
Homocysteine elicits an M1 phenotype in murine macrophages through an EMMPRIN-mediated pathwayCanadian Journal of Physiology and Pharmacology 93:577–584.https://doi.org/10.1139/cjpp-2014-0520
-
Ssrp1 promotes colorectal cancer progression and is negatively regulated by mir-28-5pJournal of Cellular and Molecular Medicine 23:3118–3129.https://doi.org/10.1111/jcmm.14134
-
An emerging role of interleukin-23 in rheumatoid arthritisImmunopharmacology and Immunotoxicology 41:185–191.https://doi.org/10.1080/08923973.2019.1610429
-
Aspirin promotes apoptosis and inhibits proliferation by blocking G0/G1 into S phase in rheumatoid arthritis fibroblast-like synoviocytes via downregulation of JAK/STAT3 and NF-κB signaling pathwayInternational Journal of Molecular Medicine 42:3135–3148.https://doi.org/10.3892/ijmm.2018.3883
Article and author information
Author details
Funding
National Natural Science Foundation of China (82172383)
- Ting-Yu Wang
National Natural Science Foundation of China (82030067)
- Di Chen
National Natural Science Foundation of China (81874011)
- Ting-Yu Wang
National Natural Science Foundation of China (82161160342)
- Di Chen
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) grants 82172383 and 81874011 to T-YW and NSFC grants 82030067 and 82161160342 to DC. This work was also partially supported by the Shanghai Municipal Science and Technology Commission [Innovation Grant 18140903502 (to T-YW)].
Ethics
Animal protocol for this study has been approved by Animal Ethics Committee of Shanghai Ninth People's Hospital (ID HKDL[2018]344).
Copyright
© 2022, Wang, Fan et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 491
- views
-
- 178
- downloads
-
- 6
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Genetics and Genomics
A glaucoma polygenic risk score (PRS) can effectively identify disease risk, but some individuals with high PRS do not develop glaucoma. Factors contributing to this resilience remain unclear. Using 4,658 glaucoma cases and 113,040 controls in a cross-sectional study of the UK Biobank, we investigated whether plasma metabolites enhanced glaucoma prediction and if a metabolomic signature of resilience in high-genetic-risk individuals existed. Logistic regression models incorporating 168 NMR-based metabolites into PRS-based glaucoma assessments were developed, with multiple comparison corrections applied. While metabolites weakly predicted glaucoma (Area Under the Curve = 0.579), they offered marginal prediction improvement in PRS-only-based models (p=0.004). We identified a metabolomic signature associated with resilience in the top glaucoma PRS decile, with elevated glycolysis-related metabolites—lactate (p=8.8E-12), pyruvate (p=1.9E-10), and citrate (p=0.02)—linked to reduced glaucoma prevalence. These metabolites combined significantly modified the PRS-glaucoma relationship (Pinteraction = 0.011). Higher total resilience metabolite levels within the highest PRS quartile corresponded to lower glaucoma prevalence (Odds Ratiohighest vs. lowest total resilience metabolite quartile=0.71, 95% Confidence Interval = 0.64–0.80). As pyruvate is a foundational metabolite linking glycolysis to tricarboxylic acid cycle metabolism and ATP generation, we pursued experimental validation for this putative resilience biomarker in a human-relevant Mus musculus glaucoma model. Dietary pyruvate mitigated elevated intraocular pressure (p=0.002) and optic nerve damage (p<0.0003) in Lmx1bV265D mice. These findings highlight the protective role of pyruvate-related metabolism against glaucoma and suggest potential avenues for therapeutic intervention.
-
- Cell Biology
G protein-coupled receptors (GPCRs) are integral membrane proteins which closely interact with their plasma membrane lipid microenvironment. Cholesterol is a lipid enriched at the plasma membrane with pivotal roles in the control of membrane fluidity and maintenance of membrane microarchitecture, directly impacting on GPCR stability, dynamics, and function. Cholesterol extraction from pancreatic beta cells has previously been shown to disrupt the internalisation, clustering, and cAMP responses of the glucagon-like peptide-1 receptor (GLP-1R), a class B1 GPCR with key roles in the control of blood glucose levels via the potentiation of insulin secretion in beta cells and weight reduction via the modulation of brain appetite control centres. Here, we unveil the detrimental effect of a high cholesterol diet on GLP-1R-dependent glucoregulation in vivo, and the improvement in GLP-1R function that a reduction in cholesterol synthesis using simvastatin exerts in pancreatic islets. We next identify and map sites of cholesterol high occupancy and residence time on active vs inactive GLP-1Rs using coarse-grained molecular dynamics (cgMD) simulations, followed by a screen of key residues selected from these sites and detailed analyses of the effects of mutating one of these, Val229, to alanine on GLP-1R-cholesterol interactions, plasma membrane behaviours, clustering, trafficking and signalling in INS-1 832/3 rat pancreatic beta cells and primary mouse islets, unveiling an improved insulin secretion profile for the V229A mutant receptor. This study (1) highlights the role of cholesterol in regulating GLP-1R responses in vivo; (2) provides a detailed map of GLP-1R - cholesterol binding sites in model membranes; (3) validates their functional relevance in beta cells; and (4) highlights their potential as locations for the rational design of novel allosteric modulators with the capacity to fine-tune GLP-1R responses.






